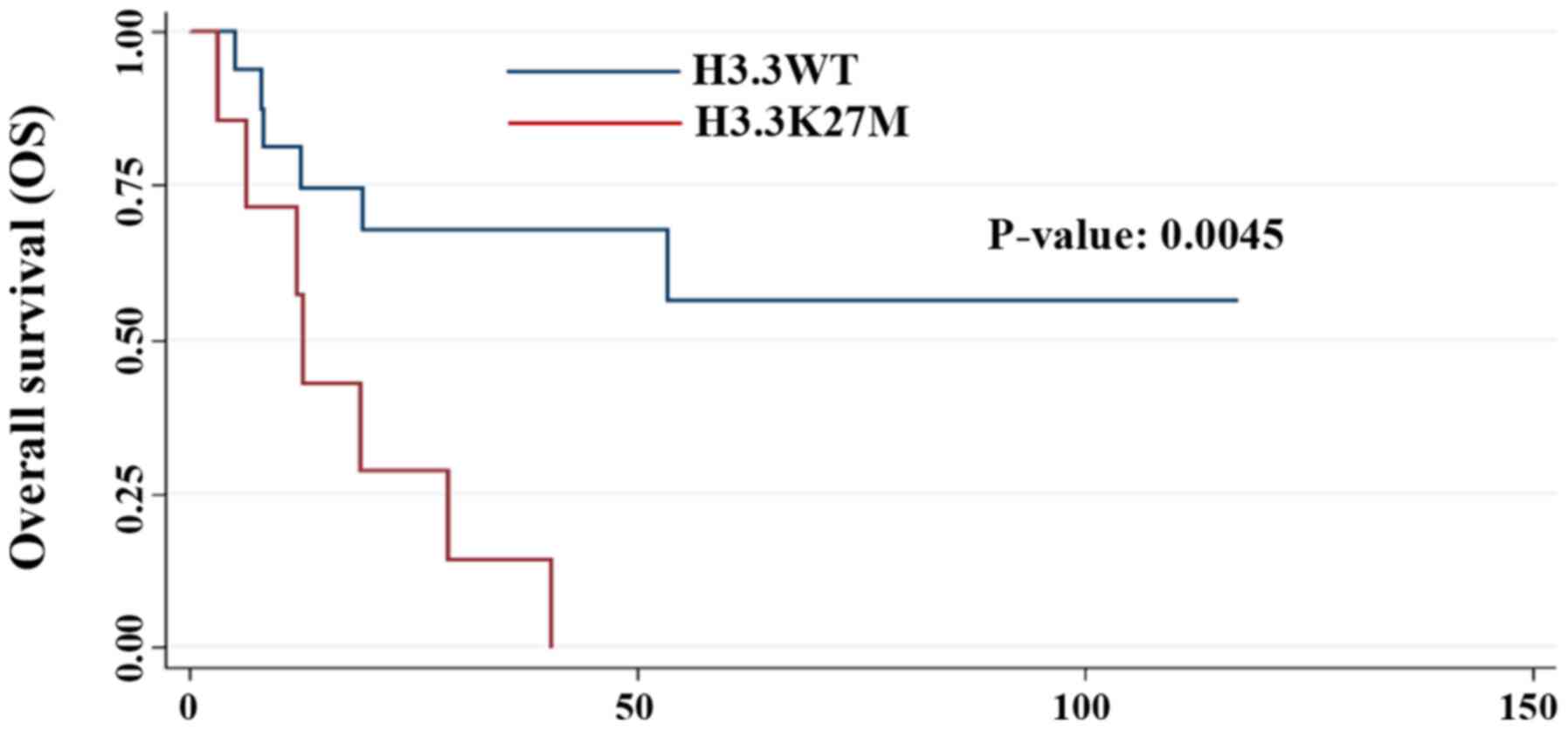
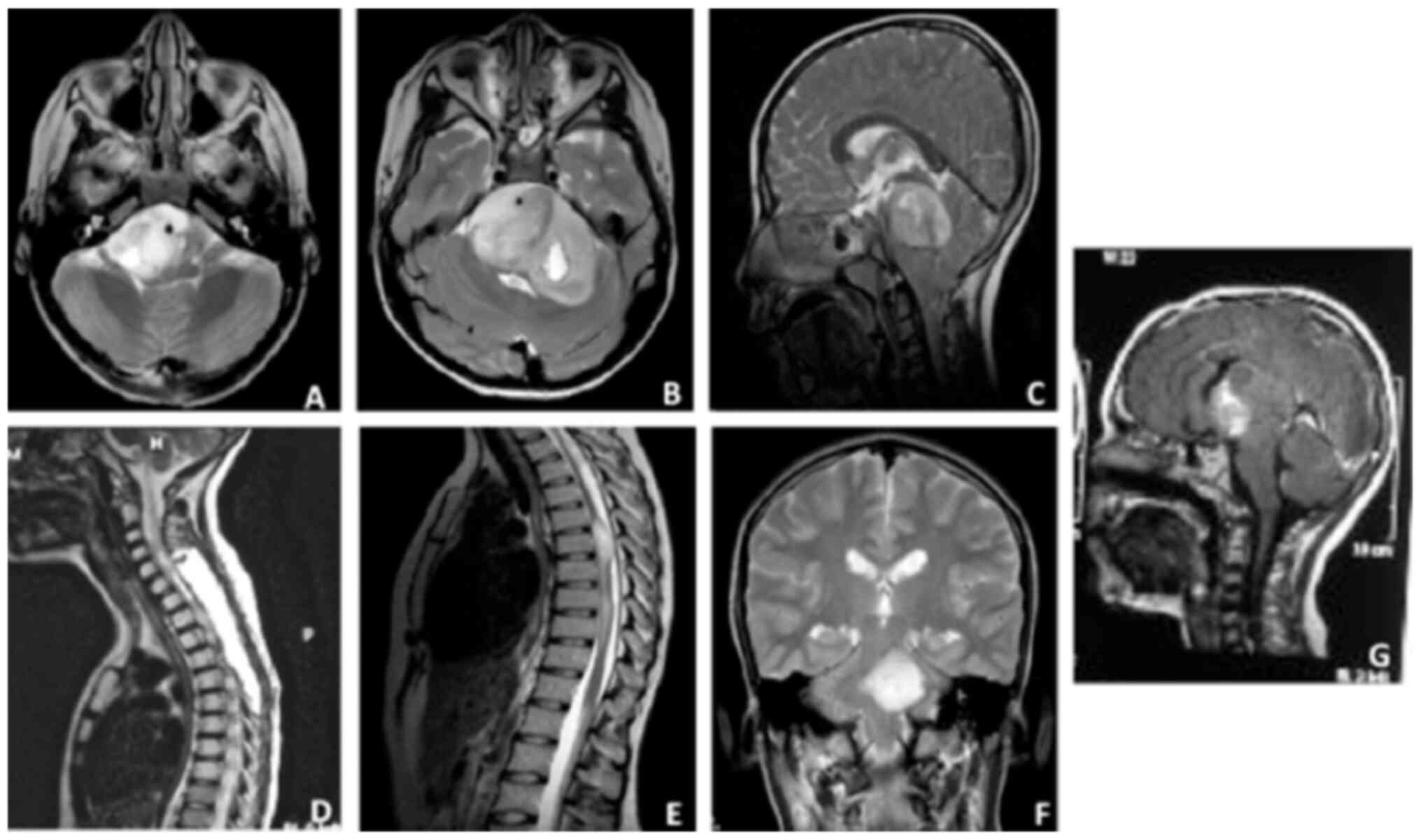
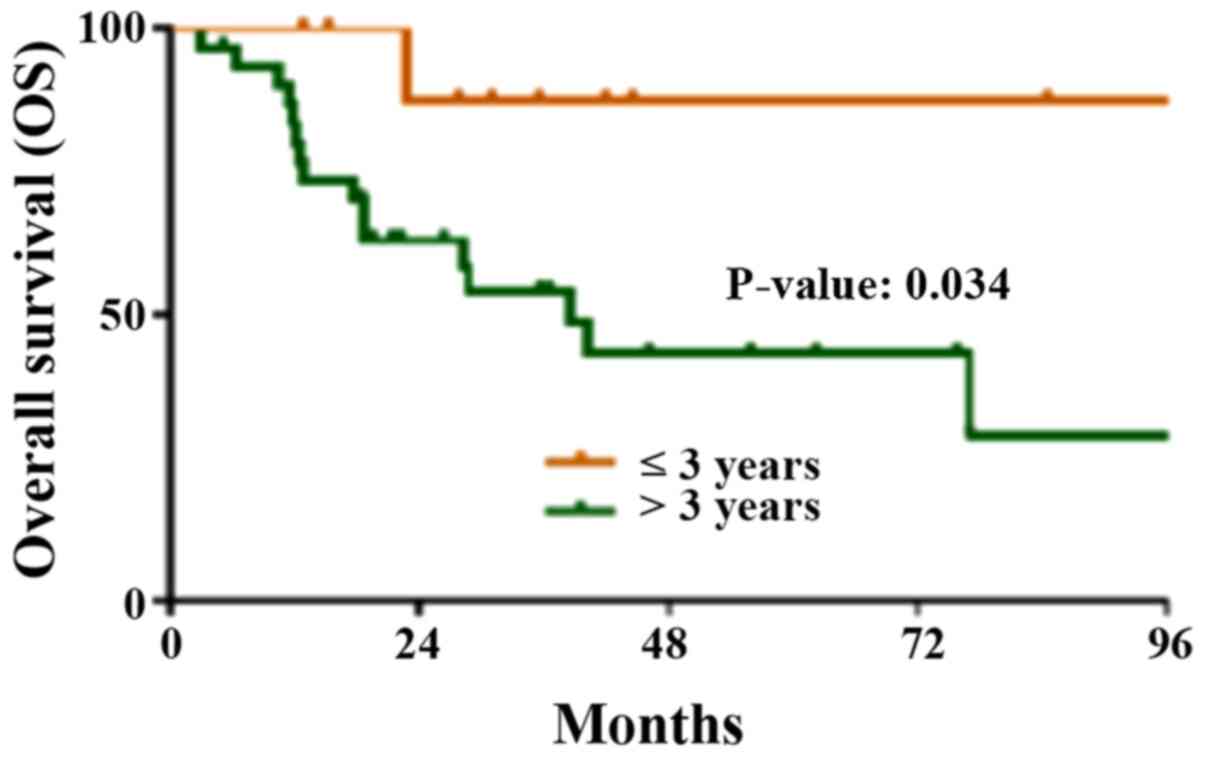
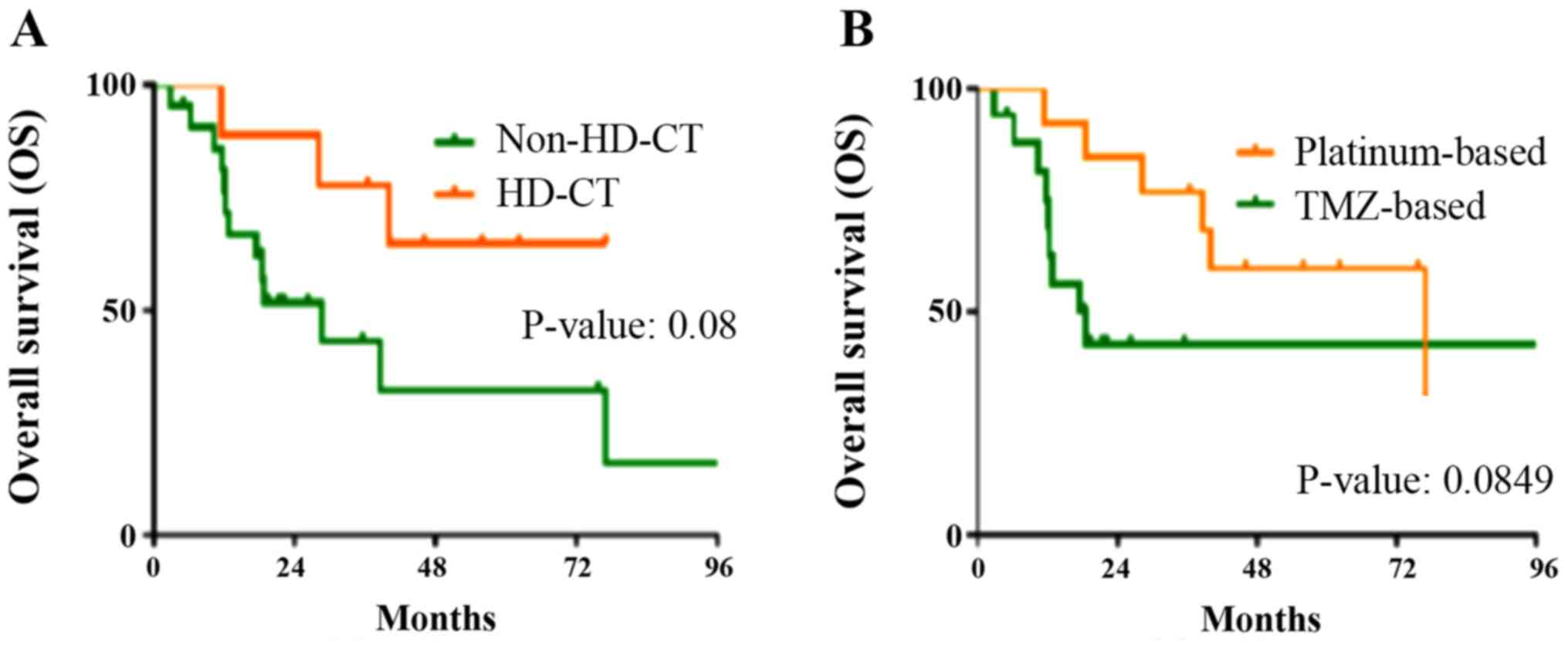
|
1
|
Merchant TE, Pollack IF and Loeffler JS:
Brain tumors across the age spectrum: Biology, therapy, and late
effects. Semin Radiat Oncol. 20:58–66. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gielen GH, Gessi M, Hammes J, Kramm CM,
Waha A and Pietsch T: H3F3A K27M mutation in pediatric CNS tumors:
A marker for diffuse high-grade astrocytomas. Am J Clin Pathol.
139:345–349. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bouffet E, Tabori U, Huang A and Bartels
U: Possibilities of new therapeutic strategies in brain tumors.
Cancer Treat Rev. 36:335–341. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Venneti S, Santi M, Felicella MM, Yarilin
D, Phillips JJ, Sullivan LM, Martinez D, Perry A, Lewis PW,
Thompson CB and Judkins AR: A sensitive and specific
histopathologic prognostic marker for H3F3A K27M mutant pediatric
glioblastomas. Acta Neuropathol. 128:743–753. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Schwartzentruber J, Korshunov A, Liu XY,
Jones DT, Pfaff E, Jacob K, Sturm D, Fontebasso AM, Quang DA,
Tönjes M, et al: Driver mutations in histone H3.3 and chromatin
remodelling genes in paediatric glioblastoma. Nature. 482:226–231.
2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bernstein BE, Mikkelsen TS, Xie X, Kamal
M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, et al:
A bivalent chromatin structure marks key developmental genes in
embryonic stem cells. Cell. 125:315–326. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Vanan MI, Underhill DA and Eisenstat DD:
Targeting epigenetic pathway in the treatment of pediatric diffuse
(high grade) gliomas. Neurotherapeutics. 14:274–283.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Castel D, Philippe C, Calmon R, Le Dret L,
Truffaux N, Boddaert N, Pagès M, Taylor KR, Saulnier P, Lacroix L,
et al: Histone H3F3A and HIST1H3B K27M mutations define two
subgroups of diffuse intrinsic pontine gliomas with different
prognosis and phenotypes. Acta Neuropathol. 130:815–827.
2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lassaletta A, Zapotocky M, Mistry M,
Ramaswamy V, Honnorat M, Krishnatry R, Guerreiro Stucklin A,
Zhukova N, Arnoldo A, Ryall S, et al: Therapeutic and prognostic
implications of BRAF V600E in pediatric low-grade gliomas. J Clin
Oncol. 35:2934–2941. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 world health organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Rheinbay E, Louis DN, Bernstein BE and
Suvà ML: A tell-tail sign of chromatin: Histone mutations drive
pediatric glioblastoma. Cancer Cell. 21:329–331. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wu G, Broniscer A, McEachron TA, Lu C,
Paugh BS, Becksfort J, Qu C, Ding L, Huether R, Parker M, et al:
Somatic histone H3 alterations in pediatric diffuse intrinsic
pontine gliomas and non-brainstem glioblastomas. Nat Genet.
44:251–253. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Khuong-Quang DA, Buczkowicz P, Rakopoulos
P, Liu XY, Fontebasso AM, Bouffet E, Bartels U, Albrecht S,
Schwartzentruber J, Letourneau L, et al: K27M mutation in histone
H3.3 defines clinically and biologically distinct subgroups of
pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol.
124:439–447. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sturm D, Witt H, Hovestadt V, Khuong-Quang
DA, Jones DT, Konermann C, Pfaff E, Tönjes M, Sill M, Bender S, et
al: Hotspot mutations in H3F3A and IDH1 define distinct epigenetic
and biological subgroups of glioblastoma. Cancer Cell. 22:425–437.
2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Fontebasso AM, Liu XY, Sturm D and Jabado
N: Chromatin remodeling defects in pediatric and young adult
glioblastoma: A tale of a variant histone 3 tail. Brain Pathol.
23:210–216. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Aihara K, Mukasa A, Gotoh K, Saito K,
Nagae G, Tsuji S, Tatsuno K, Yamamoto S, Takayanagi S, Narita Y, et
al: H3F3A K27M mutations in thalamic gliomas from young adult
patients. Neuro Oncol. 16:140–146. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Buczkowicz P, Bartels U, Bouffet E, Becher
O and Hawkins C: Histopathological spectrum of paediatric diffuse
intrinsic pontine glioma: Diagnostic and therapeutic implications.
Acta Neuropathol. 128:573–581. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Karremann M, Gielen GH, Hoffmann M, Wiese
M, Colditz N, Warmuth-Metz M, Bison B, Claviez A, van Vuurden DG,
von Bueren AO, et al: Diffuse high-grade gliomas with H3 K27M
mutations carry a dismal prognosis independent of tumor location.
Neuro Oncol. 20:123–131. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Korshunov A, Ryzhova M, Hovestadt V,
Bender S, Sturm D, Capper D, Meyer J, Schrimpf D, Kool M, Northcott
PA, et al: Integrated analysis of pediatric glioblastoma reveals a
subset of biologically favorable tumors with associated molecular
prognostic markers. Acta Neuropathol. 129:669–678. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lu VM, Alvi MA, McDonald KL and Daniels
DJ: Impact of the H3K27M mutation on survival in pediatric
high-grade glioma: A systematic review and meta-analysis. J
Neurosurg Pediatr. 23:308–316. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Pratt D, Natarajan SK, Banda A, Giannini
C, Vats P, Koschmann C, Mody R, Chinnaiyan A and Venneti S:
Circumscribed/non-diffuse histology confers a better prognosis in
H3K27M-mutant gliomas. Acta Neuropathol. 135:299–301.
2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Felix F and Fontenele J: Valproic acid may
be tested in patients With H3F3A-mutated high-grade gliomas. J Clin
Oncol. 34:3104–3105. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hashizume R, Andor N, Ihara Y, Lerner R,
Gan H, Chen X, Fang D, Huang X, Tom MW, Ngo V, et al: Pharmacologic
inhibition of histone demethylation as a therapy for pediatric
brainstem glioma. Nat Med. 20:1394–1396. 2014.PubMed/NCBI View
Article : Google Scholar
|