Introduction
Generally, surgical resection is the only
potentially curative treatment for patients with
cholangiocarcinoma. However, tumor recurrence occurs in ~70% of
patients with biliary tract cancer, even after curative resection,
and 5-year postoperative survival is less than 50% (1-3).
Long-term survival can rarely be expected once tumor recurrence
occurs because the recurrence means ‘systematic disease’. Most of
the recurrence cases, the curative resection is impossible and the
systematic chemotherapy is selected.
Gemcitabine and cisplatin (GC) combination therapy
is the first choice of treatment for recurrent or unresectable
biliary tract cancer (4), but a
strategy for second-line therapies has not yet been
established.
Several reports have suggested that conventional or
particle radiotherapy might prolong the survival of patients with
advanced biliary tract cancer (5,6). It is
known that particle therapy has a higher dose concentration
compared with conventional radiotherapy and is effective in
treating deeply located tumors. Particle therapy is divided into
proton beam radiotherapy and heavy particle therapy. Both of them
show strong cytotoxicity as a result of high linear energy
transfer, unlike the alternative X-ray beam therapy. In heavy
particle therapy, carbon ions are usually used. They have potential
advantage compared to protons. For example, they provide a better
physical dose distribution because lateral scattering is more
lessened. Furthermore, carbon ions exhibit a higher linear energy
transfer than protons. This leads to a higher relative biological
effectiveness (RBE), where damage caused by carbon ions is
clustered in the DNA, overwhelming the cellular repair system
(7). Several cohort studies showed
the effectiveness of proton beam therapy for biliary tract cancer
(8-11),
whereas heavy particle therapy for biliary tract cancer has not
been evaluated.
We experienced a case of recurrent distal bile duct
cancer that achieved long-term survival with a multimodal treatment
strategy of systematic chemotherapy and heavy particle therapy. So,
we will report this case with additional literature review.
Case report
A 65-year-old male underwent a subtotal
stomach-preserving pancreatoduodenectomy for distal bile duct
cancer. The pathological depth of tumor invasion was 5.5 mm and
there was no lymph node metastasis with a pathological stage of IIA
(T2N0M0) according to the eighth Union for International Cancer
Control staging (12). The patient
was discharged 21 days after surgery without any postoperative
complications and did not receive any adjuvant chemotherapy.
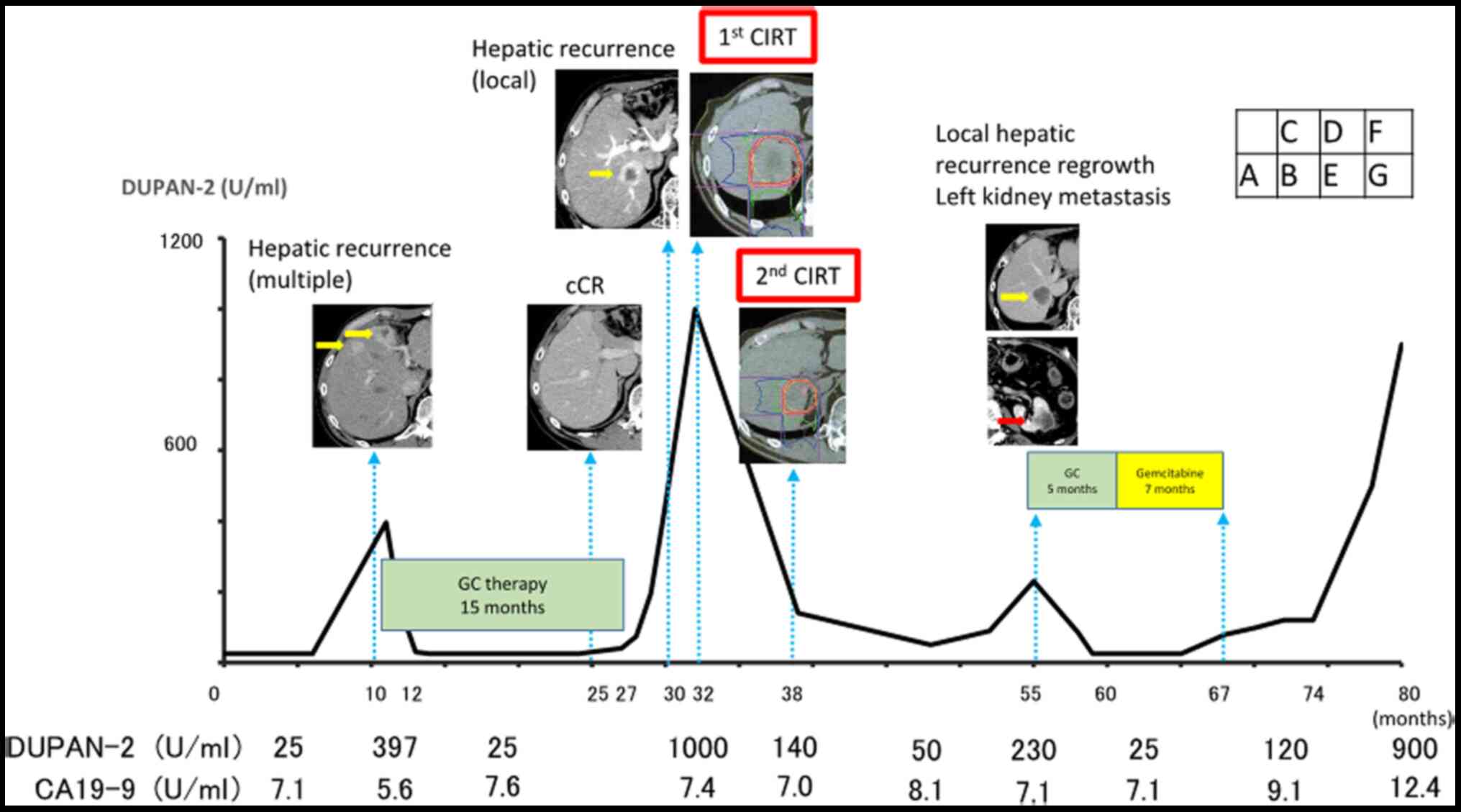
Multiple hepatic recurrences were recognized on
computed tomography (CT) performed 9 months after the surgery
(Fig. 1A), and thus GC therapy was
initiated. This therapy consisted of gemcitabine (1,000 mg per
square meter of body-surface area) and cisplatin (25 mg per square
meter of body-surface area) and was performed 10 cycles with 100%
dose. After 18 months, all hepatic tumors had completely
disappeared (Fig. 1B). The GC
therapy was discontinued at the patient's request.
Three months later, serum DUPAN-2 levels had
elevated, and a CT scan revealed a new hepatic recurrence (Fig. 1C). However, serum carbohydrate
antigen 19-9 (CA19-9) levels had not elevated. We proposed that GC
therapy should be restarted, but the patient declined because he
was suffering from peripheral neuropathy. Because no other
recurrences were detected by CT, we consulted with radiologists,
and particle therapy was recommended. The patient was treated with
heavy particle therapy [carbon ion radiotherapy (CIRT)]. The total
dose was 60 Gy (RBE) in four fractions for 4 days [15 Gy
(RBE)/day](Fig. 1D). There were no
adverse events during the first CIRT. CT scan revealed shrinkage of
the hepatic tumor. In addition, the level of standard uptake value
at the center of hepatic recurrence decreased from 8.1 to 0 by
fluorodeoxyglucose-positron emission tomography/computed tomography
(FDG-PET/CT). Serum DUPAN-2 levels also decreased.
Six months after the first CIRT, a hepatic tumor was
identified at the same site upon CT. The patient received CIRT
again, with a total dose of 66 Gy (RBE) in four fractions for 4
days [16.5 Gy (RBE)/day] (Fig. 1E).
The only adverse event was slight dermatitis. After the second
CIRT, the serum DUPAN-2 level decreased to within the normal
range.
Nineteen months after the second CIRT, CT showed a
hepatic tumor regrowth with a diameter of 45 mm (Fig. 1F). FDG-PET/CT confirmed an elevated
standardized uptake value from 0 to 7.4 in this lesion.
Furthermore, a new metastatic lesion was recognized in the left
kidney (Fig. 1G). We started GC
therapy again because it had been effective against the initial
recurrence, and the time interval since the first treatment was
approximately 35 months. We continued GC therapy for 5 months, and
then switched to gemcitabine monotherapy because of the peripheral
neuropathy and general malaise. Gemcitabine monotherapy (1,000 mg
per square meter of body-surface area) was continued administer
3-weeks on/1-week off for 7 months. The patient died 16 months
after cessation of the second chemotherapy because of disease
progression. The total survival time after the surgery was 81
months.
Discussion
We experienced a case of extrahepatic bile duct
cancer in which long-term survival was achieved with
multidisciplinary treatment including systemic chemotherapy and
CIRT after tumor recurrence. The patient survived more than 70
months after the first hepatic recurrence. Although the appearance
of a solitary recurrence is rare after complete remission of
multiple recurrences following systematic chemotherapy, our case
suggested that multiple sessions of CIRT might be able to control
the disease and contribute to prolonging overall survival.
To date, no treatment other than GC therapy has been
established for unresectable or recurrent biliary tract cancer.
Radiation therapy or chemoradiotherapy is reserved for patients who
present locally advanced unresectable disease but there is
insufficient evidence for its efficacy as a treatment for patients
with distant metastasis (13).
Recently, several case reports demonstrated the
outcomes of proton beam therapy (PBT) and CIRT for various
malignant primary or recurrent tumor types (14-18).
For example, in the research of performing
gemcitabine-concurrent proton radiotherapy (GPT) to pancreatic
cancer cases, 50 patients were divided into three groups by every
protocol of PBT. The median follow-up period was 12.5 months. The
scheduled GPT was feasible for all except 6 patients (12%) due to
acute hematologic. Grade 3 or greater late gastric ulcer and
hemorrhage were seen in 5 patients (10%). The one-year freedom from
local-progression, progression-free, and overall survival rates
were ~74% (14). Like as this
research, PBT provide useful local-regional treatment for
hepatocellular carcinoma. In this research, 76 patients were
treated and followed prospectively for treatment outcomes. Eleven
patients had multiple tumors and 46% were within the Milan
criteria. Patients received 63 Gy delivered over a 3-week period
with PBT. All cases completed the full course of treatment.
Finally, median progression-free survival for the entire group was
36 months, with a 60% 3-year progression-free survival rate for
patients within the Milan criteria. 18 patients subsequently
underwent liver transplantation; 6 (33%) explants showed
pathological complete response and 7 (39%) showed only microscopic
residual (15). The outcome of
performing CIRT for hepatocellular carcinoma is also good. In the
69 cases at National Institute of Radiological Science (NIRS), the
five-year local control rate was 81% and survival rate was 33%.
Like with the hepatocellular carcinoma, CIRT for pancreatic cancer
is effective. In the cases of locally advanced unresectable
pancreatic cancer, the combination of gemcitabine and CIRT improved
the outcomes. Second-year local control rate was 58% and
second-year overall survival was 54% in the 47 cases at NIRS. This
result is better than those reported worldwide after surgery alone
(7).
CIRT was initiated by the Heavy Ion Medical
Accelerator in Chiba, Japan, in 1993. It offers superior dose
conformity in the treatment of deeply located malignant tumors
compared with conventional X-ray therapy (18). Additionally, its biological
effectiveness (a highly localized deposition of energy) can be used
for increasing radiation exposure to tumors while minimizing
irradiation to adjacent normal tissues. PBT also has this
characteristic, but the lateral fall-off around the target is less
steep than that of carbon ion beams (19).
Despite this property for cancer treatment, no
reports have presented the benefits of CIRT for biliary tract
cancer. There have been few studies of PBT in primary, locally
recurrent, or nodal metastatic disease (Table I).
 | Table IPrevious reports regarding the
clinical outcomes of PBT for biliary tract cancer. |
Table I
Previous reports regarding the
clinical outcomes of PBT for biliary tract cancer.
| Author, year | Number of
patients | Tumor type and
characteristics | PBT dose,
fractionation, technique | Median follow-up,
months | Survival
outcomes | (Refs.) |
|---|
| Ohkawa et al,
2015 | 14 | Intrahepatic
cholangiocarcinoma. Stage II (1/14; 7%); stage IIIA (4/14; 29%);
stage IIIC (5/14; 36%); stage IV (4/14; 29%) | Median 72.6 CGE in 26
fractions | 12 | 1 y OS 50%; 1 y PFS
36%, LP in 6/14 (43%); LR in 2/14 (14%); Out-of-field recurrence in
7/14 (50%); DM in 4/14 (28%) | (8) |
| Makita et al,
2014 | 28 | Cholangiocarcinoma.
Intrahepatic (6/28; 21%); hilar (6/28; 21%); distal extrahepatic
(3/28; 11%); gallbladder (3/28; 11%); local/nodal recurrence
(10/28; 36%) | Median 68.2 CGE in 31
fractions | 12 | 1 y LC 68%, 1 y PFS
30%. 1 y OS 49%. Increased LC with BED>70 Gy (P=0.002) | (9) |
Regarding indications for radiation therapy for
biliary tract cancer, Kazuki et al (20) reported that the best indication for
curative beam therapy was locally advanced unresectable hepatic
hilar cholangiocarcinoma without distant metastasis. However,
long-term outcomes after CIRT have not yet been reported. In our
case, CIRT was indicated at the time of second recurrence, and good
local control was achieved. Hence, the patient survived for eighty
months after surgery.
In conclusion, CIRT may be a treatment option for
selected patients with solitary recurrence of biliary tract
cancer.
Acknowledgements
The authors would like to thank Dr H. Nikki March
for editing a draft of this manuscript.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
KK, IF, TT, YT, TI and YM collected the data and
assisted with data analysis. KO, YKa, ES, HT, SYas, YI and SYam
contributed to interpretation of data and manuscript preparation.
YS analyzed the data and wrote the original draft. TE conceived the
study, reviewed and revised the manuscript. JY and YKi made
substantial contributions to the conception and design. HU made
substantial contributions to analysis and interpretation of data.
JY, HU and YKi provided supervision of the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Koyama K, Ebata T, Yokoyama Y, Igami T,
Sugawara G, Mizuno T, Yamaguchi J and Nagino M: Recurrence after
curative-intent resection of perihilar cholangiocarcinoma: Analysis
of a large cohort with a close postoperative follow-up approach.
Surgery. 163:732–738. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cillo U, Fondevila C, Donadon M, Gringeri
E, Mocchegiani F, Schlitt HJ, Iizermans JNM, Vivarelli M,
Zieniewicz K, Olde Damink SWM and Groot Koerkamp B: Surgery for
cholangiocarcinoma. Liver Int. 39 (Suppl 1):S143–S155.
2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Nagino M, Ebata T, Yokoyama Y, Igami T,
Sugawara G, Takahashi Y and Nimura T: Evolution of surgical
treatment for perihilar cholangiocarcinoma: A single-center 34-year
review of 574 consecutive resections. Ann Surg. 258:129–140.
2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Valle J, Wasan H, Palmer DH, Cunningham D,
Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira
SP, et al: Cisplatin plus gemcitabine versus gemcitabine for
biliary tract cancer. N Engl J Med. 362:1273–1281. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Horgan AM, Amir E, Walter T and Knox JJ:
Adjuvant therapy in the treatment of biliary tract cancer: A
systematic review and meta-analysis. J Clin Oncol. 30:1934–1940.
2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Monson JR, Donohue JH, Gunderson LL,
Nagorney DM, Bender CE and Wieand HS: Intraoperative radiotherapy
for unresectable cholangiocarcinoma-the mayo clinic experience.
Surg Oncol. 1:283–290. 1992.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kamada T, Tsujii H, Blakely EA, Debus J,
De Neve W, Durante M, Jäkel O, Mayer R, Orecchia R, Pötter R, et
al: Carbon ion radiotherapy in Japan: An assessment of 20 years of
clinical experience. Lancet Oncol. 16:e93–e100. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ohkawa A, Mizumoto M, Ishikawa H, Abei M,
Fukuda K, Hashimoto T, Sakae T, Tsuboi K, Okumura T and Sakurai H:
Proton beam therapy for unresectable intrahepatic
cholangiocarcinoma. J Gastroenterol Hepatol. 30:957–963.
2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Makita C, Nakamura T, Takada A, Takayama
K, Suzuki M, Ishikawa Y, Azami Y, Kato T, Tsukiyama I, Kikuchiet Y,
et al: Clinical outcomes and toxicity of proton beam therapy for
advanced cholangiocarcinoma. Radiat Oncol. 9(26)2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hong TS, Wo JY, Yeap BY, Ben-Josef E,
McDonnell EI, Blaszkowsky LS, Kwak EL, Allen JN, Clark JW, Goyal L,
et al: Multi-institutional phase II study of high-dose
hypofractionated proton beam therapy in patients with localized,
unresectable hepatocellular carcinoma and intrahepatic
cholangiocarcinoma. J Clin Oncol. 34:460–468. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bush DA, Smith JC, Slater JD, Volk ML,
Reeves ME, Cheng J, Grove R and de Vera ME: Randomized clinical
trial comparing proton beam radiation therapy with transarterial
chemoembolization for hepatocellular carcinoma: Results of an
interim analysis. Int J Radiat Oncol Biol Phys. 95:477–482.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Brierley JD, Gospodarowicz MK and
Wittekind C (eds): TNM Classification of Malignant Tumors. 8th
edition. In: Union for International Cancer Control.
Wiley-Blackwell, p272, 2017.
|
|
13
|
de Jong MC, Nathan H, Sotiropoulos GC,
Paul A, Alexandrescu S, Marques H, Pulitano C, Barroso E, Clary BM,
Aldrighetti L, et al: Intrahepatic cholangiocarcinoma: An
international multi-institutional analysis of prognostic factors
and lymph node assessment. J Clin Oncol. 29:3140–3145.
2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mizumoto M, Sugahara S, Nakayama H, Hashii
H, Nakahara A, Terashima T, Okumura T, Tsuboi K, Tokuuye K and
Sakurai H: Clinical results of proton-beam therapy for
locoregionally advanced esophageal cancer. Strahlenther Onkol.
186:482–488. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Terashima K, Demizu Y, Hashimoto N, Jin D,
Mima M, Fujii O, Niwa Y, Takatori K, Kitajima N, Sirakawaet S, et
al: A phase I/II study of gemcitabine-concurrent proton
radiotherapy for locally advanced pancreatic cancer without distant
metastasis. Radiother Oncol. 103:25–31. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bush DA, Kayali Z, Grove R and Slater JD:
The safety and efficacy of high-dose proton beam radiotherapy for
hepatocellular carcinoma: A phase 2 prospective trial. Cancer.
117:3053–3059. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Schineider RA, Vitolo V, Albertini F, Koch
T, Ares C, Lomax A, Goitein G and Hug EB: Small bowel toxicity
after high dose spot scanning-based proton beam therapy for
paraspinal/retroperitoneal neoplasms. Strahlenther Onkol.
189:1020–1025. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kamata T: Clinical evidence of particle
beam therapy (carbon). Int J Clin Oncol. 17:85–88. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ohno T: Particle radiotherapy with carbon
ion beams. EPMA J. 4(9)2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kazuki T: Radiation therapy and particle
radiotherapy using proton or carbon-ion beam for biliary duct
cancer. Journal of Japan Biliary Association. 32:114–123. 2018.(In
Japanese).
|