Introduction
Among rectal cancers, rectosigmoid lesions are
infrequently associated with pelvic local recurrence, and the
recurrence mode and prognosis are similar to those of colorectal
lesions (1,2). But the survival of patients with
advanced rectal cancer other than rectosigmoid cancer is
considerably poorer than that of patients with colorectal cancer.
In men, a narrow pelvis makes it difficult to perform surgical
procedures in the deep pelvic floor. In addition, the
circumferential resection margin, anal margin and scattering of
tumor cells due to perforation during rectal surgery are reported
to influence pelvic local recurrence (3). To improve the survival rate by
reducing local recurrence, integrated approaches for advanced
rectal cancer, such as preoperative chemoradiation therapy and
intrapelvic prophylactic bilateral lymph node dissection (PBLND)
have been conducted (4-11).
Specifically, the Japanese Society for Cancer of the Colon and
Rectum guidelines 2019 for the treatment of colorectal cancer
(JSCCR guidelines 2019) (2) state
that evidence is limited for preoperative chemoradiation improving
survival rate, although it significantly reduces pelvic local
recurrence. Indeed, few studies have reported an improvement in
overall survival after preoperative chemoradiation (2,12).
Moreover, Japanese people are smaller than westerners and surgical
outcome is relatively favorable. Therefore, chemoradiation has been
conducted at a limited number of clinical sites in Japan (4-7).
PBLND is effective for treating advanced lower rectal cancer, as
reported in Japan (8,9). In 2017, the final results of the
JCOG0212 study, a randomized, multi-center study of PBLND, were
announced. Statistical non-inferiority was not demonstrated in a
longitudinal analysis of total mesorectal excision (TME) with or
without PBLND, supporting the benefits of conventional PBLND
(10,11). However, although local recurrence
rate was lower with PBLND, no differences were observed between
5-year relapse-free survival (5Y-RFS) or 5-year overall survival
(5Y-OS), or the equivalent 10-year rates, in groups treated with or
without PBLND. A retrospective study of 944 patients at Kurume
University showed no significant difference in the survival rate
but the prognosis was poor for patients with pathological (p)-stage
IIIb cancer (9).
Our hospital did not introduce radiotherapy until
2010. In addition to a longer surgical time and more bleeding,
PBLND is commonly associated with dysfunctions in bowel movement,
urination and sexual function. Because of this, we have not
conducted PBLND in our department. Instead, for patients with
clinical (c)-stage II/III cancer, our standard treatment has been
TME or tumor-specific mesorectal excision (TSME) to achieve radical
resection with no macroscopic residual tumor postoperatively (R0),
with postoperative adjuvant systemic chemotherapy (2,13-16).
Currently, pre-operative chemoradiation for downsizing and
downstaging advanced lower rectal cancer is selectively offered to
patients in whom large T3/T4 tumors occupy the pelvic floor.
Lateral lymph node dissection is conducted only on the side of the
metastasis as demonstrated by pre-operative diagnostic imaging.
The purpose of this study was to
clinicopathologically compare the overall survival, relapse rate
and recurrence mode in patients with advanced (c-stage II/III)
rectal cancer who received TME/TSME plus postoperative systemic
chemotherapy. Pre-operative CRT/Lateral dissection/RS and T4 were
excluded.
Patients and methods
Study design and patients
This retrospective, observational, single-center
study was conducted over 10 years and 9 months (from April 2003 to
December 2013) with approval by the Institutional Review Board (IRB
no. 18R-331). It included 123 patients with advanced rectal cancer
who underwent radical (R0) resection leaving no macroscopic
residual tumor. Informed consent was obtained for every diagnostic
or interventional procedure and the use of electronic medical
records. Of the 123 participants, 79 (64.2%) had c-stage II cancer
and 44 (35.8%) had c-stage III cancer (Table I). The presence or absence of
metastasis (≥10 mm in the longest diameter) at the lateral lymph
nodes was evaluated preoperatively using CT images interpreted by a
radiologist, as per the JCOG0212 study (10). Patients with lateral lymph node
metastasis or T4 multiple organ invasion (i.e. cases requiring
chemoradiation) were excluded (6-9).
Postoperative recurrence was evaluated three to four times a year
using ultrasound (US) and computed tomography (CT) scans. For
patients with at least two metastases (in the liver, lung, or
locally), calculation of 5Y-OS in the recurrence group was based on
the maximum area and the estimated volume by US/CT. The location
that the larger value of the metastasis was judged as the first
recurrence location. The location of the larger metastasis was
recorded as the first recurrence location.
 | Table IDemographic characteristics of the
study population. |
Table I
Demographic characteristics of the
study population.
| Characteristics | Value |
|---|
| Total undergoing
TME/TSME, n | 123 |
| Sex, n (%) | |
|
Male | 86 (69.9) |
|
Female | 37 (30.1) |
| Age, years | |
|
Median
(range)/mean | 67 (36-92)/67 |
| Clinical stage, n
(%) | |
|
II | 79 (64.2) |
|
III | 44 (35.8) |
| Tumor location
without rectosigmoid lesion, n (%) | |
|
Ra (above
the peritoneal reflection) | 61 (49.6) |
|
Rb (below
the peritoneal reflection) | 62 (50.4) |
| Tumor distance from
anal verge, cma | |
|
Median
(range)/mean | 2.5 (0.5-12)/3.3 |
| Tumour size, n
(%) | |
|
≤5 cm | 70 (56.9) |
|
>5
cm | 53 (43.1) |
| Type of surgery
(D2/D3 resection), n (%) | |
|
Anterior
resection | 1 (0.8) |
|
Low anterior
resection | 89 (72.4) |
|
Abdominoperineal
resection (Miles' operation) | 31 (25.2) |
|
Hartmann's
procedure | 2 (1.6) |
| Surgical method, n
(%) | |
|
Conventional
laparotomy | 55 (44.7) |
|
Hand-assisted
laparoscopic surgery | 68 (55.3) |
For patients with p-stage II cancer, the anti-cancer
agent tegafur-uracil (UFT) was administered orally for 6-12 months
postoperatively. Patients with p-stage III cancer received modified
FOLFIRI chemotherapy (5-fluorouracil + leucovorin) for 6 months,
followed by oral UFT for at least 6 months (6-9).
Excluding rectosigmoid lesions, the tumor was located above the
peritoneal reflection (Ra) in 61 patients (49.6%) and below (Rb) in
62 patients (50.4%). The median distance from the anal verge was
2.5 cm (range, 0.5-12.0 cm; mean, 3.3 cm) (Table I). Operative methods were low
anterior resection in 89 patients (72.4%), the Miles operation in
31 patients (25.2%) and other methods in 3 patients (2.4%).
Conventional laparotomy (CL) was conducted in 55 patients (44.7%)
and hand-assisted laparoscopic surgery (HALS) was conducted in 68
patients (55.3%) (Table I)
(6-9).
Pathological T category distributions were as follows: T2, 36
patients (29.3%); T3, 74 patients (60.2%); others, 13 patients
(10.6%). Pathological N category distributions were as follows: N0,
70 patients (56.9%); N1, 45 patients (36.6%); others, 8 patients
(6.5%) (Table II). Forty-two
patients (34.1%) had p-stage II cancer, 53 (43.1%) had p-stage III,
and 28 (22.8%) had cancers classed as other stages. Of all
resections, 118 (95.9%) were R0 and 5 (4.1%) were R1 (Table II).
 | Table IIClinicopathological features of the
study population. |
Table II
Clinicopathological features of the
study population.
| Clinicopathological
feature | Value |
|---|
| Total undergoing
TME/TSME, n | 123 |
| Time, min | |
|
Median
(range)/mean | 230 (88-432)/243 |
| Blood loss, ml | |
|
Median
(range)/mean | 439 (5-4293)/569 |
| Pathological T
category, n (%) | |
|
pT1 | 4 (3.2) |
|
pT2 | 36 (29.3) |
|
pT3 | 74 (60.2) |
|
pT4 | 9 (7.3) |
| Pathological N
category, n (%) | |
|
pN0 | 70 (56.9) |
|
pN1 | 45 (36.6) |
|
pN2 | 7 (5.7) |
|
pN3 | 1 (0.8) |
| Pathological stage,
n (%) | |
|
I | 27 (22.0) |
|
II | 42 (34.1) |
|
III | 53 (43.1) |
|
IV | 1 (0.8) |
| Pathological
residual tumor, n (%) | |
|
R0 | 118 (95.9) |
|
R1 | 5 (4.1) |
|
R2 | 0 (0) |
Operations and procedures
CL was standard midline laparotomy. For HALS, a
small incision of 50 mm was created for hand access. In accordance
with the JSCCR guidelines 2019(2)
and classification of colorectal carcinoma (17), central vascular ligation was
conducted at the root of the inferior mesenteric artery as distal
D3 lymph node dissection or left colic artery-preserving D2
ligation (17-20).
TME was conducted for rectosigmoid tumors by transection of the
mesorectum immediately above the aortic bifurcation. Lateral
dissection was performed from the anterior sacrum to the medial
region of the bilateral internal iliac artery. TME was also
conducted from the anterior region of the pelvic floor muscles and
from the lower part of the bladder to the posterior face of the
prostate or the rear wall of the vagina (18). Patients with Ra rectal cancer
underwent TSME. After securing a minimum safety margin of
approximately 20 mm from the posterior face of the tumor to the
anal side, the lower rectum was clamped. Then, the inner part of
the rectum was carefully washed, and the incision was anastomosed
and closed using the double-staple technique (21).
5Y-RFS and 5Y-OS were calculated for patients with
c-stage II/III cancer. Early post-operative complication rate was
evaluated using the National Cancer Institute's Common Terminology
Criteria for Adverse Events version 4.0, and the correlation
between recurrence modes and prognosis was investigated. 5Y-OS was
also calculated for the group of patients whose tumors
recurred.
Statistical analysis
We retrospectively evaluated operation time, blood
loss, postoperative morbidity (grade 3 or 4), and hospital
mortality. 5Y-RFS and 5Y-OS were obtained by Kaplan-Meier
estimation and log-rank test. Pearson's Chi-square test, Fisher's
exact test and the Mann-Whitney U test were used to compare patient
demographics. Statistical analysis was conducted using IBM SPSS
Statistics for Windows Version 25.0 (IBM Corp.) and P<0.05 was
considered significant.
Results
Survival rates
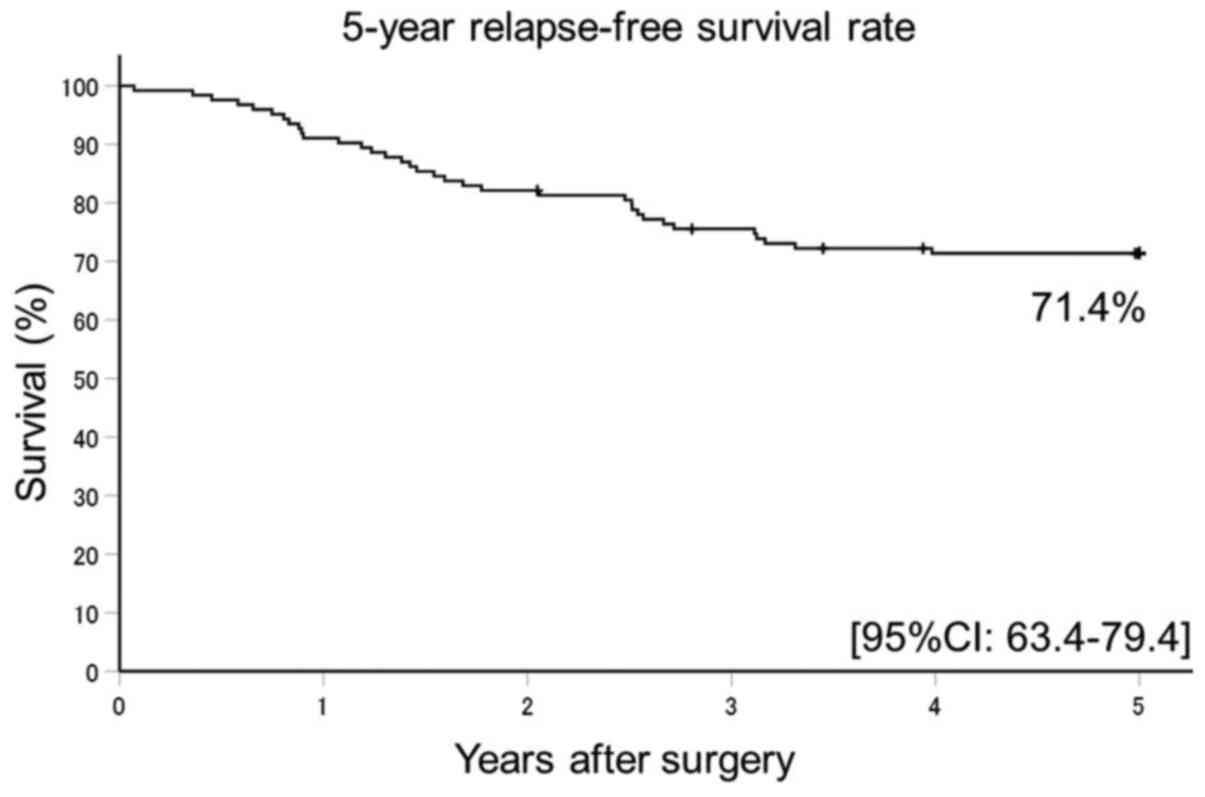
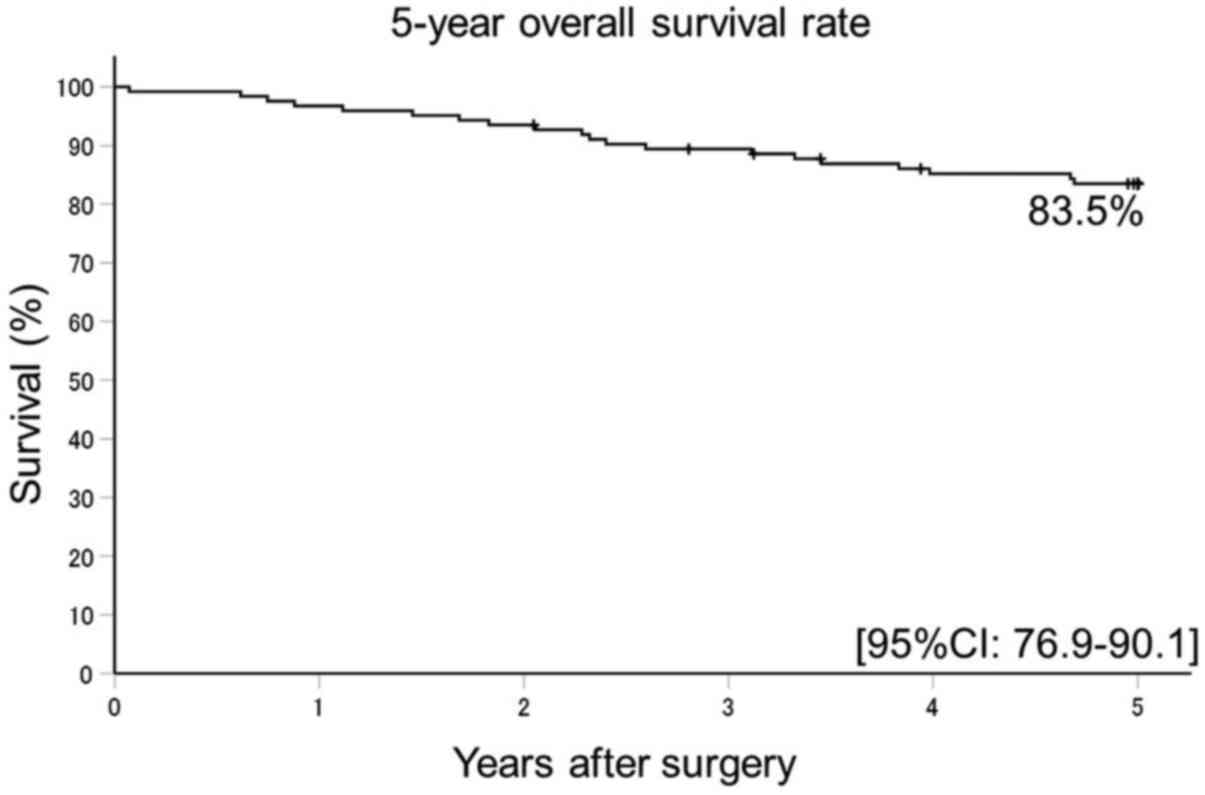
The 5Y-RFS rate was 71.4% (95% confidence interval
[CI]: 63.4-79.4) (Fig. 1) and 5Y-OS
rate was 83.5% (95% CI: 76.9-90.1). The follow up rate for both
estimates was 94.3% (Fig. 2).
Complication rates
Grade 3-4 postoperative complications were observed
in 12 patients (9.8%); anastomotic leakage in 9 patients (7.3%);
urinary retention or injury in 7 patients (5.7%); pelvic abscess in
5 patients (4.1%); and wound infection in 33 patients (26.9%). The
33 patients with wound infection comprised 17 (44.7%) of the 38
patients who had undergone CL, and 16 (30.8%) of the 52 patients
who had undergone HALS, with no significant difference between
operative method (P=0.358, Pearson's Chi-square test). All wound
infections were grades 1 or 2 and healed fully after conservative
treatment (Table III).
 | Table IIIAdverse events. |
Table III
Adverse events.
| Adverse event | Number (%) |
|---|
| Any grade 3-4
complicationa | 12 (9.8) |
| Anastomotic
leakageb | 9 (10.0) |
| Urinary
retention/injury | 7 (5.7) |
| Hemorrhage after
surgery | 0 (0) |
| Wound
infection | 33 (26.8) |
| Pelvic abscess | 5 (4.1) |
| Bowel
obstruction | 13 (10.6) |
| Othersc | 10 (8.1) |
Recurrence rates
Recurrence occurred in 28/123 patients (22.8%).
Distant metastases occurred in 17/123 patients (13.8%), of whom 8
(6.5%) had metastasis in the lung, 5 (4.1%) in the liver, and 4
(3.3%) elsewhere (Table IV). Local
recurrence occurred in 11/123 patients (8.9%), of whom 7 (5.7%) had
metastasis anterior to the sacrum, 2 (1.6%) at the anastomosis, and
2 (1.6%) in the medial part of the pelvis (Table IV).
 | Table IVPatterns of recurrence. |
Table IV
Patterns of recurrence.
| Recurrence | Number (%) |
|---|
| Total recurrence
rate | 28 (22.8) |
| Distant
metastasis | 17 (13.8) |
|
Lung | 8 (6.5) |
|
Liver | 5 (4.1) |
|
Others | 4 (3.3) |
|
Peritoneal
dissemination | 3 (2.4) |
|
Inguinal
lymph node | 1 (0.8) |
| Local pelvic
recurrence | 11 (8.9) |
|
Median
presacral | 7 (5.7) |
|
Anastomosis | 2 (1.6) |
|
Pelvic
lateral lymph node | 2 (1.6) |
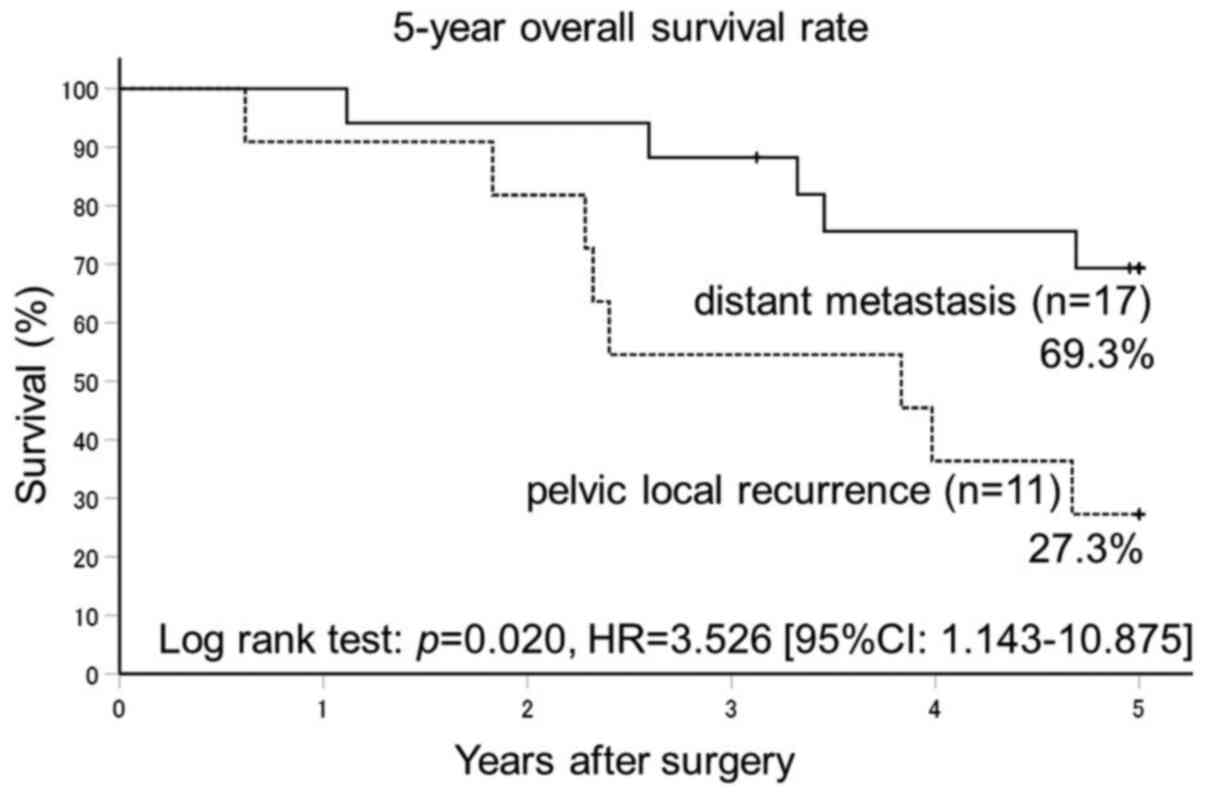
Survival rate of patients with
relapsed tumors
The 5Y-OS rate for patients whose cancer had
recurred was 69.3% in the 17 patients with remote metastasis and
27.3% in the 11 patients with local recurrence (P=0.02), from a
follow-up rate of 92.9% (Fig. 3).
No significant differences were observed in patient demographics
between local recurrence and distant metastasis (Table V).
 | Table VClinical backgrounds of patients with
distant and local recurrence. |
Table V
Clinical backgrounds of patients with
distant and local recurrence.
| Variable | Total cases
(n=28) | Distant metastasis
(n=17) | Pelvic local
recurrence (n=11) | P-value |
|---|
| Sex, n (%) | | | | 0.191a |
|
Male | 21 (75.0) | 11 (64.7) | 10 (90.9) | |
|
Female | 7 (25.0) | 6 (35.3) | 1 (9.1) |
>0.999b |
| Age, years; median
(range) | 70 (44-86) | 70 (44-86) | 70 (46-81) | |
| Tumor location, n
(%) | | | |
>0.999a |
|
Ra | 9 (32.1) | 6 (35.3) | 3 (27.3) | |
|
Rb | 19 (67.9) | 11 (64.7) | 8 (72.7) | |
| Clinical stage, n
(%) | | | | 0.404b |
|
II | 12 (42.9) | 6 (35.3) | 6 (54.5) | |
|
III | 16 (57.1) | 11 (64.7) | 5 (45.5) | |
Discussion
The prognosis following rectal cancer surgery is
considerably poorer than after colon cancer, for the following
possible reasons: i) The pelvic cavity narrows toward the anal
region, making surgery difficult and pelvic local recurrence
relatively common (3). ii)
Preoperative chemoradiation and PBLND are conducted to lower the
rate of recurrence and improve survival but, although these
preoperative treatments significantly lower local recurrence rate,
there is little evidence that they improve survival rate. These two
reasons do not seem to add up at present (4-11).
An alternative approach could be to control fatal hematogenous
metastasis in the lung and liver, which may be more beneficial than
reducing local recurrence in extending progression-free and overall
survival rates (3,18,19).
However, in the present study, the prognosis of the remote
metastasis group was better than that of the local recurrence
group. Remote metastatic lesions can be resected and re-resected
without negatively affecting quality of life. In addition, a
variety of treatment options exist, from first-line to later-line
chemotherapy, and radiofrequency ablation for liver metastasis,
which may explain the present results.
In contrast, for pelvic local recurrence, R0
re-resection while preserving quality of life is often difficult.
Some success has been achieved with sacrococcygeal resection and
total removal of the bladder and prostate (double stoma), but
treatment options other than surgical re-resection and
chemoradiation are limited. Furthermore, in the present study, when
pelvic recurrence occurred first, the prognosis was very poor.
Seven of eleven cases had local recurrence with distant secondary
metastasis (lung metastasis: 6 cases, both lung and liver
metastasis: 1 case). There were no distant metastasis cases with
secondary local recurrence case (0/17). In the 11 patients with
pelvic local recurrence, six cases occurred within 2.5 years, and
two occurred in less than 1 year. Three patients had late local
recurrence that occurred after 2.5 years. Remote metastasis after
chemoradiation was fatal. The two patients who experienced
anastomotic recurrence underwent a second Miles operation, which
helped control local lesions. The prognosis of local recurrence is
generally favorable. But pelvic local recurrence that occurs
relatively soon after surgery (within 2.5 years) may initiate a new
metastatic cascade to the lung, liver and other organs, with
malignant potential. Therefore, we recommend a large-scale survival
analysis in patients whose first relapse is pelvic local
recurrence, and the adoption of MRI and FDG-PET should be
considered over CT (12,22).
Recently, pre-operative chemoradiation for lower
rectal cancer has become popular in Japan (4-7).
However, in secondary cancers, such as metachronous prostate and
uterine cancer, which are becoming more prevalent due to an aging
society, treatment options may be limited. Moreover, after
pre-operative chemoradiation, the disease is already at a clinical
stage. The pathological staging system by which stage II/III is
determined is based on the Dukes classification, and has no
category for the presence or absence of metastasis to lymph nodes.
In addition, the number of metastases to lymph nodes cannot be
calculated accurately (20). PBLND,
which is uniquely conducted in Japan for controlling local relapse,
does not affect survival rate. The JCOG0212 study showed that the
lateral lymph node metastasis rate was 15.4% (4/26 patients) after
lymph node dissection. The most common central pelvic, anterior to
the sacrum, recurrence was 27.3% (12/44 patients) in that study's
TME-only group and 42.3% (11/26 patients) in the group that also
underwent lateral lymph node dissection. Non-inferiority was not
demonstrated for 7-year progression-free survival (P=0.064)
(10,11). Therefore, although preoperative
chemoradiation and PBLND lower the local recurrence rate, the
decision to use these approaches must be decided after careful
consideration of functional impairment, adverse reactions, and
treatment options after recurrence.
Full-endoscopic surgery has become popular in Japan
in recent years. However, for operating on advanced lower rectal
cancer, complications relating to the dissection margin at the
posterior and rectal side of the tumor have been observed using
this technique (23,24). Accordingly, in 2007, we began
developing HALS, a hybrid technique between standard laparotomy and
full-endoscopic surgery, and have reported favorable outcomes
(18,25,26).
Three-port HALS for rectal cancer achieved better results than
standard laparotomy (18).
Requiring only a small incision of approximately 50 mm, HALS is
considered a sound technique, characterized by low invasiveness,
low risk, low cost, and good outcomes. In addition, it places less
burden on patients, doctors and hospitals than standard laparotomy
(18,25,26).
Three intra-operative factors can lower the rate of pelvic local
recurrence: i) Maintaining a thick resection margin (>1 mm)
around the tumor, which must be avoided so as not to disperse
cancer cells into the intestine by perforating the tumor during
surgery. In men with tumors at the anterior wall of the lower
rectum, the prostate is present and it is not easy to ensure such a
thick margin. Combined resection may carry a high risk of bleeding
and functional impairment. In women, partial combined resection of
the posterior vaginal wall may be effective. Male patients with Rb
anterior wall rectal cancer with a resection margin of ≤1 mm are
considered at high risk of local recurrence. In the present study,
we identified no cases of recurrence from the lower part of the
bladder to the nearby area of the prostate. ii) Pelvic local
recurrence occurred in the anterior part of the sacrum in 7/11
patients (63.6%) in the present study. TME/TSME using
layer-by-layer surgery from the anterior region to the internal
iliac artery region was considered the most important procedure for
avoiding recurrence. Specifically, for male patients with a small
pelvic cavity and high body mass index, HALS was considered
suitable for dissection anterior to the sacrum. By using the left
hand to apply strong traction to the rectum, pulling it towards the
outside of the pelvic cavity to enlarge the endoscopic view,
accurate TME/TSME can be conducted for sharp dissection from the
region anterior to the sacrum and to expose the posterior wall of
the rectum. We observed a better survival rate for HALS than for CL
in the present study (data not shown: 5Y-RFS, P=0.166; 5Y-OS,
P=0.013). iii) Careful intraoperative washing of the anorectal area
prior to dissection is important. To avoid mechanical implantation
of cancer cell clusters in the rectum upon dissection, iodine and
physiological saline washes and aspiration must be conducted under
endoscopy. In addition, an anal-side stump length of ≥20 mm should
be retained if possible (21).
Together, these factors will contribute to the prevention of local
recurrence. For patients at high risk of pelvic recurrence, such as
those with massive lymph node metastasis (>N2b) and anterior
sacrum R1, postoperative pathological staging (without modification
by radiation therapy) indicates that an integrated approach
consisting of aggressive combination therapy, such as the early use
of a molecular target drug and radiotherapy to the pelvic floor or
anterior sacrum, is considered effective.
The prognosis becomes poor if postoperative
complications occur in patients with lower rectal cancer. Among
patients with early postoperative complications in this study,
wound infection was most common (33/123 patients; 26.8%). With CL,
the rate was 17/55 patients (30.9%) (27), but the infections we observed in the
present study were mild (grades 1-2). Together, the present and
previous data indicate that to improve relapse-free and overall
survival, the following points should be adhered to: i) During
surgery, to prevent local recurrence, a HALS-based low-invasiveness
R0 resection should be conducted. A ‘one surgery, one chance’
approach could reduce postoperative complications; ii) shortly
after surgery (1-2 months), postoperative adjuvant chemotherapy
should be conducted for 6 months (18,19).
In conclusion, advanced rectal cancer and control of local pelvic
recurrence are manageable by R0 resection and postoperative
multi-combinational chemotherapy. However, for patients whose
initial relapse is pelvic recurrence, the relapsed tumor initiated
a new metastatic cascade to distant organs and significantly
affected prognosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SU and DY performed the research for the present
study, contributed to data analysis and wrote the manuscript. MM,
HM and TT designed the research for the present study, provided
surgical advice and supervised the present study. KK, SH and TK
analyzed the data. KT, TM, and TH diagnosed and judged the
radiological findings, particularly the CT examinations. EN
acquired the surgical data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Ethics approval was provided by the Research and
Study Program of Tokai University Educational System General
Research Organization (IRB no. 18R-331). Written informed consent
to participate was obtained from all patients and/or their family
members.
Patient consent for publication
Written informed consent for publication of the
present study was obtained from the patients and/or family
members.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mukai M, Kishima K, Yamazaki M, Aoki H,
Izumi H, Yamamoto S, Tajima T, Tobita K, Sadahiro S, Yasuda S and
Ogoshi K: Stage II/III cancer of the rectosigmoid junction: An
independent tumor type? Oncol Rep. 26:737–741. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hashiguchi Y, Muro K, Saito Y, Ito Y,
Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M,
et al: Japanese Society for Cancer of the Colon and Rectum(JSCCR)
guidelines 2019 for the treatment of colorectal cancer. Int J Clin
Oncol. 25:1–42. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mukai M, Nakamura M, Kishima K, Ninomiya
H, Nomura N, Sato H, Kato N, Machida T, Nakasaki H and Makuuchi H:
Local recurrence and occult neoplastic cells in the extranodal fat
of dissected lymph nodes in patients with curatively resected
primary colorectal cancer. Oncol Rep. 17:1365–1369. 2007.PubMed/NCBI
|
|
4
|
Cammà C, Giunta M, Fiorica F, Pagliaro L,
Craxì A and Cottone M: Preoperative radiotherapy for resectable
rectal cancer. A meta-analysis. JAMA. 284:1008–1015.
2000.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Colorectal Cancer Collaborative Group.
Adjuvant radiotherapy for rectal cancer: A systematic overview of
8,507 patients from 22 randomised trials. Lancet. 358:1291–1304.
2001.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bosset JF, Collete L, Calais G, Mineur L,
Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A and Ollier
JC: EORTC Radiotherapy Group Trial 22921. Chemotherapy with
preoperative radiotherapy in rectal cancer. N Engl J Med.
355:1114–1123. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gérard JP, Conroy T, Bonnetain F, Bouché
O, Chapet O, Closon-Dejardin MT, Untereiner M, Leduc B, Francois E,
Maurel J, et al: Preoperative radiotherapy with or without
concurrent fluorouraciland leucovorin in T3-4 rectal cancers:
Results of FFCD 9203. J Clin Oncol. 24:4620–4625. 2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ozawa H, Kotake K, Hosaka M, Hirata A and
Sugihara K: Impact of lateral pelvic lymph node dissection on the
survival of patients with T3 and T4 low rectal cancer. World J
Surg. 40:1492–1499. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kinugasa T, Akagi Y and Shirouzu K:
Benefit of lateral lymph node dissection for rectal cancer:
Long-term analysis of 944 cases undergoing surgery at a single
center (1975-2004). Anticancer Res. 34:4633–4639. 2014.PubMed/NCBI
|
|
10
|
Fujita S, Mizusawa J, Kanemitsu Y, Ito M,
Kinugasa Y, Komori K, Ohue M, Ota M, Akazai Y, Shiozawa M, et al:
Mesorectal excision with or without lateral lymph node dissection
for clinical stage II/III lower rectal cancer (JCOG0212): A
multicenter, randomized controlled, noninferiority trial. Ann Surg.
266:201–207. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tsukamoto S, Fujita S, Ota M, Mizusawa J,
Shida D, Kanemitsu Y, Ito M, Shiomi A, Komori K, Ohue M, et al:
Long-term follow-up of the randomized trial of mesorectal excision
with or without lateral lymph node dissection in rectal cancer
(JCOG0212). Br J Surg. 107:586–594. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Folkesson J, Birgisson H, Pahlman L,
Cedermark B, Glimelius B and Gunnarsson U: Swedish rectal cancer
trial: Long lasting benefits from radiotherapy on survival and
local recurrence rate. J Clin Oncol. 23:5644–5650. 2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Mukai M, Tajima T, Nakasaki H, Sato S,
Ogoshi K and Makuuchi H: Efficacy of postoperative adjuvant oral
immunochemotherapy in patients with Dukes' B colorectal cancer. Ann
Cancer Res Ther. 11:201–214. 2003.
|
|
14
|
Lembersky BC, Wieand HS, Petrelli NJ,
O'Connell MJ, Colangelo LH, Smith RE, Seay TE, Giguere JK, Marshall
ME, Jacobs AD, et al: Oral uracil and tegafur plus leucovorin
compared with intravenous fluorouracil and leucovorin in stage II
and III carcinoma of the colon: Results from National Surgical
Adjuvant Breast and Bowel Project Protocol C-06. J Clin Oncol.
24:2059–2064. 2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ito I, Mukai M, Ninomiya H, Kishima K,
Tsuchiya K, Tajima T, Oida Y, Nakamura M and Makuuchi H: Comparison
between intravenous and oral postoperative adjuvant
immunochemotherapy in patients with stage II colorectal cancer.
Oncol Rep. 20:1189–1194. 2008.PubMed/NCBI
|
|
16
|
Mukai M, Okada K, Fukumitsu H, Yazawa N,
Hoshikawa T, Tajima T, Hirakawa H, Ogoshi K and Makuuchi H:
Efficacy of 5-FU/LV plus CPT-11 as first-line adjuvant chemotherapy
for stage IIIa colorectal cancer. Oncol Rep. 22:621–629.
2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Japanese Society for Cancer of the Colon
and Rectum. Japanese Classification of Colorectal, Appendiceal, and
Anal Carcinoma. 9th edition. Kanehara Shuppan Co. Ltd., Tokyo,
pp21-27, 2018.
|
|
18
|
Tajima T, Mukai M, Koike T, Yokoyama D,
Uda S, Yoshii H, Higami S, Izumi H, Hasegawa S, Nomura E and
Makuuchi H: Better survival after hand-assisted laparoscopic
surgery than conventional laparotomy for rectal cancer: Five year
results from a single center in Japan. Clin Surg.
2(1368)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Mukai M, Tajima T, Hasegawa S, Yokoyama D,
Koike T, Hiraiwa S, Sugiyama T and Tajiri T: Recent molecular
immunological chemotherapy for gastrointestinal malignancies. Clin
Oncol. 2(1233)2017.
|
|
20
|
Dukes CE: The classification of cancer of
the rectum. J Pathol (Bacteriol). 35:323–332. 1932.
|
|
21
|
Furuhata T, Hata F, Tsuruma T, Nshimori H,
Yamaguchi K, Mizuguchi T, Kimura Y, Katsuramaki T, Mukaiya M,
Sasaki K and Hirata K: Endo-Bowel clamp (PL540S) for safe rectal
irrigation in laparoscopy-assisted rectal resection. Surg Today.
34:882–884. 2004.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cerny M, Dunet V, Prior JO, Hahnloser D,
Wagner AD, Meuli RA and Schmidt S: Initial staging of locally
advanced rectal cancer and regional lymph nodes comparison of
diffusion-weighted MRI with 18F-FDG-PET/CT. Clin Nucl Med.
41:289–295. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Fleshman J, Branda M, Sargent DJ, Boller
AM, George V, Abbas M, Peters WR Jr, Maun D, Chang G, Herline A, et
al: Effect of laparoscopic-assisted resection vs open resection of
stage II or III rectal cancer on pathologic outcomes: The ACOSOG
Z6051 randomized clinical trial. JAMA. 314:1346–1355.
2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Stevenson AR, Solomon MJ, Lumley JW,
Hewett P, Clouston AD, Gebski VJ, Davies L, Wilson K, Hague W and
Simes J: ALaCaRT Investigators. Effect of laparoscopic-assisted
resection vs. open resection on pathological outcomes in rectal
cancer: The ALaCaRT randomized clinical trial. JAMA. 314:1356–1363.
2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mukai M, Kishima K, Tajima T, Hoshikawa T,
Yazawa N, Fukumitsu H, Okada K, Ogoshi K and Makuuchi H: Efficacy
of hybrid 2-port hand-assisted laparoscopic surgery (Mukai's
operation) in patients with colorectal cancer. Oncol Rep.
22:893–899. 2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tajima T, Mukai M, Yokoyama D, Higami S,
Uda S, Hasegawa S, Nomura E, Sadahiro S, Yasuda S and Makuuchi H:
Comparison of hand-assisted laparoscopic surgery (HALS) and
conventional laparotomy in patients with colorectal cancer: Final
results from a single center. Oncol Lett. 13:4953–4958.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kitano S, Inomata M, Mizusawa J, Katayama
H, Watanabe M, Yamamoto S, Ito M, Saito S, Fujii S, Konishi F, et
al: Survival outcomes following laparoscopic versus open D3
dissection for stage II or III colon cancer (JCOG0404): A phase 3,
randomized controlled trial. Lancet Gastroenterol Hepatol.
2:261–268. 2017.PubMed/NCBI View Article : Google Scholar
|