Introduction
Anaplastic thyroid cancer (ATC) accounts for 1-2% of
all thyroid cancers, but has a very poor prognosis (1,2). It is
an aggressive disease with a mean survival time of 3-6 months after
initial diagnosis, and a one-year survival prognosis of 5-20%
(3).
The poor prognosis of ATC results from a tumor
biology that promotes aggressive tumor growth with rapid invasion
of the surrounding structures of the thyroid gland, including
muscles, trachea, esophagus and recurrent laryngeal nerve, in
addition to the development of distant metastasis (4,5).
Unfortunately, no established treatment strategies exist for ATC.
Although radical resection of the tumor is a common therapeutic
option, previous studies suggest that concurrent postoperative
radiotherapy and chemotherapy may offer further survival benefits
(6,7). However, these treatments do not
greatly improve the prognosis of ATC.
Importantly, there are some patients who have had
long-term survival. In these patients, improved prognosis was
related to treatment by radical resection. Currently, reported
prognostic factors for ATC include acute symptoms, tumor size
>50 mm, presence of distant metastasis, WBC count of over
10,000/mm2, T4b and age over 70(3). However, these reports are few and in
between, thus, we investigated the key prognostic factors for ATC
in 17 patients of our own.
Materials and methods
Background
Seventeen patients with histologically confirmed ATC
treated between 1990 and 2019, were identified from the thyroid
databases of the Nara Medical University in Japan. The inclusion
criteria was evidence of ATC at histopathologic examination of the
primary tumor, lymph node involvement, or distant metastases. ATCs
were classified into three types: Common type, incidental type and
anaplastic transformation type. In this study, we investigated only
common type ATCs, and excluded incidental type and anaplastic
transformation type.
Preoperative staging
Preoperative staging of patients included
laryngoscopy, neck ultrasonography and computed tomography (CT) of
head, neck and thorax to determine the extent of tumor
infiltration. Only patients with a confirmed histopathological
diagnosis of ATC by the surgical specimen were included. Poorly
differentiated thyroid carcinomas were excluded. Patients were
allocated to surgery, radiotherapy, chemotherapy, a combination of
treatment modalities, or to best supportive care based on the
preoperative staging.
Prognostic factors
Follow-up time started from the date of initial
diagnosis, beginning in January 1990, and ended at either death, or
December 2019. The primary endpoint of the study was the
disease-related OS. To investigate prognostic factors for ATC, we
compared survival rates by age, tumor size, WBC count, presence of
distant metastasis, treatment by radical resection and
tracheostomy.
Statistical analysis
Statistical analysis was performed with StatMate V
statistical software (ATMS Co., Ltd.). Overall survival after ATC
diagnosis was analyzed using the Kaplan-Meier method, and groups
were compared using the log-lank test. P-values <0.05 were
considered statistically significant. A multivariable analysis was
not performed, due to the small number of the patients.
Results
Clinical characteristics
The clinical characteristics of the 17 evaluated
patients are shown in Table I. Nine
female and 8 male patients ranging from 58 to 84 years of age were
included, with a median age of 74 years.
 | Table ICharacteristics of patients with
anaplastic thyroid cancer. |
Table I
Characteristics of patients with
anaplastic thyroid cancer.
| Characteristics | Value |
|---|
| Number of
patients | 17 |
| Sex, n | |
|
Female | 9 |
|
Male | 8 |
| Age, years
(range) | 74 (58-84) |
| Median tumor size, mm
(range) | 64 (38-100) |
| Median white blood
cell count, mm2 (range) | 10,800
(3,800-26,300) |
| Distant metastasis,
n | 5 |
| Radical resection,
n | 6 |
| Tracheostomy, n | 5 |
Treatment strategies
The treatment strategies are shown in Table II. Six of 17 patients with IVA/B
carcinomas underwent potentially curative treatments. Two patients
received radical resection, three patients radical resection with
radiotherapy and one patient radical resection with chemotherapy.
Four patients received radiotherapy only. Only one of 17 patients
received best support care. In five of 17 patients, tracheostomies
were performed.
 | Table IIPerformed treatment in patients with
anaplastic thyroid cancer. |
Table II
Performed treatment in patients with
anaplastic thyroid cancer.
| Potentially curative
treatment | No. of patients
(%) |
|---|
| Radical
resection | 6(35) |
|
Radical
resection only | 2(12) |
|
Radical
resection + RT | 3(18) |
|
Radical
resection + PTX | 1(6) |
| RT | 4(24) |
| CT | 6(35) |
| Best supportive
care | 1(6) |
Prognostic factors
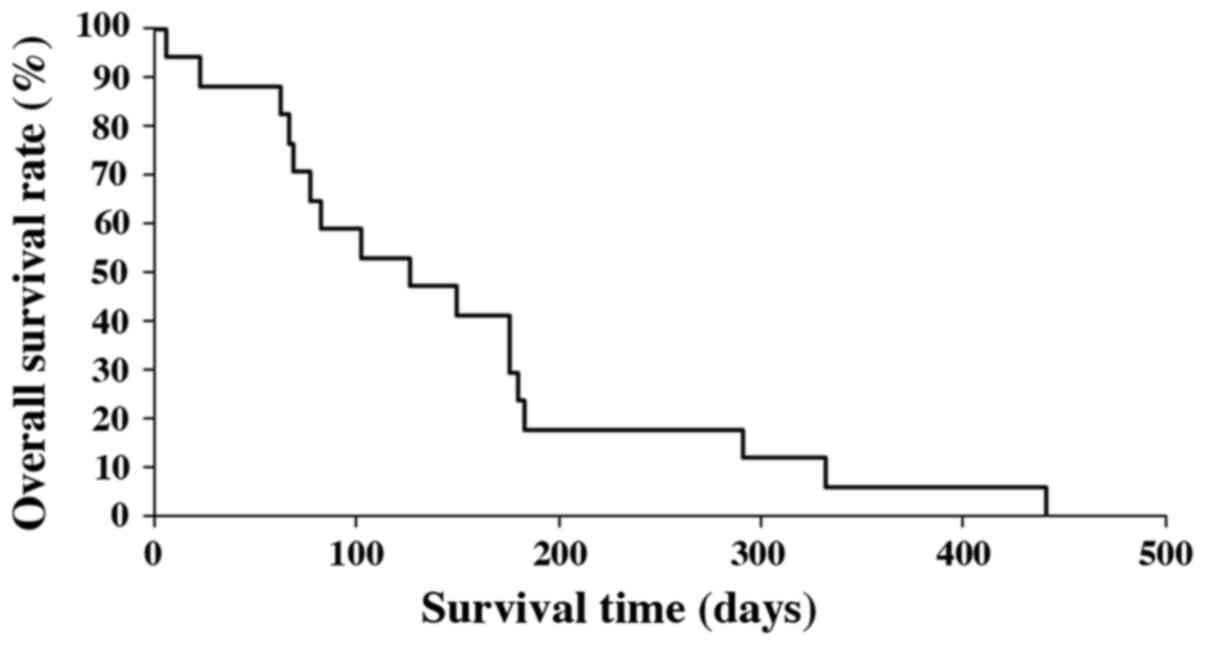
Median OS of all patients was 3.8 months (range
6-442 days). Survival rates at 6 and 12 months after the initial
diagnosis were 17.6 and 5.9%, respectively (Fig. 1). The median OS of patients under
the age of 70, without distant metastasis and who had radical
resection, were significantly longer compared to those patients
over the age of 70, with distant metastasis and no treatment by
radical resection (P=0.010, P=0.00022 and P=0.0036, respectively;
Figs.
2-4). There was no significant effect on OS from tumor size or
WBC count. Moreover, tracheostomy did not contribute to improvement
of prognosis (P=0.71). The prognostic factors of ATC in this study
are shown in Table III.
 | Table IIIPrognostic factors of anaplastic
thyroid cancer. |
Table III
Prognostic factors of anaplastic
thyroid cancer.
| Factors | P-value |
|---|
| Age (≥70 vs. <70
years) | 0.01000 |
| Tumor size (≥50 vs.
<50 mm) | 0.38000 |
| White blood cell
count (≥10,000 vs. <10,000 mm2) | 0.12000 |
| Distant metastasis (+
or -) | 0.00022 |
| Radical resection (+
or -) | 0.00360 |
| Tracheostomy (+ or
-) | 0.71000 |
Discussion
ATC accounts for 1-2% of all thyroid carcinomas and
is characteristic for fast progression, local invasion and a high
rate of distant metastasis, all which result in poor prognosis
(8). A generally accepted treatment
strategy for ATC is not yet established (9,10).
Despite prolonged overall survival obtained by a combination
therapy consisting of surgery, radiotherapy and chemotherapy
(median survival of 9.9 months), more clinical research is still
needed for the treatment of ATC (11). Radical treatment may worsen the
quality of life, and occasionally even shorten the survival.
Selecting patients who will benefit from either aggressive therapy
or only supportive care is important in the management of ATC.
In 2001, Sugitani et al (12) devised a prognostic index (PI) based
on four unfavorable prognostic factors present in patients with
ATC. The factors were, i) acute symptoms (duration of severe
complaints such as dysphonia, dysphagia, dyspnea and rapid growth
of the tumor <1 month); ii) WBC count >10,000
/mm2; iii) tumor size >5 cm, and iv) distant
metastasis. In the present study, patients with PI=1 showed a 62%
survival rate at 6 months, whereas no patients with PI=3 or 4
survived longer than 6 months. All patients with PI=4 died within 3
months. Moreover, according to the ATC Research Consortium of Japan
(ATCCJ) established in January 2009, that in addition to PI, both
aged over 70 and T4b-stage tumor were confirmed to be significant
risk factors for cause-specific death from ATC (3).
We found the median OS of all patients was 3.8
months with 6 and 12 months survival rates of 17.6 and 5.9%,
respectively, in agreement with previous reports. Median OS was
significantly longer in patients under the age of 70, without
distant metastasis, and who had radical resection. These results
also confirm the opinion of the ATCCJ. In contrast, we found no
significant differences regarding tumor size and WBC count, even
though they have previously been identified as prognostic factors
and included in the prognostic index for ATC. This may be due to an
insufficient number of cases in this study. Regarding T4b-stage
tumors and acute symptoms, we were unable to thoroughly investigate
these 17 cases. Moreover, tracheostomy did not improve prognosis,
thus we recommend that it should be avoided, whenever possible, to
preserve the quality of life of ATC patients.
Clinicians should take both the Union for
International Cancer Control (UICC) stage as an indication of
disease extent, and other prognostic factors representing the grade
of biological malignancy, into consideration when determining
treatment and prognosis. A distant metastasis exceedingly worsens
the prognosis of ATC patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TM, HU, IO and TKit conceived and designed the
study. TM, HU, IO, TKim, DN, TY, KY and TKit acquired data. TM, HU,
IO, TKim and DN analyzed and interpreted data. TM and TKit wrote
the original draft of the manuscript. TM, HU, IO and TKit reviewed
and edited the manuscript. All authors have accepted their
responsibility for the entire content of this manuscript and
approved the submission. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by Ethics Committee
of Nara Medical University Hospital, and written informed consent
was obtained from the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wachter S, Vorlander C, Schabram J,
Mintziras I, Fulber I, Manoharan J, Holzer K, Bartsch DK and Maurer
E: Anaplastic thyroid carcinoma: Changing trends of treatment
strategies and associated overall survival. Eur Arch
Otorhinolaryngol. 277:1507–1514. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Shinohara S, Kikuchi M, Naito Y, Fujiwara
K, Hori S, Tona Y, Yamazaki H, Kobayashi H and Ishihara T:
Successful treatment of locally advanced anaplastic thyroid
carcinoma by chemotherapy and hyperfractionated radiotherapy. Auris
Nasus Larynx. 36:729–732. 2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sugitani I, Onoda N, Ito KI and Suzuki S:
Management of anaplastic carcinoma: The fruits from the ATC
research consortium of Japan. J Nippon Med Sch. 85:18–27.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cabanillas ME, Zafereo M, Gunn GB and
Ferrarotto R: Anaplastic thyroid carcinoma: Treatment in the age of
molecular targeted therapy. J Oncol Pract. 12:511–518.
2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sykorova V, Dvorakova S, Vcelak J,
Vaclavikova E, Halkova T, Kodetova D, Lastuvka P, Betka J, Vlcek P,
Reboun M, et al: Search for new genetic biomarkers in poorly
differentiated and anaplastic thyroid carcinoma using next
generation sequencing. Anticancer Res. 35:2029–2036.
2015.PubMed/NCBI
|
|
6
|
Kwon J, Kim BH, Jung HW, Besic N, Sugitani
I and Wu HG: The prognostic impacts of postoperative radiotherapy
in the patients with resected anaplastic thyroid carcinoma: A
systematic review and meta-analysis. Eur J Cancer. 59:34–45.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pierie JP, Muzikansky A, Gaz RD, Faquin WC
and Ott MJ: The effect of surgery and radiotherapy on outcome of
anaplastic thyroid carcinoma. Ann Surg Oncol. 9:57–64.
2002.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kebebew E, Greenspan FS, Clark OH, Woeber
KA and McMillan A: Anaplastic thyroid carcinoma. Treatment outcome
and prognostic factors. Cancer. 103:1330–1335. 2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Granata R, Locati L and Licitra L:
Therapeutic strategies in the management of patients with
metastatic anaplastic thyroid cancer: Review of the current
literature. Curr Opin Oncol. 25:224–228. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Smallridge RC, Marlow LA and Copland JA:
Anaplastic thyroid cancer: Molecular pathogenesis and emerging
therapies. Endocr Relat Cancer. 16:17–44. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Haymart MR, Banerjee M, Yin H, Worden F
and Griggs JJ: Marginal treatment benefit in anaplastic thyroid
cancer. Cancer. 119:3133–3139. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sugitani I, Kasai N, Fujimoto Y and
Yanagisawa A: Prognostic factors and therapeutic strategy for
anaplastic carcinoma of thyroid. World J Surg. 25:617–622.
2001.PubMed/NCBI View Article : Google Scholar
|