Introduction
In Japan, the elderly comprised 27.7% of the total
population in 2017, making it the world's most rapidly aging
society. Moreover, 19.89 million women in Japan are aged 65 years
or over and account for 30.6% of the female population. By 2040,
the elderly populations of other Asian countries such as South
Korea, Singapore, and Thailand are predicted to reach levels
similar to the current level in Japan (1).
Given this background, gynecologists are expected to
treat an increasing number of older patients in the future. Among
the elderly, there is wide individual variation in health status,
ranging from robust individuals to frail elderly with serious
underlying conditions. Frail individuals who develop gynecological
cancer are often treated surgically, but postoperative
complications may arise and affect their activities of daily
living. Greater efforts to reduce such complications are required
on the part of gynecologists. However, sufficient evidence
supporting the prediction of elderly gynecological patients who are
more likely to develop postoperative complications has not yet been
obtained.
During assessment of the indications for surgery,
gynecologists often rely on ambiguous methodology, thereby risking
under- or over-treatment. Preoperative frailty assessments have
been useful for predicting postoperative complications, but these
have been conducted in a variety of ways and no standard assessment
has been established. This multicenter study was conducted to
identify the preoperative and intraoperative risk factors for
postoperative complications in older patients with gynecological
cancer. In this study, we focused on postoperative complications
and short-term prognosis.
Materials and methods
Patients
The study subjects included elderly patients aged
≥65 years who were diagnosed with primary gynecological cancer
between January 2015 and December 2015 at four Japanese university
hospitals located in major cities (Osaka University Hospital and
Yokohama City University Hospital) and in rural areas (Ryukyu
University Hospital and Fukui University Hospital). Age, family
composition, number of children, type of cancer, cancer stage,
medical history, Charlson comorbidity score (CCS), body mass index
(BMI), subjective global assessment (SGA), fall risk assessment,
American Society of Anesthesiologists physical status
classification, surgical Apgar score (SAS), type of surgery, and
1-year postoperative mortality were investigated retrospectively
and compared between subjects who did and did not develop
complications.
The SAS, which was developed by Gawande et al
(2) for patients undergoing open
colectomy, is an index for assessing a patient's general condition
during surgery and has been shown to be useful for predicting
complications after general and vascular surgery. Similar to the
Apgar score used for neonatal evaluation at birth, the SAS is
scored out of 10, with a higher score indicating a better
condition. It is very simple to use, and evaluates patients on the
basis of the three parameters of estimated blood loss, lowest mean
blood pressure, and lowest heart rate. It can be calculated
postoperatively from anesthesia records. One tool for assessing the
probability of postoperative complications is the web-based
National Surgical Quality Improvement Program Surgical Risk
Calculator, which was developed by the American College of Surgeons
(3) and can show the percentage
probability of the occurrence of serious complications for each
parameter. Although it has allowed patients to understand the
assessment results more easily, it has been reported by some
studies to not be particularly well suited to gynecological cancer
surgery (4,5).
Family composition was classified as living with
others, living alone, or other, such as institutional resident or
unknown. The type of cancer was classified as cervical cancer,
uterine cancer, uterine sarcoma, ovarian cancer, or vaginal/vulvar
cancer. Staging was according to the International Federation of
Gynecology and Obstetrics clinical staging system or the surgical
staging system (6-8).
In the medical history, hypertension was defined as a patient who
had undergone medical treatment, excluding cases that were treated
only with diet or exercise. Coronary artery disease was defined as
a definitive diagnosis obtained by cardiac catheterization or other
testing. Arrhythmia was defined as present only if the patient had
undergone medical treatment. Heart dysfunction was defined as grade
2 or worse aortic valve insufficiency, aortic stenosis, mitral
insufficiency, or tricuspid insufficiency, as confirmed by
echocardiography, excluding grade 1 conditions that did not affect
daily life. Cerebrovascular disorder was defined as noted in the
medical records. Rheumatoid arthritis was defined as treatment with
antirheumatic drugs. Mental illness was defined as schizophrenia,
major depressive disorder, or a similar disorder that had been
diagnosed and treated with oral psychotropic agents, excluding
treatment only with sleep medications. Dementia was defined as
noted in the patient's medical history, regardless of oral
medication intake. Asthma and dyslipidemia were defined as noted in
the patient's medical history. Diabetes mellitus was defined as
treatment with oral medication or insulin, excluding treatment only
with diet or exercise. Osteoporosis was defined as noted in the
patient's medical history, based on treatment that had been
provided, or when strongly suspected due to femoral neck fracture
or a similar event in the patient's clinical history.
The CCS was formulated as a preoperative comorbidity
score in 1987 by Charlson et al (9). In the present study, the version
revised by Quan et al (10)
in 2011 was used. The CCS classifies patients according to the
weight of 12 different medical conditions, with a higher score
indicating more severe comorbidities. The BMI was classified as
normal or obese, based on a cutoff value of 25 kg/m2.
Using the malnutrition risk score upon hospital admission, the SGA
was scored on a 3-point scale as low (1), moderate (2), or high (3). The postoperative fall risk was
assessed using somewhat different criteria among the different
institutions, but an assessment that broadly conformed to the Fall
Assessment Score Sheet, which was included in the Medical Safety
Manual for Healthcare Professionals (11) published by the Japan Medical
Association, was used.
As a measure of the patient's general condition
during surgery, the SAS was calculated on the basis of estimated
blood loss, lowest mean blood pressure, and lowest heart rate.
Similar to the neonatal evaluation at birth, it was scored out of
10, with a higher score indicating a better condition (Table I). Receiver operator characteristic
(ROC) curve analysis was performed to assess the ability of the SAS
to predict postoperative complications in the elderly; the
determined SAS cutoff values were used to divide the patients into
3 groups, including high risk (8-10), moderate risk (4-7), and low
risk (0-3) for postoperative complications.
 | Table ISurgical Apgar score. |
Table I
Surgical Apgar score.
| | Score |
|---|
| Variable | 0 | 1 | 2 | 3 | 4 |
|---|
| Intraoperative
estimated amount of bleeding, ml | >1,000 | 601-1,000 | 101-600 | ≤100 | |
| Minimal mean arterial
blood pressure, mmHg | <40 | 40-54 | 55-69 | ≥70 | |
| Minimum heart rate,
beats/minutes | >85 | 76-85 | 66-75 | 56-65 | ≤55 |
Postoperative complications were analyzed using the
Clavien-Dindo classification, following the Japan Clinical Oncology
Group criteria for postoperative complications (12). Complications were defined as
Clavien-Dindo classification grade III or above and requirement for
surgical, endoscopic, or interventional radiology treatment or
intensive care. Cases that improved with medication alone were
excluded.
Statistical analysis
Statistical analysis was performed using Fisher's
exact test for comparisons between the 2 groups, and logistic
regression analysis was performed for univariate and multivariate
analysis. A P-value <0.05 was considered to indicate statistical
significance. Sensitivity and specificity were evaluated using ROC
curves. All data were analyzed using SPSS version 21 statistical
software (IBM Inc., Armonk, NY, USA).
Results
Patients' characteristics
The patients' characteristics are shown in Table II. A total of 173 patients (mean
age 72.54 years) were enrolled from the four institutions. Overall,
76.3% of the patients were living with their husband or other
family members, and 87.3% had children.
 | Table IIPatient characteristics. |
Table II
Patient characteristics.
| Characteristic | Value |
|---|
| Number | 173 |
| Age, years (mean ±
SD) | 72.54±6.289 |
| Family composition, n
(%) | |
|
Living
alone | 41 (23.7) |
|
Living with
others | 102 (59.0) |
|
Other | 30 (17.3) |
| Children, n (%) | |
|
No | 21 (12.1) |
|
Yes | 151 (87.3) |
|
Unknown | 1 (0.6) |
| Cervical cancer, n
(%) | 16 (9.2) |
| Endometrial cancer, n
(%) | 81 (46.8) |
| Uterine sarcoma, n
(%) | 12 (6.9) |
| Ovarian cancer, n
(%) | 50 (28.9) |
| Vaginal/vulvar
cancer, n (%) | 14 (8.1) |
| Stage, n (%) | |
|
I | 108 (62.4) |
|
II | 15 (8.7) |
|
III | 38 (22.0) |
|
IV | 11 (6.4) |
|
Metastatic | 1 (0.6) |
| Surgical procedure, n
(%) | |
|
No open
surgery | 11 (6.4) |
|
Open surgery
without retroperitoneal operations | 80 (46.2) |
|
Open surgery
with retroperitoneal operations | 82 (47.4) |
| G3 or worse
complications, n (%) | |
|
No | 159 (91.9) |
|
Yes | 14 (8.1) |
| Death within 1 year
postoperatively, n (%) | |
|
No | 148 (85.5) |
|
Yes | 10 (5.8) |
|
Unknown | 15 (8.7) |
The most common types of cancer were endometrial
cancer (46.8%) and ovarian cancer (28.9%), both of which are
increasing in Japan. In 62.4% of cases, the cancer was identified
as stage I. The medical history of many patients included
hypertension (46.2%), dyslipidemia (26.0%), and diabetes (14.5%),
all of which are lifestyle diseases. The incidence of dementia was
1.2%, which was significantly lower than the estimated prevalence
of 17.9% among Japanese people aged ≥65 years in 2012. This was
most likely because of the presence of undiagnosed cases or because
surgery was not indicated for patients with dementia. Of those
patients who underwent abdominal surgery, the number of cases who
underwent retroperitoneal surgery was about the same as the number
who did not (46.2 vs. 47.4%). Ten patients (5.8%) who underwent
surgery died within 1 year postoperatively.
Postoperative prognosis
Grade 3 or worse postoperative complications
occurred in 14 patients (8.1%). There was 1 case of grade 5
complication of postoperative death caused by sepsis. Postoperative
death within 1 year was significantly more frequent in patients
with postoperative complications than in those without
postoperative complications (Table
III). Univariate analysis was carried out to investigate the
parameters associated with postoperative complications and death
within 1 year postoperatively. On univariate analysis (Table IV), BMI, mental illness, and SAS
were significantly associated with postoperative complications,
whereas cancer stage, CSS, and SAS were significantly associated
with death within 1 year postoperatively. Multivariate analysis by
logistic regression (Table V)
revealed that BMI and mental illness were risk factors for
postoperative complications, whereas low SAS increased the risk for
both postoperative complications and death within 1 year
postoperatively.
 | Table IIIAssociation between postoperative
complications and death within 1 year postoperatively. |
Table III
Association between postoperative
complications and death within 1 year postoperatively.
| | Death within 1 year
postoperatively | |
|---|
| G3 or worse
postoperative complications | No, n | Yes, n | Total, n | P-value |
|---|
| No | 139 | 7 | 146 | 0.030a |
| Yes | 9 | 3 | 12 | |
| Total | 148 | 10 | 158 | |
 | Table IVUnivariate analysis. |
Table IV
Univariate analysis.
| | G3 or worse
complications | Death within 1 year
postoperatively |
|---|
| Variable | Value | P-value | Odds ratio (95%
CI) | P-value | Odds ratio (95%
CI) |
|---|
| Patient
characteristics | | | | | |
|
Age, years
(mean ± SD) | 72.54±6.289 | 0.91 | 1.00
(0.91-1.09) | 0.24 | 1.06
(0.96-1.17) |
|
Children, n
(%) | 21 (12.1) | 0.98 | 1.01
(0.23-15.19) | 0.98 | 0.97
(0.11-8.26) |
|
Obesity (BMI
≥25 kg/m2) | | 0.03 | 3.54
(1.16-10.78) | 0.29 | 0.32
(0.04-2.62) |
|
Living with
other family members | | 0.46 | 2.53
(0.22-29.29) | 0.15 | 8.75
(0.45-168.60) |
| Medical history,
prevalence (%) | | | | | |
|
Hypertension | 80 (46.2) | 0.40 | 1.61
(0.54-4.86) | 0.39 | 1.77
(0.478-6.51) |
|
Coronary
artery disease | 11 (6.4) | >0.99 | - | >0.99 | - |
|
Arrhythmia | 11 (6.4) | >0.99 | - | 0.47 | 2.24
(0.248-20.22) |
|
Cardiac
dysfunction | 12 (6.9) | 0.98 | 1.04
(0.12-8.66) | 0.47 | 2.24
(0.248-20.22) |
|
Cerebrovascular
impairment | 15 (8.7) | >0.99 | - | 0.22 | 2.83
(0.54-14.87) |
|
Rheumatoid
arthritis | 1 (0.6) | >0.99 | - | >0.99 | - |
|
Mental
illness | 5 (2.9) | 0.03 | 8.67
(1.32-56.97) | 0.24 | 4.00
(0.40-39.60) |
|
Dementia | 2 (1.2) | >0.99 | - | >0.99 | - |
|
Asthma | 8 (4.6) | 0.64 | 1.67
(0.19-14.64) | >0.99 | - |
|
Diabetes | 25 (14.5) | 0.99 | 0.99
(0.21-4.69) | 0.71 | 0.67
(0.08-5.58) |
|
Dyslipidemia | 45 (26.0) | 0.82 | 1.15
(0.34-3.87) | 0.38 | 1.80
(0.48-6.71) |
|
Osteoporosis | 14 (8.1) | 0.89 | 0.86
(0.11-7.14) | >0.99 | - |
| Stage (1 or 2 vs. 3
or 4) | | 0.24 | 2.01
(0.69-6.12) | 0.01 | 7.47
(1.83-30.42) |
| Surgical procedure
(retroperitoneal operations) | | 0.73 | 0.86
(0.35-2.07) | 0.12 | 0.70
(0.26-1.87) |
| Assessment score,
mean ± SD | | | | | |
|
CCS
sore | 2.54±1.45 | 0.40 | 1.14
(0.84-1.54) | 0.01 | 1.43
(1.10-1.86) |
|
SGA
score | 1.10±0.40 | 0.22 | 2.14
(0.63-7.25) | 0.07 | 0.29
(0.93-12.53) |
|
Fall risk
assessment | 1.61±0.56 | 0.08 | 0.37
(0.13-1.12) | 0.06 | 0.27
(0.07-1.07) |
|
ASA
preoperative risk assessment | 1.88±0.53 | 0.84 | 0.90
(0.32-2.54) | 0.16 | 2.39
(0.71-8.07) |
|
SAS
score | 6.83±1.96 | 0.02 | 0.72
(0.55-0.94) | 0.01 | 0.64
(0.46-0.89) |
 | Table VMultivariate analysis using logistic
regression analysis. |
Table V
Multivariate analysis using logistic
regression analysis.
| | G3 or worse
complications | Death within 1 year
postoperatively |
|---|
| Variable | P-value | Odds ratio (95%
CI) | P-value | Odds ratio (95%
CI) |
|---|
| SAS score | 0.04 | 0.74
(0.55-0.99) | 0.05 | 0.69
(0.47-0.99) |
| Obesity (BMI ≥25
kg/m2) | 0.02 | 4.35
(1.27-14.88) | 0.56 | 0.52
(0.06-4.68) |
| CCS score | 0.87 | 1.03
(0.72-1.48) | 0.23 | 1.21
(0.89-1.66) |
| Mental illness | 0.02 | 11.68
(1.43-95.48) | 0.47 | 2.96
(0.16-55.88) |
| Stage 1 or 2 vs. 3
or 4 | 0.45 | 1.69
(0.43-6.64) | 0.11 | 3.65
(0.75-17.76) |
Surgical apgar score
The number and percentage of patients in each SAS
score are shown in Table VI. ROC
analysis of the SAS for postoperative complications showed an area
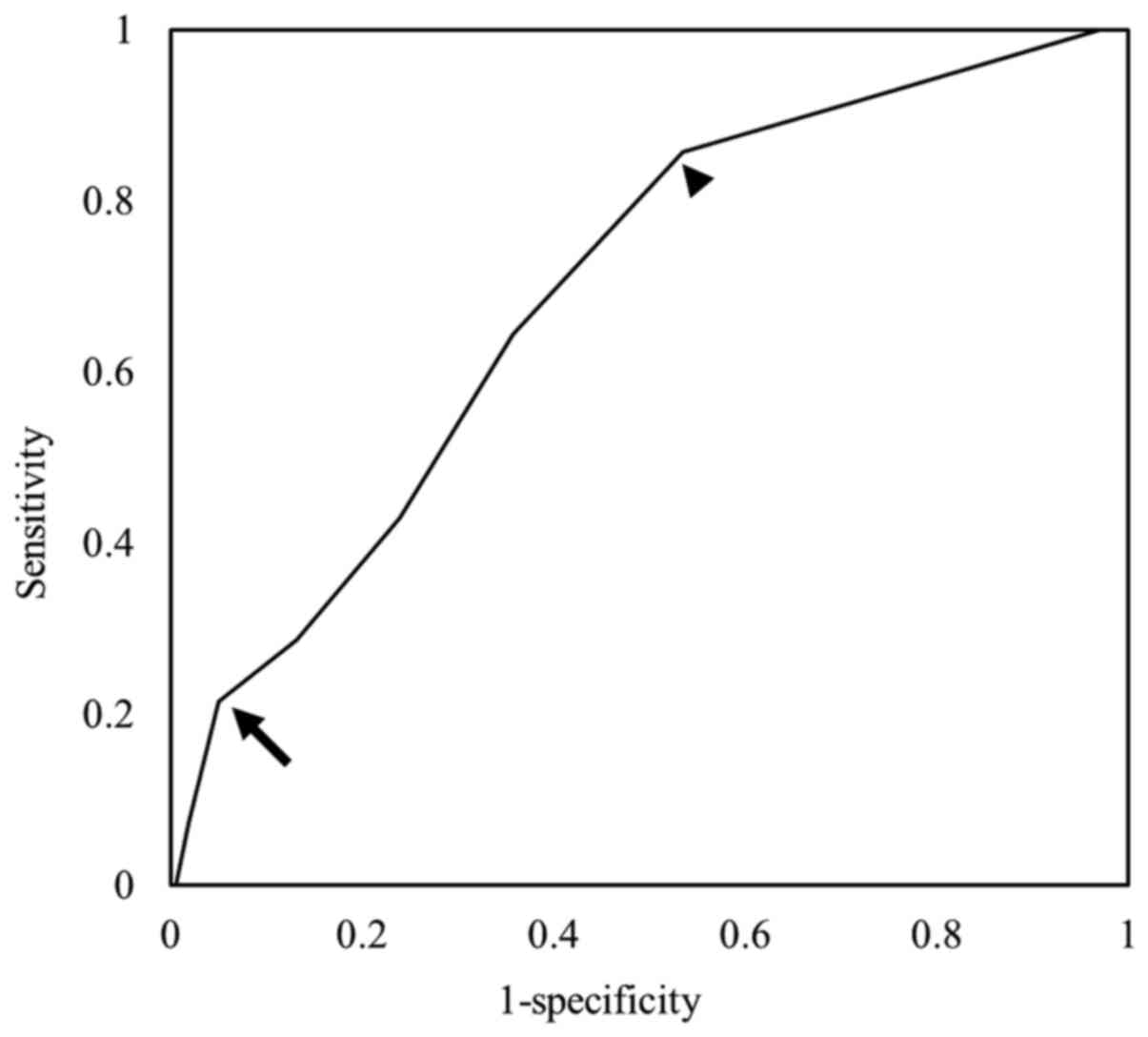
under the curve (AUC) of 0.649 (P=0.016). An SAS of ≤6 points
predicted postoperative complications with a sensitivity of 64.3%
and specificity of 64.2%. The SAS risk classification predicted
high risk with sensitivity of 85.7% and specificity of 46.5% and
low risk with sensitivity of 21.4% and specificity of 95% (Fig. 1).
 | Table VINumber and percentage of patients
with each SAS. |
Table VI
Number and percentage of patients
with each SAS.
| SAS score | Number | Percentage |
|---|
| 1 | 1 | 0.6 |
| 2 | 3 | 1.7 |
| 3 | 7 | 4.0 |
| 4 | 14 | 8.1 |
| 5 | 19 | 11.0 |
| 6 | 22 | 12.7 |
| 7 | 31 | 17.9 |
| 8 | 36 | 20.8 |
| 9 | 35 | 20.2 |
| 10 | 5 | 2.9 |
Discussion
In the present study, older gynecological cancer
patients with a preoperative history of mental illness, high CCS,
obesity, or low intraoperative SAS were found to be more likely to
develop postoperative complications, and patients with a low SAS
and those who developed postoperative complications were more
likely to die within 1 year postoperatively. In particular, low SAS
was an independent risk factor not only for the development of
postoperative complications, but also for death within 1 year
postoperatively.
A previous study reported that the lower the SAS,
the higher the probability of major complications or death
(2). Subsequently, the SAS was used
in a large-scale study (13) and
for gynecological surgery (14,15).
In a previous single-center study, we found that intraoperative SAS
was useful for predicting postoperative complications in
gynecological patients, including those without cancer (16). Moreover, the World Health
Organization recommended the use of the SAS for safe surgery
guidelines in the World Alliance for Patient Safety documents
(17). The SAS cannot be used to
assess the capability of patients to withstand surgery, because it
is based on intraoperative data; however, it may be helpful in
determining the requirement for postoperative intensive care. Based
on our ROC analysis, high-risk patients had a high probability of
requiring treatment for postoperative complications, whereas
low-risk patients had >95% probability of not developing
postoperative complications. Although the present study did not
distinguish between open and laparoscopic surgery, it is important
to note that SAS was originally developed for use in open surgery.
Moreover, attempts have been made to determine the utility of the
SAS for predicting perioperative complications during
robotic-assisted radical hysterectomy, in which blood loss is low
(18). Especially in endometrial
cancer, the usefulness of minimally invasive surgery in the elderly
has been reported (19,20), and it is necessary to examine the
usefulness of SAS in minimally invasive surgery in the future.
In the present study, history of mental illness,
obesity, and low SAS were all risk factors for developing
postoperative complications. Even a serious mental illness may not
always be detected on standard preoperative tests, such as blood
tests, urinalysis, electrocardiography, and plain radiography.
Therefore, clinicians are likely to be unaware that such patients
are at high risk. However, patients with psychiatric disorders tend
to complain less actively about their symptoms because of the
nature of their illness. Furthermore, the analgesic and sedative
effects of psychotropic medications may have led these patients to
have higher pain thresholds and to be less aware of postoperative
abnormalities. In fact, around half of patients who were less
likely to seek medical attention were reported to have physical
disorders, and many cases were not correctly diagnosed (21). Patients on psychotropic medications
were reported to have a high risk for venous thromboembolism (VTE)
(22,23), and those with schizophrenia were
known to have an increased risk for not only VTE, but also
respiratory failure (24). In a
pooled analysis of 3 GINECO phase 2 trials by Tinquaut et al
(25), depression and the Hospital
Anxiety and Depression Scale score were associated with survival.
In the present study, although mental illness was not found to have
a direct association with death within 1 year postoperatively, it
increased the risk for postoperative complications. These results
suggest that the presence of mental illness in older patients with
a gynecological malignancy may be associated with resistance to
treatment. It is important to note that the present study defined
postoperative complications as those with grade III or higher
Clavien-Dindo classification, thereby excluding medically treated
postoperative complications such as delirium. Therefore, the
incidence of complications in patients with mental illness may have
been underestimated. Clinicians should manage such patients with
greater caution. To the best of our knowledge, no study has
identified the association of postoperative complications with
widely used antidepressants, such as selective serotonin reuptake
inhibitors, serotonin-norepinephrine reuptake inhibitors, or
noradrenergic and specific serotonergic antidepressants. This is an
important topic for future research.
As previously mentioned, the present study showed
that patients with low SAS and those who developed postoperative
complications were more likely to die within 1 year
postoperatively. However, the direct causality between
postoperative complications and increased risk for death, as well
as the possible confounding effect of a poor general condition on
death within 1 year as a result of postoperative complications, was
unclear. Nevertheless, because our analysis showed that SAS was an
independent risk factor for death within 1 year postoperatively,
patients with low SAS scores may require careful postoperative
follow-up. Of the 3 patients who developed postoperative
complications and died within 1 year, 1 patient was classified as
CCS 2, SAS 3, and did not have a mental illness; 1 patient was CCS
2, SAS 4, and did not have a mental illness; and 1 patient was CCS
9, SAS 5, and had a mental illness.
Based on our results, SAS may be useful for
assessing the risks for postoperative complications and death
within 1 year postoperatively. In the present study, the only
predictor of death within 1 year postoperatively was the SAS score,
which is an intraoperative assessment, and no preoperative
predictive factor was identified. The Vulnerable Elders Survey-13
(VE-13) has been shown to be useful for pre-chemotherapy assessment
(26), but other studies have found
that the preoperative Comprehensive Geriatric Assessment and GA-GYN
scores were not predictive of postoperative outcomes (27,28).
Therefore, a preoperative risk assessment score for the treatment
of older patients with gynecological cancer has yet to be
established. New preoperative assessment tools that can anticipate
low SAS scores and reflect the postoperative prognosis of older
gynecologic patients must be addressed and developed.
This study had a number of limitations. First, this
study did not collect data on postoperative treatment. However,
this study analyzes the short-term prognosis of one year, and we
believe that the impact of the choice of postoperative treatment on
the prognosis is limited. Although this was a multicenter analysis,
it included only 173 patients from four hospitals. More
gynecological institutions in Japan need to take part in clinical
studies. In addition, alternative questions were used in the survey
about postoperative complications. Therefore, in the 14 patients
who developed postoperative complications, the detailed breakdown
of conditions, such as infection, ileus, and thrombosis, was
unknown. With the expected rise in the number of older patients who
require treatment for gynecological cancer, the effects of newer
psychotropic medications and the increasing use of laparoscopic
surgery necessitate further study.
In conclusion, the SAS may be useful for assessing
the risks of postoperative complications and death within 1 year
postoperatively. It is important to develop a preoperative
assessment tool that can predict a low SAS score and reflect the
postoperative prognosis of older gynecological cancer patients.
Acknowledgements
The authors would like to thank Dr Tetsuji Kurokawa,
Dr Yoko Chino and Dr Akiko Shinagawa (all University of Fukui,
Fukui, Japan) for their advice on data analysis and assistance with
the study.
Funding
The present study was partly supported by a
Grant-in-Aid for Scientific Research from the Japan Society for the
Promotion of Science (grant no. 18H02944).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KK, MY, and YY wrote the manuscript. KK, MY, MAS,
MS, YU, YA and YY collected and analyzed data, and edited the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was reviewed and approved by the
institutional review boards of University of Fukui, Yokohama City
University Graduate School of Medicine, Osaka University Graduate
School of Medicine and University of the Ryukyus before the start
of the present study. Informed consent was obtained in the form of
an opt-out option on the website.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Annual Report on the Aging Society: 2018
(Summary). Cabinet Office, Tokyo, 2018.
|
|
2
|
Gawande AA, Kwaan MR, Regenbogen SE,
Lipsitz SA and Zinner MJ: An apgar score for surgery. J Am Coll
Surg. 204:201–208. 2007.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bilimoria KY, Liu Y, Paruch JL, Zhou L,
Kmiecik TE, Ko CY and Cohen ME: Development and evaluation of the
universal ACS NSQIP surgical risk calculator: A decision aid and
informed consent tool for patients and surgeons. J Am Coll Surg.
217:833–842. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Szender JB, Frederick PJ, Eng KH, Akers
SN, Lele SB and Odunsi K: Evaluation of the national surgical
quality improvement program universal surgical risk calculator for
a gynecologic oncology service. Int J Gynecol Cancer. 25:512–520.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rivard C, Nahum R, Slagle E, Duininck M,
Vogel RI and Teoh D: Evaluation of the performance of the ACS NSQIP
surgical risk calculator in gynecologic oncology patients
undergoing laparotomy. Gynecol Oncol. 141:281–286. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pecorelli S: Revised FIGO staging for
carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol
Obstet. 105:103–104. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Prat J: FIGO Committee on Gynecologic
Oncology. Staging classification for cancer of the ovary, fallopian
tube, and peritoneum. Int J Gynaecol Obstet. 124:1–5.
2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Prat J: FIGO staging for uterine sarcomas.
Int J Gynaecol Obstet. 104:177–178. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Charlson ME, Pompei P, Ales KL and
MacKenzie CR: A new method of classifying prognostic comorbidity in
longitudinal studies: Development and validation. J Chronic Dis.
40:373–383. 1987.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Quan H, Li B, Couris CM, Fushimi K, Graham
P, Hider P, Januel JM and Sundararajan V: Updating and validating
the charlson comorbidity index and score for risk adjustment in
hospital discharge abstracts using data from 6 countries. Am J
Epidemiol. 173:676–682. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kobayashi K, Imagama S, Ando K, Inagaki Y,
Suzuki Y, Nishida Y, Nagao Y and Ishiguro N: Analysis of falls that
caused serious events in hospitalized patients. Geriatr Gerontol
Int. 17:2403–2406. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Katayama H, Kurokawa Y, Nakamura K, Ito H,
Kanemitsu Y, Masuda N, Tsubosa Y, Satoh T, Yokomizo A, Fukuda H and
Sasako M: Extended clavien-dindo classification of surgical
complications: Japan clinical oncology group postoperative
complications criteria. Surg Today. 46:668–685. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Regenbogen SE, Ehrenfeld JM, Lipsitz SR,
Greenberg CC, Hutter MM and Gawande AA: Utility of the surgical
apgar score: Validation in 4119 patients. Arch Surg. 144:30–36.
2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zighelboim I, Kizer N, Taylor NP, Case AS,
Gao F, Thaker PH, Rader JS, Massad LS, Mutch DG and Powell MA:
‘Surgical Apgar Score’ predicts postoperative complications after
cytoreduction for advanced ovarian cancer. Gynecol Oncol.
116:370–373. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Clark RM, Lee MS, Rauh-Hain JA, Hall T,
Boruta DM, del Carmen MG, Goodman A, Schorge JO and Growdon WB:
Surgical apgar score and prediction of morbidity in women
undergoing hysterectomy for malignancy. Gynecol Oncol. 136:516–520.
2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kurata K, Chino Y, Shinagawa A, Kurokawa T
and Yoshida Y: Surgical apgar score predicts 30-day morbidity in
elderly patients who undergo non-laparoscopic gynecologic surgery:
A retrospective analysis. Int J Surg. 48:215–219. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
World Health Organization (WHO): World
Alliance for Patient Safety: forward programme 2008-2009, 1st
edition. WHO, Geneva, 2008. urihttps://apps.who.int/iris/handle/10665/70460simplehttps://apps.who.int/iris/handle/10665/70460.
Accessed June 25, 2008.
|
|
18
|
Park SH, Lee JY, Nam EJ, Kim S, Kim SW and
Kim YT: Prediction of perioperative complications after
robotic-assisted radical hysterectomy for cervical cancer using the
modified surgical apgar score. BMC Cancer. 18(908)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Uccella S, Bonzini M, Palomba S, Fanfani
F, Malzoni M, Ceccaroni M, Seracchioli R, Ferrero A, Berretta R,
Vizza E, et al: Laparoscopic vs. open treatment of endometrial
cancer in the elderly and very elderly: An age-stratified
multicenter study on 1606 women. Gynecol Oncol. 141:211–217.
2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Berretta R, Merisio C, Melpignano M, Rolla
M, Ceccaroni M, DE Ioris A, Patrelli TS and Nardelli GB: Vaginal
versus abdominal hysterectomy in endometrial cancer: A
retrospective study in a selective population. Int J Gynecol
Cancer. 18:797–802. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Goldman LS: Medical illness in patients
with schizophrenia. J Clin Psychiatry. 60 (Suppl 21):S10–S15.
1999.PubMed/NCBI
|
|
22
|
Liperoti R, Pedone C, Lapane KL, Mor V,
Bernabei R and Gambassi G: Venous thromboembolism among elderly
patients treated with atypical and conventional antipsychotic
agents. Arch Intern Med. 165:2677–2682. 2005.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Parker C, Coupland C and Hippisley-Cox J:
Antipsychotic drugs and risk of venous thromboembolism: Nested
case-control study. BMJ. 341(c4245)2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Daumit GL, Pronovost PJ, Anthony CB,
Guallar E, Steinwachs DM and Ford DE: Adverse events during medical
and surgical hospitalizations for persons with schizophrenia. Arch
Gen Psychiatry. 63:267–272. 2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tinquaut F, Freyer G, Chauvin F, Gane N,
Pujade-Lauraine E and Falandry C: Prognostic factors for overall
survival in elderly patients with advanced ovarian cancer treated
with chemotherapy: Results of a pooled analysis of three GINECO
phase II trials. Gynecol Oncol. 143:22–26. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ferrero A, Villa M, Tripodi E, Fuso L and
Menato G: Can vulnerable elders survey-13 predict the impact of
frailty on chemotherapy in elderly patients with gynaecological
malignancies? Medicine (Baltimore). 97(e12298)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Suh DH, Kim JW, Kim HS, Chung HH, Park NH
and Song YS: Pre- and intra-operative variables associated with
surgical complications in elderly patients with gynecologic cancer:
The clinical value of comprehensive geriatric assessment. J Geriatr
Oncol. 5:315–322. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ahmed A, Deng W, Tew W, Bender D, Mannel
RS, Littell RD, DeNittis AS, Edelson M, Morgan M, Carlson J, et al:
Pre-Operative assessment and post-operative outcomes of elderly
women with gynecologic cancers, primary analysis of NRG CC-002: An
NRG oncology group/gynecologic oncology group study. Gynecol Oncol.
150:300–305. 2018.PubMed/NCBI View Article : Google Scholar
|