Introduction
The age-adjusted incidence and mortality of thyroid
cancer was 15.03 and 0.51 per 100,000 individuals, respectively, in
2015 (https://seer.cnacer.gov/csr/1975_2115/). The
increasing incidence of thyroid cancer is mostly because of
increasing surveillance and overdiagnosis (1,2).
In Japan, the age-adjusted incidence of thyroid
cancer was 8.7 per 100,000 person-years in 2017, whereas the
age-adjusted mortality rate was 0.3 per 100,000 person-years in
2018 (https://ganjoho.jp/reg_stat/index.html). Since the
introduction of tyrosine kinase inhibitors (TKIs) for cancer
treatment, their efficacy and adverse events (AEs) have been
reported for various carcinomas (3,4). TKI
treatment has also been introduced as a guideline for treating
patients with radioactive iodine (RAI)-refractory differentiated
thyroid carcinoma (DTC) (5).
Patients with recurrent distant metastases of advanced thyroid
cancer are refractory to RAI; another option for TKI treatment is
external irradiation, but there are limited sites and frequency of
this alternative treatment. The therapeutic effect of TKIs depends
on the target lesion (6). For
instance, the treatment results for pulmonary metastases are
excellent, whereas those for bone metastases and unresectable local
lesions are poor. The efficacy of TKIs represented a breakthrough
for cancer treatment, and these drugs have attracted the attention
of several clinicians. However, their clinical utility is limited
by frequent AEs (7,8). High blood pressure, cutaneous
symptoms, and digestive tract symptoms can be treated with
appropriate management. Conversely, tumor necrosis-induced
cavitation resulting in macrovascular rupture (9) and rapid tumor regrowth after treatment
discontinuation (10) are
considered as life-threatening AEs. However, the prognosis of TKIs
is favorable despite the appearance of AEs. In particular, the
appearance of hand-foot syndrome (HFS) is reportedly correlated
with prognosis for several TKIs (11-13).
This retrospective study aimed to verify the AEs of lenvatinib in
the real-world setting and clarify whether life-threatening AEs and
HFS are markers of good prognosis as previously reported.
Materials and methods
Patients' selection
Between June 2015 and May 2020, 111 patients were
treated at Kanagawa Cancer Center, Japan with lenvatinib, including
79 patients with radioactive iodine-refractory DTC and 32 patients
with anaplastic thyroid cancer (ATC). This study was approved by
the Institutional Review Board of Kanagawa Cancer Center (IRB
approval number 27-61 for DTC and 28-49 for ATC). All patients
provided a comprehensive consent stating that their samples
collected for medical examination could be utilized for
investigation and clinical research.
Treatment dose and AE evaluation
The initial dose of lenvatinib was 24 mg/day, and
the dose could be reduced for the following reasons: Body weight
<40 kg, age ≥80 years, performance status ≥2, heart failure, and
chronic kidney disease. Interruption or reduction of dose (to 20,
14, 10 or 8 mg/day) was permitted in cases of toxic effects. The
dose reduction criteria for proteinuria were as reported previously
(14). We classified the radiologic
response to TKI therapy on the basis of the RECIST 1.1 criteria
(15) as follows: Complete
remission of the disease (CR), partial response (PR), stable
disease (SD), and progressive disease (PD). To evaluate safety, the
occurrence of any AE and the time for treatment discontinuation
were recorded. AEs were graded from 1 to 5 according to the Common
Terminology Criteria for Adverse Events (CTCAE) version 5.0
(http://www.jcog.jp/doctor/tool/ctcaev5.html), and the
maximum value was totaled for each patient. Table I shows the frequency of all grades
and that of ≥grade 3. The frequency of ≥grade 3 was low. Grade 4
indicated a life-threatening AE, which included skin fistula,
cavitation, embolism, GI perforation, and regrowth, requiring drug
discontinuation.
 | Table IAEs observed in the DTC and ATC
groups. |
Table I
AEs observed in the DTC and ATC
groups.
| | DTC (n=79) | ATC (n=32) |
|---|
| AEs | All-grade | Grade ≥3 | All-grade | Grade ≥3 |
|---|
| Hypertension, % | 91.1 | 8.9 | 75.0 | 9.4 |
| Proteinuria, % | 64.6 | 15.2 | 40.6 | 3.1 |
| Appetite loss, % | 43.0 | 5.1 | 46.9 | 6.2 |
| HFS, % | 40.5 | 5.1 | 18.8 | 6.2 |
| Liver dysfunction,
% | 16.5 | 1.3 | 12.5 | 3.1 |
| Fatigue, % | 15.2 | 0.0 | 12.5 | 3.1 |
| Alopecia, % | 12.7 | 0.0 | 0.0 | 0.0 |
| Stomatitis, % | 10.1 | 0.0 | 9.4 | 0.0 |
| Diarrhea, % | 8.9 | 0.0 | 6.2 | 0.0 |
| Serious AEs, n
(%) | | | | |
|
Skin
fistula | 7 (8.9) | 11 (34.4) |
|
Cavitation | 1 (1.3) | 2 (6.3) |
|
Embolism | 4 (5.1) | 2 (6.3) |
|
GI
perforation | 2 (2.6) | 0 (0.0) |
|
Regrowth | 7 (8.9) | 0 (0.0) |
Statistical analysis
The median values between the two groups were
compared using the Fisher's test for nominal variables and the
Student's t-test for continuous variables. The statistically
significant difference was set at P<0.05. All statistical data
were analyzed using EZR software version 2.4. (16) Furthermore, survival analysis for
DTCs was performed using the Cox hazard model for the AEs listed in
Table I. Overall survival (OS)
after lenvatinib treatment and 12-month survival rates were
calculated using the Kaplan-Meier method with SPSS software
(version 24; IBM Corp.), and the log-rank and Bonferroni tests were
applied. P<0.05 was considered statistically significant.
Results
Patient characteristics
Table II presents
the patient characteristics of the ATC and DTC groups. Patients
with ATC were significantly older, and they had a worse treatment
outcome. In addition, patients with ATC had shorter treatment
periods and survival.
 | Table IIPatient characteristics in the ATC and
DTC groups. |
Table II
Patient characteristics in the ATC and
DTC groups.
| | Group | |
|---|
| Parameter | ATC | DTC | P-value |
|---|
| Number | 32 | 79 | |
| Age, years | 77 (42-89) | 72 (41-87) | 0.020a |
| Female, n (%) | 18 (56.2) | 49 (62.0) | 0.669 |
| Male, n (%) | 14 (43.8) | 30 (38.0) | |
| Height, cm | 157.2
(133.9-174.1) | 154.9
(135.1-174.0) | 0.645 |
| BW, kg | 53.1 (41.5-88.0) | 55.9
(32.7-101.4) | 0.275 |
| PR, n (%) | 6 (18.8) | 29 (36.7) |
<0.001b |
| SD, n (%) | 8 (25.0) | 37 (46.8) | |
| NE, n (%) | 6 (18.8) | 0 (0.0) | |
| PD, n (%) | 12 (37.5) | 13 (16.5) | |
| OS, months | 3.2 (0.5-28.9) | 19.9 (1.0-62.3) |
<0.001a |
| Initial dose, mg | 24.0 (10-24) | 24.0 (8-24) | 0.750 |
| Duration, months | 3.2 (0.2-27.9) | 18.2 (0.7-62.3) |
<0.001a |
Frequency and characteristics of
AEs
Meanwhile, the frequency of AEs is presented in
Table I. The most common AE was
hypertension (HT) in both groups, and approximately half of such
patients had HT as a pre-existing disease before treatment that was
exacerbated by lenvatinib therapy. The second most common AE was
proteinuria, followed by anorexia and HFS, while diarrhea occurred
less frequently because antidiarrheal drugs were often prescribed
at the beginning of treatment. HT and HFS were observed as an
early-phase AE in all patients within 1 month following lenvatinib
treatments. The frequencies of HFS and proteinuria were higher in
patients with DTC who had long treatment periods, whereas alopecia
was only observed as a late-phase AE in patients with DTC who used
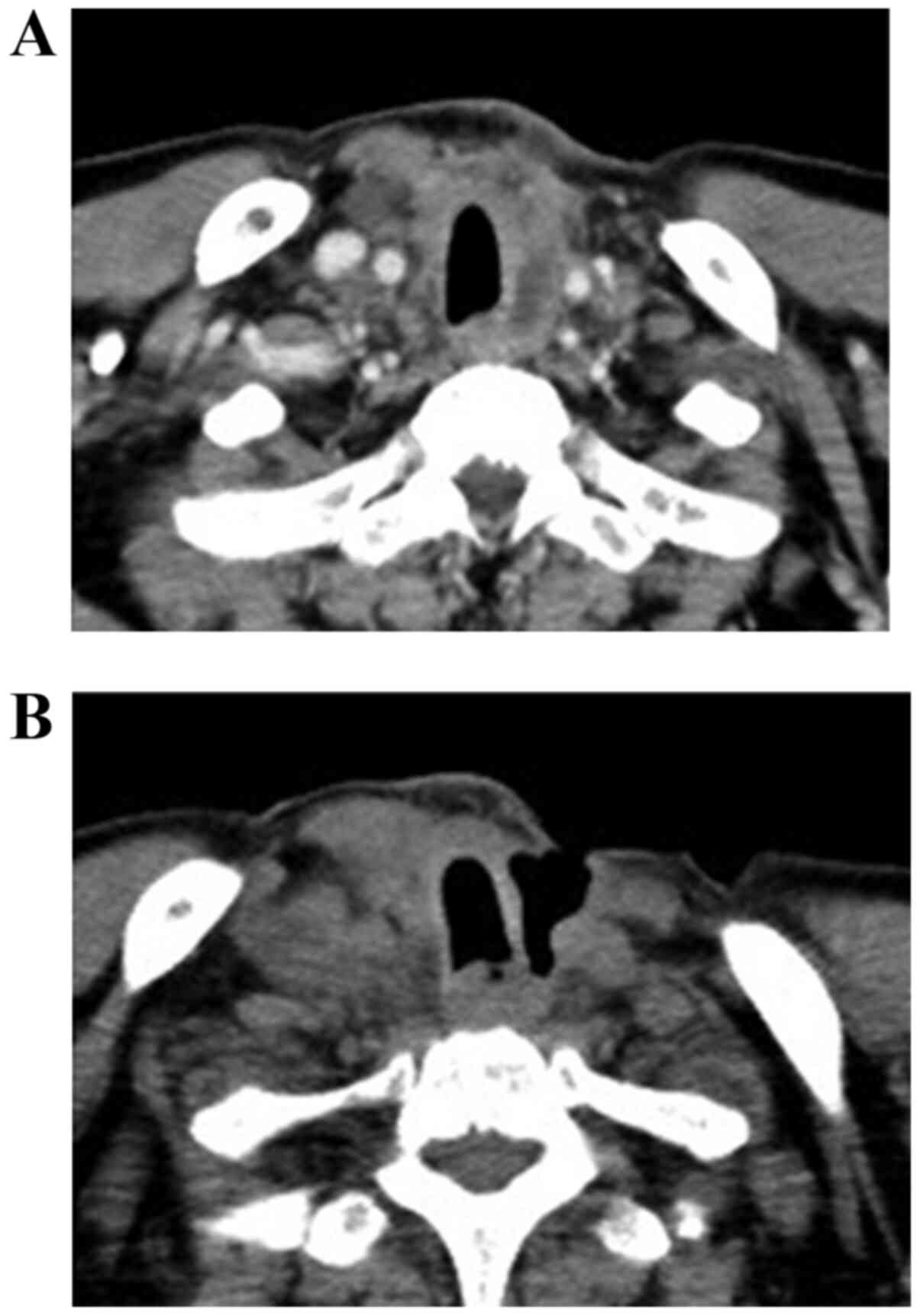
lenvatinib for >1 year. Concerning serious AEs, skin fistula
appeared in 11 patients with DTC (34.4%) and 7 patients with ATC
(8.9%). In both groups, fistulae were caused by tumor necrosis in
the lesion adjacent to the trachea or skin. One typical case is
presented in Fig. 1. The fatality
rate associated with this AE was 38.9% (7/18), including three
deaths because of major bleeding and four deaths attributable to
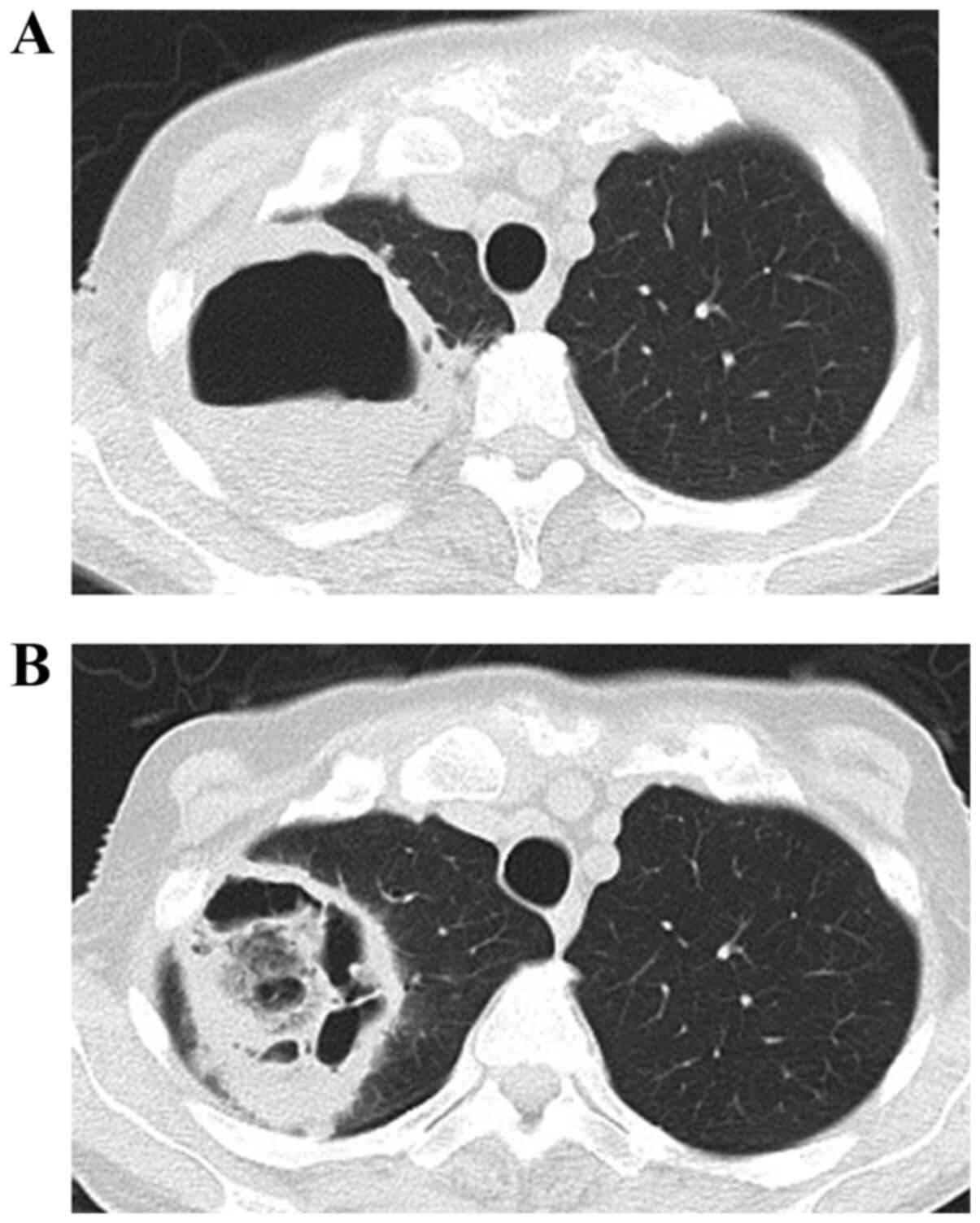
successive pneumonia and mediastinitis. Three patients (two
patients with ATC and one patient with DTC) displayed cavity
formation in the lungs that prevented further treatment with
lenvatinib. One particular case is shown in Fig. 2. Embolism was observed in four
patients with DTC (pulmonary embolism, cerebral infarction,
thrombotic cholecystitis, and deep vein thrombosis) and two
patients with ATC (cerebral infarction and thrombotic
cholecystitis). Gastrointestinal tract perforation was recognized
in two patients with DTC. During treatment, irreversible regrowth
occurred in seven patients with DTC who discontinued treatment
because of this AE, and all these patients died. The AE
characteristics are summarized in Table III.
 | Table IIICharacteristics of adverse events of
lenvatinib treatment for patients with differentiated thyroid
cancer. |
Table III
Characteristics of adverse events of
lenvatinib treatment for patients with differentiated thyroid
cancer.
| Characteristics of
AEs | AE |
|---|
| Early phase AE
(within 1 month) | Hypertension, HFS,
diarrhea |
| Late phase AE
(>1 year) | Alopecia |
| All phase AE | Proteinuria,
appetite loss, liver dysfunction, fatigue, stomatitis |
| AE with poor
prognosis | Serious
AEa, liver
dysfunction |
| AE with favorable
prognosis | HFS |
OS curves
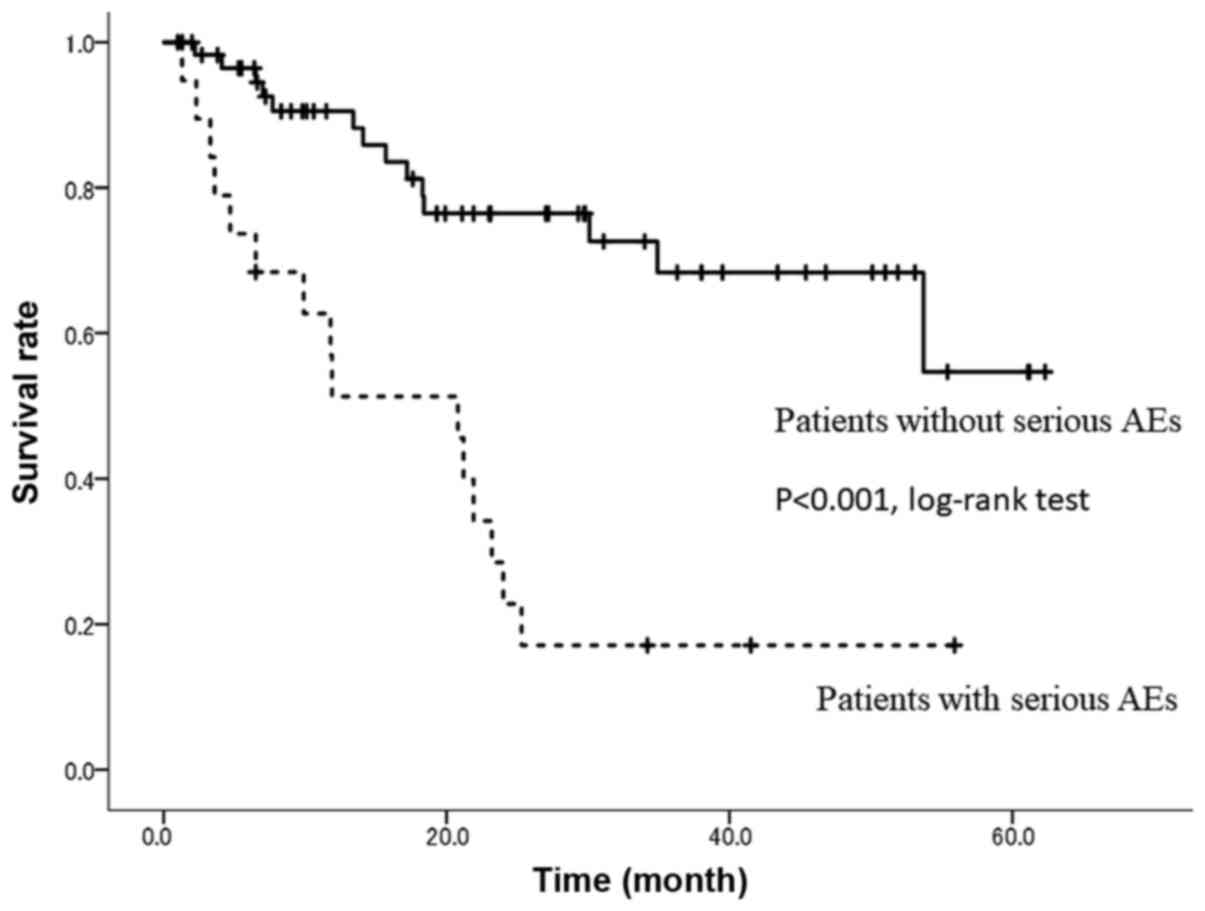
As shown in Fig. 3,
19 patients (including two overlapping cases) with serious AEs had
poor outcomes, including a significantly lower 24-month OS rate
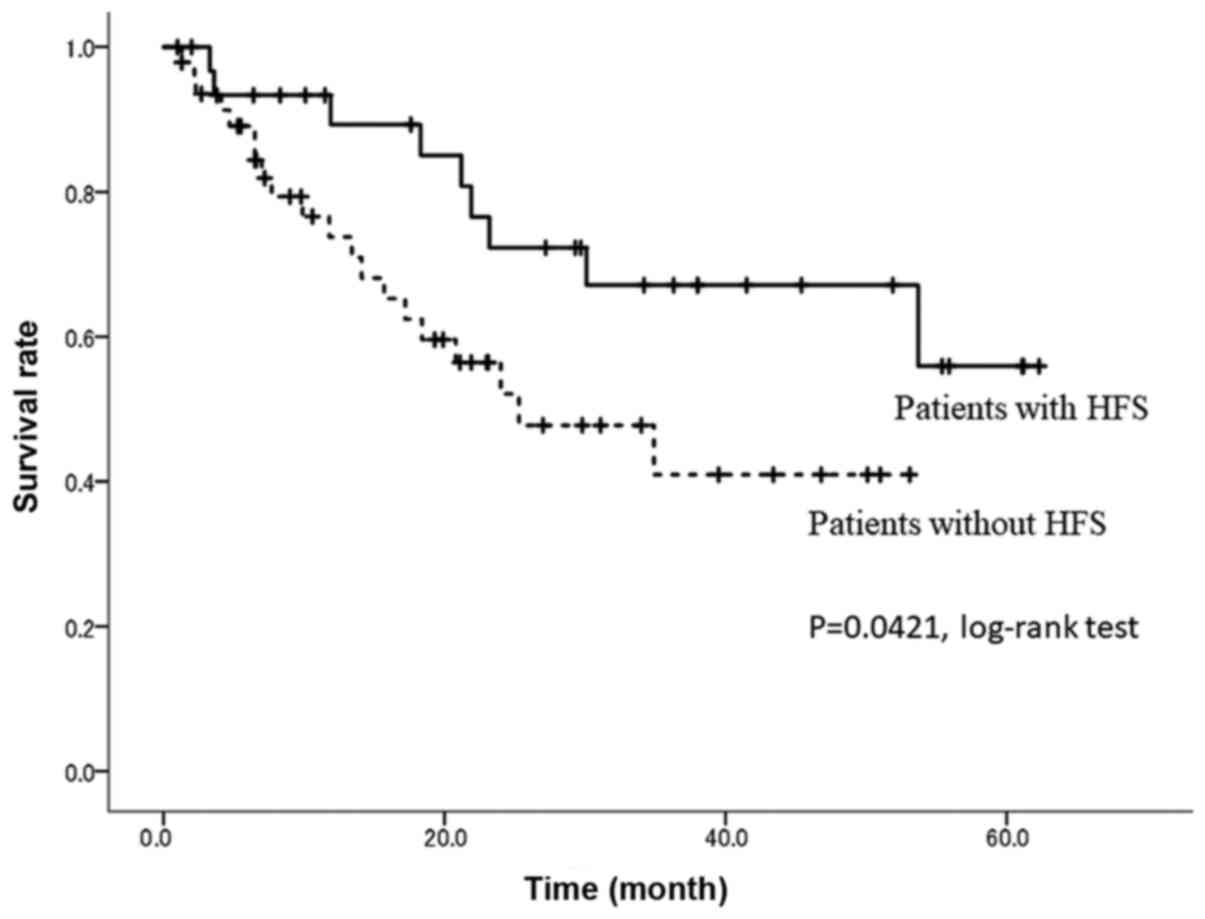
(P<0.001). Meanwhile, the 24-month OS rate was 73.2% in patients
with HFS compared with 52.1% in patients without HFS (P=0.0421,
Fig. 4).
Discussion
We previously reported the results of lenvatinib for
the treatment of ATC (16,17). In this prior study, 19/32 lesions
were diagnosed by biopsy only, and total thyroidectomy was not
performed. Skin fistula was recognized in 11/19 patients (57.8%).
The oral treatment period was too short to consider other AEs. In
many cases, even if AEs occurred, the disease progressed quickly,
and the lenvatinib dose could not be reduced. With DTC, the
administration period was long, and hence it was possible to
evaluate AEs other than skin fistula. HT of 91.1%, which was most
common, did not correlate with prognosis because of its high
frequency. Proteinuria and anorexia also appeared during the entire
administration period, and no correlation with prognosis was
observed. HT was the most common AE; nonetheless, HT of all
patients was well controlled by administering 1-3 types of
antihypertensive drugs and adjusting the dose. Regarding
proteinuria, renal failure could be prevented by reducing the dose
of lenvatinib and withdrawing this drug adequately (14). Meanwhile, anorexia and weight loss
due to lenvatinib improved with drug suspension and resumption of
dose reduction.
HT and HFS appeared within 1 month of administration
in all cases, and a correlation with prognosis was observed in HFS.
No correlation between HFS and prognosis was observed in prior
studies.
In the real world, the lenvatinib therapy outcome
for DTC was worse than that in the Select trial because of disease
progression in the patients (18),
in line with other reports (19).
Of course, the management of non-serious AEs, such as hypertension,
proteinuria, and HFS, is also necessary; however, as observed in
the present study, prognosis will be poor if serious AEs are
present. Therefore, careful management to avoid these AEs is
essential to improve patient outcomes.
TKIs inhibit angiogenesis, and as such, careful
administration is required because lesions adjacent to large blood
vessels are at risk of bleeding (9). In particular, there is a risk of blood
vessel wall rupture in lesions with a history of external
irradiation (20) or for fistulae
that develop in the digestive tract or skin (21). Although TKIs are not curative, they
can be administered continuously. If a fistula develops, it is
necessary to halt treatment or reduce the dose, whereas dose
reduction may be preferable to treatment discontinuation to prevent
irreversible tumor regrowth.
Anticoagulant therapy should be administered to
patients at risk of embolism. For gastrointestinal perforation and
cavity formation in the lungs, early detection, dose reduction, and
drug interruption may be necessary for symptoms, such as abdominal
pain and chest pain.
Avoiding serious AEs is paramount for improving the
treatment outcomes. If a skin fistula develops, especially if it is
close to a large blood vessel, the drug needs to be discontinued.
Dose reduction is necessary to avoid fistula development in the
digestive tract or trachea. Moreover, although embolism is
difficult to prevent because of its sudden onset, d-dimer should be
monitored in the high-risk groups, and anticoagulants should be
administered as per requirement. Lenvatinib treatment has also been
initiated for hepatic cancer; with an average dosing period of 6
months, the overall response and disease control rates were 57.1
and 71.4%, respectively (22).
The frequency of HFS is high in Japan, as has been
reported for several TKIs (13).
Although the precise mechanisms by which multikinase inhibitors
cause HFS remain unknown, the antiangiogenic activity of these
drugs may inhibit vascular repair mechanisms in high-pressure
areas, such as the palms and soles, which are repeatedly exposed to
subclinical trauma (23).
This study was retrospective. Because the subjects
were patients with ATC or DTC, which have different biological
prognoses, the frequencies of AEs were also different. To examine
whether the prognosis of DTC can be predicted by the presence or
absence of HFS, a median treatment period of 18.2 months is not
sufficient. Moreover, prognosis was significantly worse for
patients with serious AEs.
This study, reported from real-world experience,
showed that lenvatinib therapy could result in severe AEs requiring
dose reduction or treatment discontinuation. It also indicated that
the appearance of HFS portended a good prognosis in patients
treated with lenvatinib, which may be characteristic of the
Japanese population. We expect additional reports from other
facilities regarding these two points.
Acknowledgements
The authors would like to thank Dr Hiroyuki Hayashi
(Department of Pathology, Yokohama City Hospital, Yokohama, Japan)
for assistance in pathological diagnosis.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HI and ST designed the study. HI, ST, SK and DM
designed the study and investigation. SK and AM analyzed the data
and contributed to data curation. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The Cancer Board of Kanagawa Cancer Center Hospital
(Japan) approved lenvatinib treatment, including surgery, for
patients with ATC or DTC. The study was approved by the
Institutional Review Board of Kanagawa Cancer Center Hospital
(Japan). All patients provided a comprehensive consent stating that
their samples that were collected for medical examination could be
utilized for investigation and clinical research.
Patient consent for publication
All patients signed a consent form stating that
their personal data and samples could be used for academic
presentation or paper presentation while ensuring complete
anonymity before receiving the treatment.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
HI is an endocrine surgeon working at Kanagawa
Cancer Center and has extensive experience of several surgeries for
ATC, as well as ATC treatment.
References
|
1
|
Seib CD and Sosa JA: Evolving
understanding of the epidemiology of thyroid cancer. Endocrinol
Metab Clin North Am. 48:23–35. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Olson E, Wintheiser G, Wolfe KM, Droessler
J and Silberstein PT: Epidemiology of thyroid cancer: A review of
the National Cancer Database, 2000-2013. Cureus.
11(e4127)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Melosky B, Leighl NB, Rothenstein J,
Sangha R, Stewart D and Papp K: Management of EGFR TKI-induced
dermatologic adverse events. Curr Oncol. 22:123–132.
2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Eskazan AE and Ozmen D: Tyrosine kinase
inhibitor (TKI) therapy for newly-diagnosed patients with chronic
myeloid leukemia: Focusing on TKI discontinuation due to adverse
events-is better always good? Expert Rev Hematol. 10:583–586.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Haugen BR: 2015 American Thyroid
Association Management Guidelines for adult patients with thyroid
nodules and differentiated thyroid cancer: What is new and what has
changed? Cancer. 123:372–381. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Iwasaki H, Yamazaki H, Takasaki H,
Suganuma N, Sakai R, Nakayama H, Hatori S, Toda S and Masudo K:
Treatment outcomes of differentiated thyroid cancer with distant
metastasis improve by tyrosine kinase inhibitors. Oncol Lett.
17:5292–5300. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kamba T and McDonald DM: Mechanisms of
adverse effects of anti-VEGF therapy for cancer. Br J Cancer.
96:1788–1795. 2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sonpavde G, Bellmunt J, Schutz F and
Choueiri TK: The double edged sword of bleeding and clotting from
VEGF inhibition in renal cancer patients. Curr Oncol Rep.
14:295–306. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Machiels JP, Henry S, Zanetta S, Kaminsky
MC, Michoux N, Rommel D, Schmitz S, Bompas E, Dillies AF, Faivre S,
et al: Phase II study of sunitinib in recurrent or metastatic
squamous cell carcinoma of the head and neck: GORTEC 2006-01. J
Clin Oncol. 28:21–28. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mancuso MR, Davis R, Norberg SM, O'Brien
S, Sennino B, Nakahara T, Yao VJ, Inai T, Brooks P, Freimark B, et
al: Rapid vascular regrowth in tumors after reversal of VEGF
inhibition. J Clin Invest. 116:2610–2621. 2006.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Liu L, Wang E, Li L, Chen D, Peng K, Wang
M and Han G: As clinical markers, hand-foot-skin reaction and
diarrhea can predict better outcomes for hepatocellular carcinoma
patients receiving transarterial chemoembolization plus sorafenib.
Can J Gastroenterol Hepatol. 2019(2576349)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Falcone G, Arrigoni C, Dellafiore F,
Gallucci F, Milani V, Boveri S, Ausili D and Caruso R: A systematic
review and meta-analysis on the association between hand-foot
syndrome (HFS) and cancer chemotherapy efficacy. Clin Ter.
170:e388–e395. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kobayashi K, Kawakami K, Yokokawa T,
Aoyama T, Suzuki K, Wakatsuki T, Suenaga M, Sato H, Sugiyama E,
Yamaguchi K and Hama T: Association of hand-foot skin reaction with
regorafenib efficacy in the treatment of metastatic colorectal
cancer. Oncology. 96:200–206. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Iwasaki H, Yamazaki H, Takasaki H,
Suganuma N, Sakai R, Nakayama H, Toda S and Masudo K: Renal
dysfunction in patients with radioactive iodine-refractory thyroid
cancer treated with tyrosine kinase inhibitors: A retrospective
study. Medicine (Baltimore). 98(e17588)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Iwasaki H, Yamazaki H, Takasaki H,
Suganuma N, Nakayama H, Toda S and Masudo K: Lenvatinib as a novel
treatment for anaplastic thyroid cancer: A retrospective study.
Oncol Lett. 16:7271–7277. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Iwasaki H, Toda S, Suganuma N, Murayama D,
Nakayama H and Masudo K: Lenvatinib vs. palliative therapy for
stage IVC anaplastic thyroid cancer. Mol Clin Oncol. 12:138–143.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Schlumberger M, Tahara M, Wirth LJ,
Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff
AO, et al: Lenvatinib versus placebo in radioiodine-refractory
thyroid cancer. N Engl J Med. 372:621–630. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Locati LD, Piovesan A, Durante C, Bregni
M, Castagna MG, Zovato S, Giusti M, Ibrahim T, Puxeddu E, Fedele G,
et al: Real-world efficacy and safety of lenvatinib: Data from a
compassionate use in the treatment of radioactive iodine-refractory
differentiated thyroid cancer patients in Italy. Eur J Cancer.
118:35–40. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hui EP, Ma BBY, King AD, Mo F, Chan SL,
Kam MKM, Loong HH, Ahuja AT, Zee BCY and Chan ATC: Hemorrhagic
complications in a phase II study of sunitinib in patients of
nasopharyngeal carcinoma who has previously received high-dose
radiation. Ann Oncol. 22:1280–1287. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Blevins DP, Dadu R, Hu M, Baik C,
Balachandran D, Ross W, Gunn B and Cabanillas ME: Aerodigestive
fistula formation as a rare side effect of antiangiogenic tyrosine
kinase inhibitor therapy for thyroid cancer. Thyroid. 24:918–922.
2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Katsura Y, Takeda Y, Ohmura Y, Sakamoto T,
Kawai K, Inatome J, Murakami K, Naito A, Kagawa Y, Masuzawa T, et
al: Experience of treatment with lenvatinib in patients with
advanced HCC-A in a single institution. Gan To Kagaku Ryoho.
46:2101–2103. 2019.PubMed/NCBI(In Japanese).
|
|
23
|
Yada M, Masumoto A, Motomura K, Tajiri H,
Morita Y, Suzuki H, Senju T and Koyanagi T: Indicators of sorafenib
efficacy in patients with advanced hepatocellular carcinoma. World
J Gastroenterol. 20:12581–12587. 2014.PubMed/NCBI View Article : Google Scholar
|