Introduction
Immune checkpoint blockade (ICB) has proven
effective for prolonging the prognosis in gastric cancer (1). The main mechanism of action in ICB
therapy is the anti-tumor immune response of tumor-infiltrating
lymphocytes (TILs) against cancer cells. Many studies have reported
that TILs are associated with a good prognosis (2-6).
Meanwhile, several studies have reported that tertiary lymphoid
structures (TLSs) are associated with favorable clinical outcomes
in patients with various types of cancer, including lung, gastric,
colorectal and breast cancer (7-10),
and we previously reported that TLSs are positively associated with
TILs (11). Moreover, recent
studies have shown that TLSs might play an important role in
sustaining the anti-tumor immune response to ICB therapy (12,13).
TLSs are transient ectopic lymphoid organizations that are detected
in the invasive margin of the tumor and/or in the stroma of most
cancers and display an overall organization similar to that
observed in secondary lymphoid organs, such as the lymph nodes
(14). TLSs are composed of B-cell
follicles, T-cell zones, follicular dendritic cells and high
endothelial venules (15,16).
On the other hand, as the tumor grows, non-specific
inflammatory responses caused by cancer cells or surrounding tissue
tend to increase the peripheral blood neutrophil count and reduce
the lymphocyte count (17). Thus,
systemic inflammatory responses, including the high
neutrophil-to-lymphocyte ratio (NLR), are related to tumor
development and progression (18)
and have been shown to be associated with outcomes in patients with
various malignancies, including esophageal squamous cell carcinoma,
gastric cancer, colorectal cancer, and hepatocellular carcinoma
(19-23).
The NLR could also be useful in the diagnosis of thyroiditis
(24) and as an indicator to
differentiate malignant from benign thyroid nodules in the
preoperative period (25). In
addition, it could be associated with glucose control and
correlated with the HbA1c level in type 2 diabetes mellitus
(26,27), and it could serve as a diagnostic
tool for various other inflammatory conditions, such as ulcerative
colitis, irritable bowel syndrome, and nonalcoholic fatty liver
disease (28-30).
According to the above reasons, the NLR can be a
useful biomarker for various cancers, and TLSs have a vital role in
the anti-tumor immune response, such as in the prevention of tumor
progression by increasing the numbers of TILs, and may be an
independent prognostic marker in various cancers. Therefore, we
hypothesized that the NLR, an indicator of the systemic
inflammatory response, might reflect TLSs in the tumor
microenvironment, and investigated the association between the
preoperative NLR and clinicopathological features and their
relevance to the TLS density surrounding the tumor in gastric
cancer.
Patients and methods
Patients
This retrospective study included all 199
consecutive patients with stage IB-IV gastric cancer who had
undergone initial surgical resection without preoperative
chemotherapy or radiotherapy between 2007 and 2010 at Osaka City
University Hospital, Japan. Patients with stage IA disease were
excluded from this study because the tumor had been resected by
Endoscopic mucosal resection (EMR) or because of the small size of
the tumor, which makes it difficult to assess TLSs. All patients
were followed up regularly until April 2015 or until their death.
Follow-up examinations were scheduled for every three months for
the first two years, every six months during the third to fourth
years, and annually thereafter. The median follow-up period after
surgery was 49 (1-92) months. Overall survival (OS) was defined as
the time between the date of surgery and death, and disease-free
survival (DFS) was defined as the time between the date of surgery
and recurrence. This study was approved by the Osaka City
University Ethics Committee. Informed consent was obtained from all
patients.
Data collection
Clinicopathological information was extracted
retrospectively from hospital data. The patient data included age,
sex, smoking history, tumor staging (TNM), histological type,
lymphatic invasion, venous invasion, preoperative NLR and TLS
density. Pathological TNM staging was recorded for all patients
based on the UICC TNM classification, 7th edition. The preoperative
NLR, which is calculated by dividing the absolute neutrophil count
by the absolute lymphocyte count, was determined based on routine
test results from peripheral blood samples that were collected
within two weeks before the operation. In cases with multiple blood
samples, the sample from the first hospital visit was used to
calculate the NLR. Then, to examine the impact of the preoperative
NLR on the clinicopathological features, we divided the patients
into 2 groups according to the NLR, and compared the low and high
NLR groups.
Immunohistochemistry
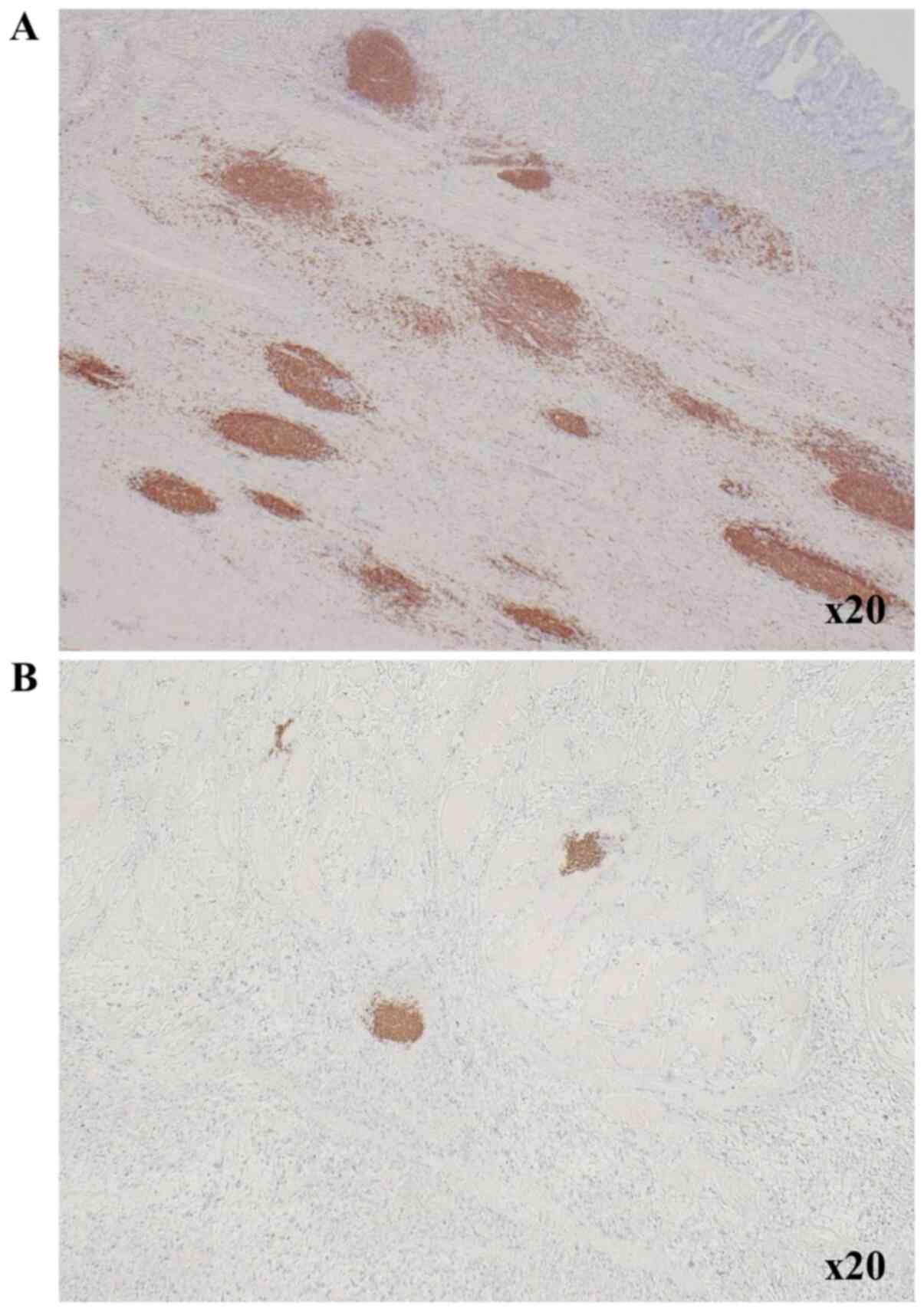
The centers of TLSs surrounding tumor were located
in B cells that formed clusters. We therefore counted B cell
clusters as TLSs, as we previously reported (11). Immunohistochemistry was performed on
4-µm thick sections from formalin-fixed paraffin-embedded (FFPE)
tumor blocks, which were obtained from patients with gastric cancer
and fixed with 10% formalin at room temperature for 6-48 h. After
incubation at 60˚C for 10 min, the sections were deparaffinized
using xylene and rehydrated in a graded ethanol series (70, 80, 90
and 100%) for 3 min each time, twice. Endogenous peroxidase
activity was blocked with absolute methanol containing 3% hydrogen
peroxide at room temperature for 15 min. After washing the sections
in PBS, they were microwaved for 10 min to achieve antigen
retrieval. Non-specific binding was blocked using the non-specific
staining blocking reagent, Target Retrieval Solution (Dako; Agilent
Technologies, Inc.), which was diluted 10 times with sterile
distilled water, and the samples incubated at 95˚C for 45 min. The
sections were subsequently incubated with the primary antibody
overnight at 4˚C, following which, they were incubated with the
secondary antibody, histofine reagent (pre-diluted; Nichirei
Biosciences, Inc.) at room temperature for 10 min, and the signal
was visualized using 3-3'-diaminobenzidine, and finally
counter-stained with hematoxylin at room temperature for 20 sec
before mounting. The primary antibodies used for the
immunohistochemical analyses were mouse anti-CD20 for B cells
(clone L26; pre-diluted; Dako; Agilent Technologies, Inc.). The
primary antibodies were diluted with 5% BSA (Sigma-Aldrich, Inc.;
Merck KGaA) in PBS. Then, we measured the area (mm2) of
CD20-positive cells and calculated the CD20-positive area (%) of
each field using the ImageJ software program (version 15.1;
National Institutes of Health). The CD20+ B cell density
was determined as the mean CD20-positive area in three fields.
Fig. 1 shows TLS-high and TLS-low
images in one low-power field of view.
Statistical analysis
All statistical analyses were performed using the
JMP software program (version 13; SAS Institute, Inc.). The
receiver operating characteristic (ROC) curve and area under the
ROC curve were used to select the best cut-off values for the
preoperative NLR and TLS density. Categorical variables were
compared using the chi-squared test. Correlation analysis was
performed using Pearson's correlation analysis. The Kaplan-Meier
method and log-rank test were used to compare survival curves.
Univariate and multivariate analyses were performed using a Cox
proportional hazards regression model. P-values of <0.05 were
considered to indicate statistical significance.
Results
The association of the preoperative
NLR with the clinicopathological features
The median and mean values of the preoperative NLR
were 2.18 and 2.7, respectively, with a standard deviation (SD) of
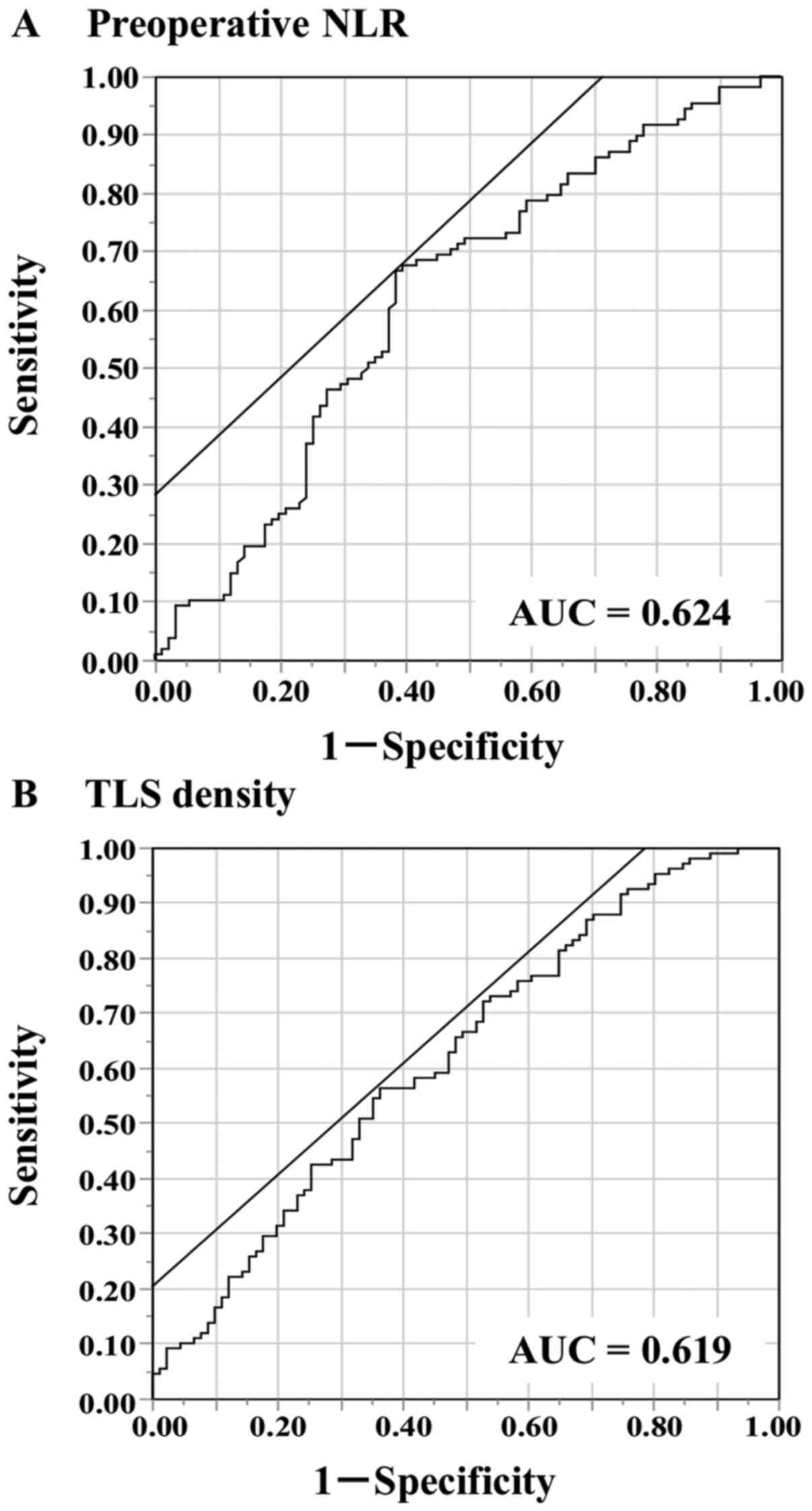
2.04 and a range of 0.59-15.17. The ROC analysis showed that the
optimal cut-off value of the preoperative NLR was 2.33 (area under
the curve [AUC] 0.625) (Fig. 2A).
Based on the cutoff value, the diagnostic sensitivity and
specificity were 66.7 and 61.5%, respectively. This value was then
used to divide the patients into 2 groups: The low NLR group
(<2.33; n=108) and the high NLR group (≥2.33; n=91). There were
no significant differences in age, sex, smoking history, T
category, N category, TNM stage, histological type or incidence of
venous invasion between the groups (Table I). The high NLR group had a higher
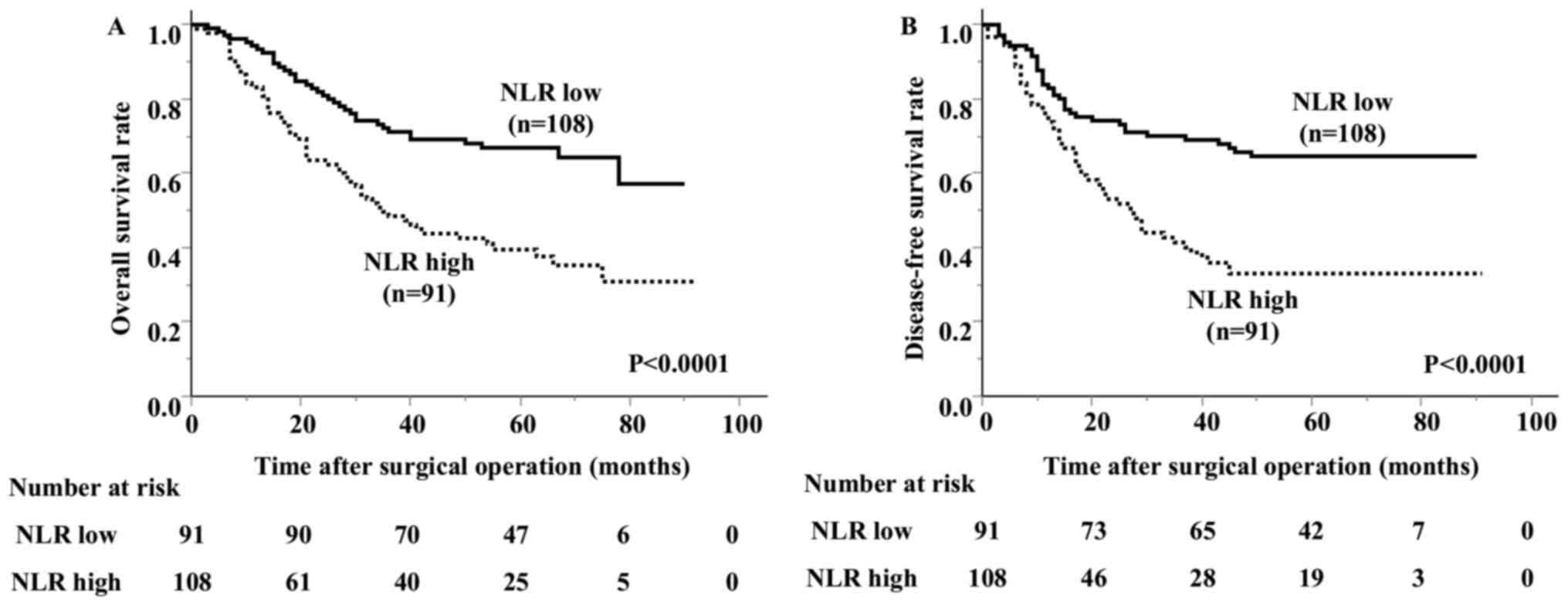
incidence of lymphatic invasion than the low NLR group. The overall
survival of the high NLR group was significantly worse than that of
the low NLR group (Fig. 3). The
5-year survival rate was 66.9% in the low NLR group and 39.5% in
the high NLR group.
 | Table IAssociation of the preoperative NLR
with the clinicopathological characteristics of patients with
gastric cancer (n=199). |
Table I
Association of the preoperative NLR
with the clinicopathological characteristics of patients with
gastric cancer (n=199).
|
Characteristics | No. of
patients | NLR low
(n=108) | NLR high
(n=91) | P-value |
|---|
| Age, years | | | | |
|
<60 | 39 | 22 | 17 | |
|
≥60 | 160 | 86 | 74 | 0.7649 |
| Sex | | | | |
|
Male | 143 | 78 | 65 | |
|
Female | 56 | 30 | 26 | 0.9013 |
| Smoking
history | | | | |
|
No | 122 | 61 | 61 | |
|
Yes | 77 | 47 | 30 | 0.1279 |
| pT category | | | | |
|
T1 | 15 | 11 | 4 | |
|
T2 | 48 | 26 | 22 | |
|
T3 | 45 | 29 | 16 | |
|
T4 | 91 | 42 | 49 | 0.0901 |
| pN category | | | | |
|
N0 | 65 | 39 | 26 | |
|
N1-N3 | 134 | 69 | 65 | 0.2586 |
| pStage | | | | |
|
Ib | 38 | 21 | 17 | |
|
II | 58 | 37 | 21 | |
|
III | 72 | 39 | 33 | |
|
IV | 31 | 11 | 20 | 0.0880 |
| Histological
type | | | | |
|
tub1, tub2,
pap | 82 | 50 | 32 | |
|
por, sig,
muc | 115 | 57 | 58 | |
|
Othersa | 2 | 1 | 1 | 0.2828 |
| Lymphatic
invasion | | | | |
|
Negative | 33 | 24 | 9 | |
|
Positive | 166 | 84 | 82 | 0.0198 |
| Venous
invasion | | | | |
|
Negative | 144 | 80 | 64 | |
|
Positive | 55 | 28 | 27 | 0.5563 |
The association of the TLS with the
NLR and the impact on survival
The median TLS density was 1.78 (average 2.96±2.72).
The ROC analysis showed that the optimal cut-off value of the TLS
density was 2.00 (AUC 0.619) (Fig.
2B). Based on the cutoff value, the diagnostic sensitivity and
specificity were 56.5 and 63.7%, respectively. A scatter chart
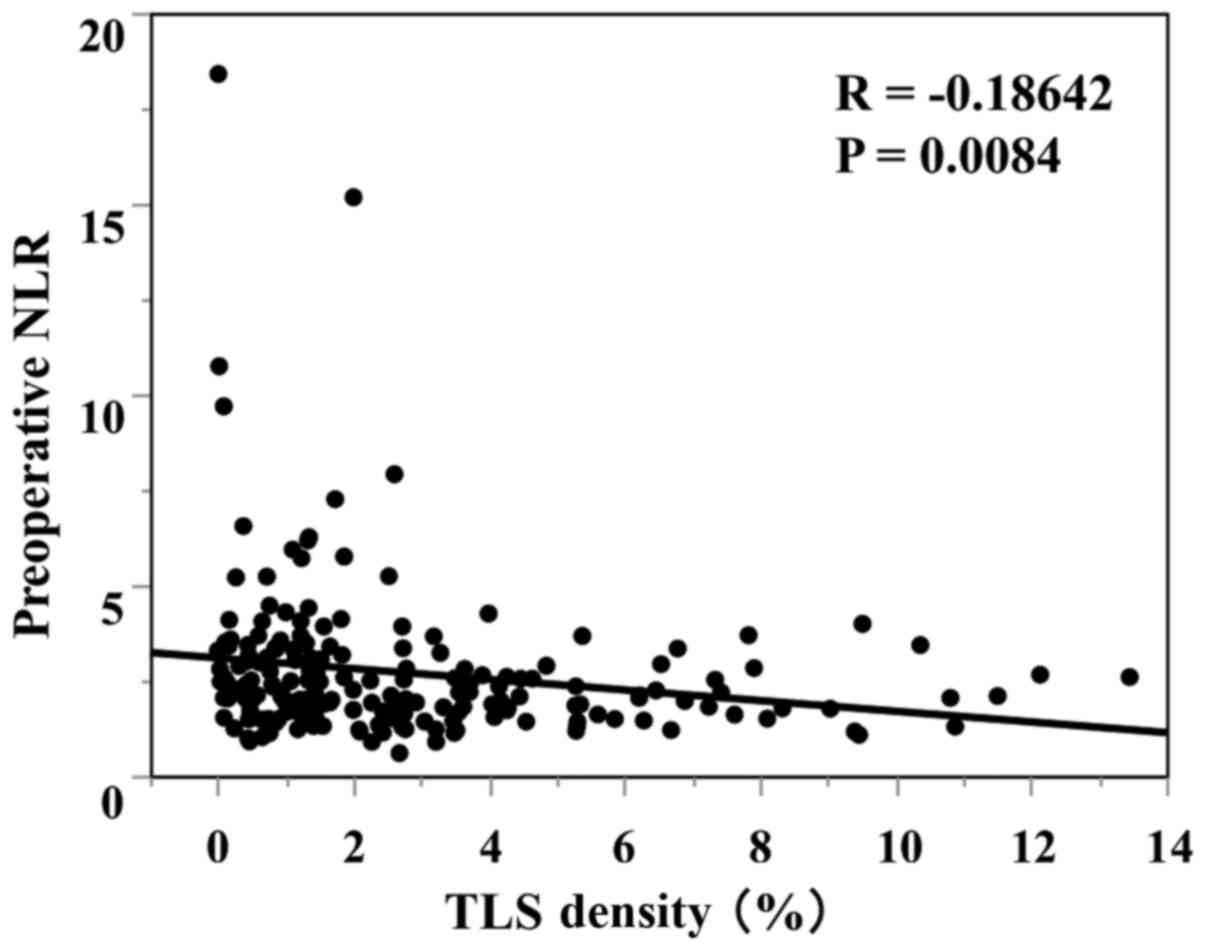
plotting the preoperative NLR and TLS density revealed a marginal
negative association; however, this association did not reach
statistical significance (P=0.0084, R=-0.1864) (Fig. 4). The comparison of the 2 groups
revealed that 66% of the high NLR group had fewer TLSs, and 58% of
the low NLR group had more TLSs (Table
II). Regarding the survival curves according to the combination
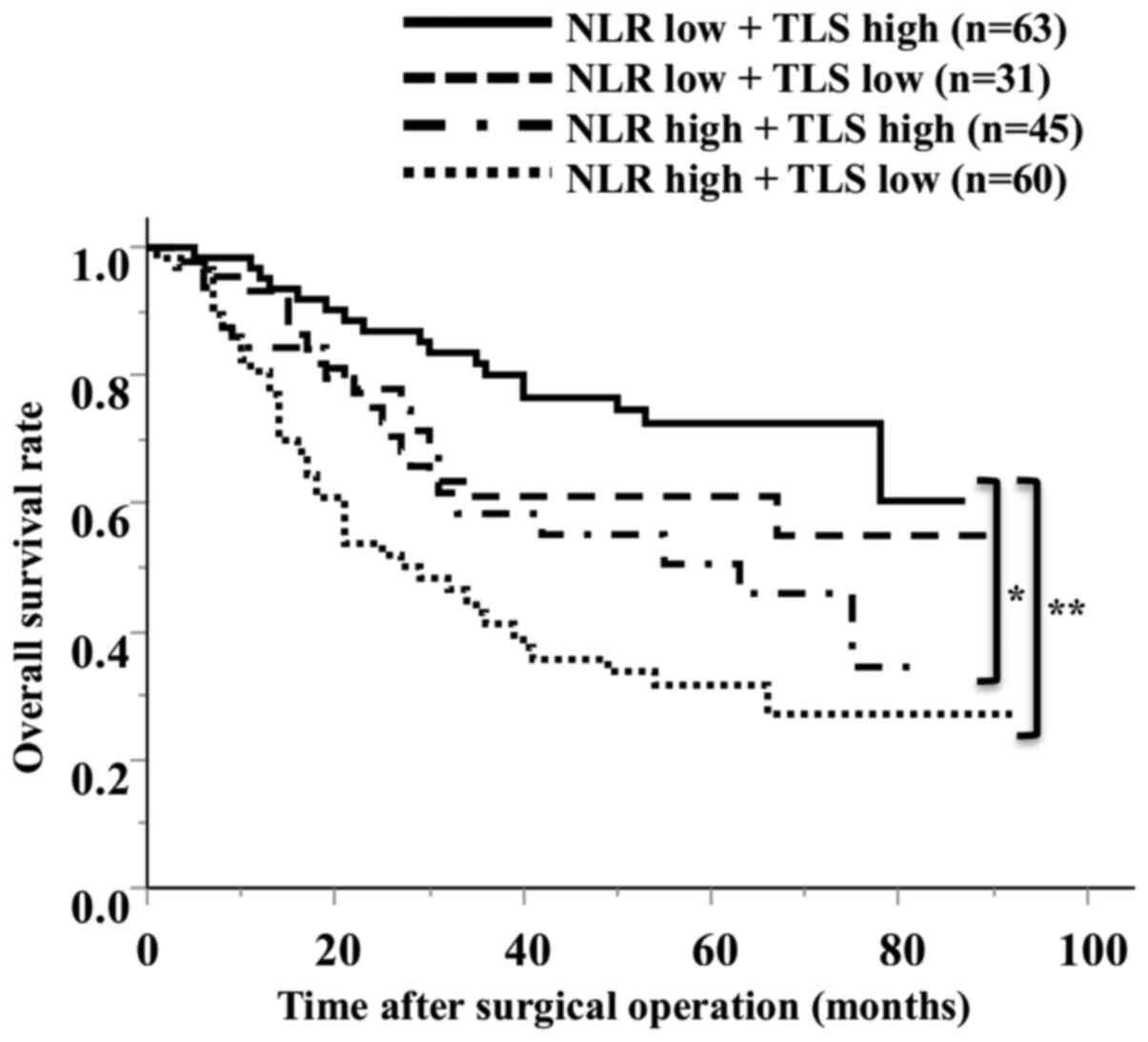
of the preoperative NLR and TLS density, the OS of the low NLR/high
TLS density groups was significantly better than that of the other
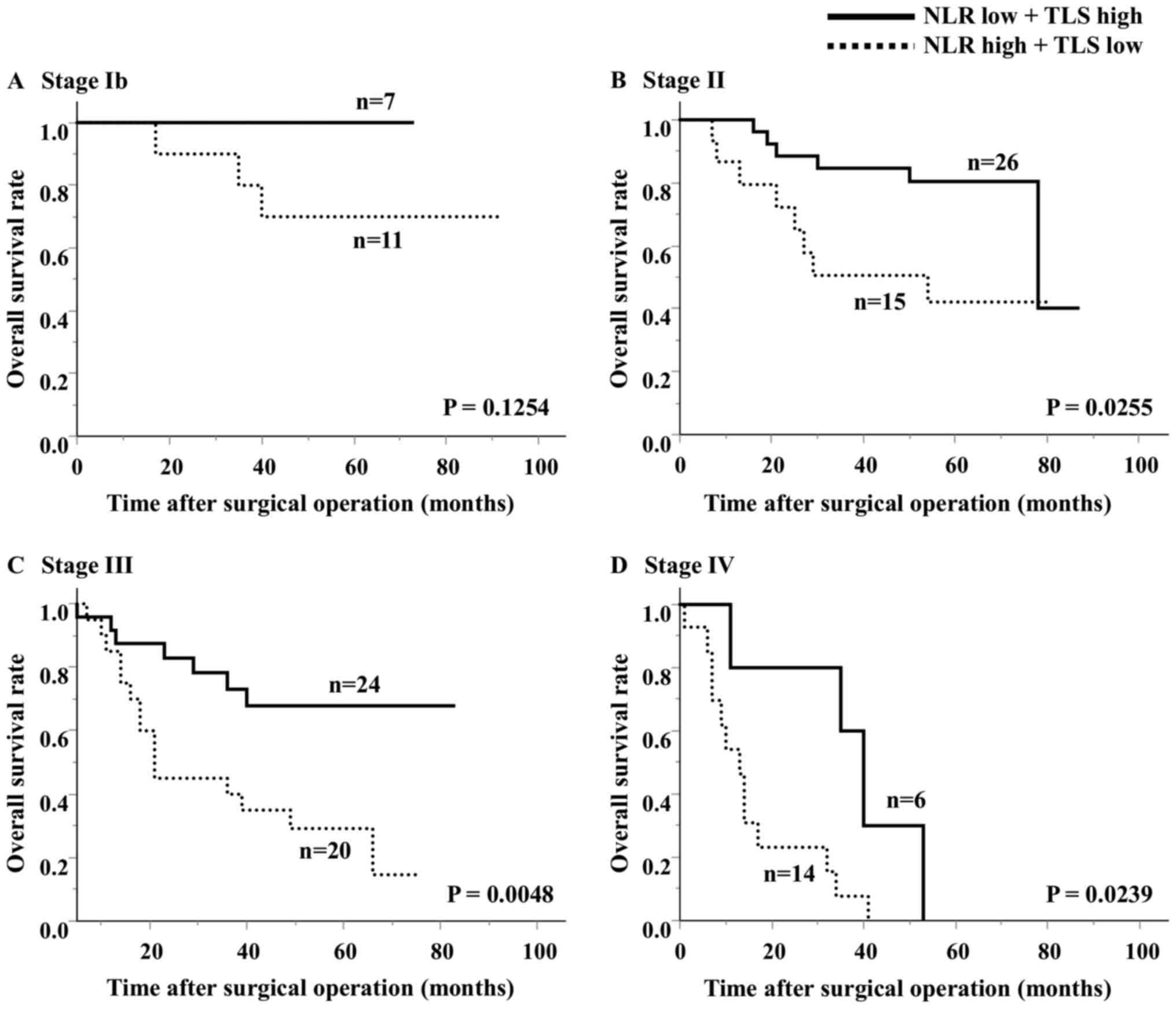
groups (Fig. 5). In an analysis
according to stage, the high NLR/low TLS group also had a
significantly worse prognosis than the low NLR/high TLS group
(Fig. 6).
 | Table IIAssociation between the preoperative
NLR and TLS density in the tumor microenvironment. |
Table II
Association between the preoperative
NLR and TLS density in the tumor microenvironment.
| TLS density | No. of
patients | NLR low | NLR high | P-value |
|---|
| All cases | 199 | 108 | 91 | |
| TLS low | 105 | 45 | 60 | |
| TLS high | 94 | 63 | 31 | 0.0006 |
Regarding the prognostic factors, the univariate
analysis showed that the T stage, N stage, TNM stage, histological
type, lymphatic invasion, NLR and TLS density were associated with
overall survival. The multivariate analysis results showed that the
T stage, histological type, NLR and TLS density were independently
associated with the overall survival rate (Table III).
 | Table IIIResults of the univariate and
multivariate analyses of the prognostic factors of the overall
survival for patients with gastric cancer. |
Table III
Results of the univariate and
multivariate analyses of the prognostic factors of the overall
survival for patients with gastric cancer.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (<60/≥60
years) | 1.273
(0.762-2.265) | 0.3693 | NA | NA |
| Sex
(male/female) | 1.111
(0.687-1.795) | 0.6653 | NA | NA |
| pT category
(T1+T2/T3+T4) | 3.153
(1.866-5.708) | <0.0001 | 2.7
(1.552-5.014) | 0.0002 |
| pN category
(N0/N1-3) | 2.423
(1.492-4.134) | 0.0002 | 1.627
(0.979-2.844) | 0.0609 |
| pStage
(Ib+II/III+IV) | 3.328
(2.142-5.315) | <0.0001 | NA | NA |
| Histological type
(tub1,tub2,pap/por,sig,muc) | 1.809
(1.171-2.848) | 0.0071 | 1.569
(1.016-2.479) | 0.0422 |
| Lymphatic invasion
(negative/positive) | 7.629
(2.861-31.081) | <0.0001 | 2.901
(0.992-12.367) | 0.0520 |
| Venous invasion
(negative/positive) | 1.092
(0.676-1.710) | 0.7102 | NA | NA |
| NLR (low/high) | 2.300
(1.516-3.531) | <0.0001 | 1.65
(1.068-2.579) | 0.0241 |
| TLS (high/low) | 2.003
(1.314-3.104) | 0.0012 | 2.042
(1.311-3.227) | 0.0015 |
Discussion
In the present study, we investigated the
relationships between the preoperative NLR and TLSs in the tumor.
We showed that the NLR was potentially correlated with the TLS
density, and both the NLR and TLSs were independent prognostic
factors. Our results suggested that the systemic NLR might reflect
the TLS density in the tumor microenvironment.
Tumor-associated neutrophils (TANs) exhibit
plasticity and are capable of polarization into either an
anti-tumorigenic ‘N1’ phenotype or a pro-tumorigenic ‘N2’ phenotype
(31,32). The ‘N2’ phenotype produces
pro-tumorigenic factors, including vascular endothelial growth
factor, inflammatory mediators and matrix metalloproteinases, and
promotes tumor growth and progression (33). We previously reported that an
increase in neutrophils at the tumor site was found within the
primary tumor and lymph node metastasis, with a poor prognosis in
patients with a high neutrophil count (34). Furthermore, we demonstrated
experimentally that neutrophils exhibited an increased PD-L1
expression when they reacted with cancer cells and exerted an
immunosuppressive function, such as the suppression of T cell
proliferation by TANs (35).
The NLR is a systemic inflammation marker reported
that has been to be an independent prognostic factor for survival
in several malignancies (36-40).
Among the many reports on gastric cancer, several studies have
reported that the NLR may be a useful marker not only for surgery
but also for chemotherapy and metastasis (41-45).
In this study, we showed that the preoperative NLR was an
independent prognostic factor for overall survival in gastric
cancer patients, suggesting that an elevated NLR might reflect the
host immune status. We previously reported that TANs were
associated with tumor progression, and that high TAN infiltration
was correlated with the preoperative NLR (46), with TANs exerting an
immunosuppressive function (35).
On the other hand, Choi et al showed that, within the tumor
microenvironment, the NLR was associated with the density of
CD4+ T cells, supporting the prognostic value of
systemic inflammation in gastric cancer (47). Tanaka et al also showed that
in biliary tract cancer, the preoperative NLR was negatively
correlated with CD8+ TILs, and that it may predict
CD8+ TILs infiltrating in the tumor microenvironment
(48). Furthermore, it has been
reported that the high pre-treatment NLR was significantly
associated with high neutrophil infiltration and low
CD3+ T cell infiltration into tumors in patients with
glioblastoma (49), and that the
preoperative NLR might originate from proinflammatory conditions
such as tumor necrosis or absence of TILs in hepatocellular
carcinoma (50).
ICB to unleash an antitumor immune response results
in a durable effect in gastric cancer (51). However, because some patients do not
respond to ICB therapy, case selection will be necessary in the
future. Increased local antitumor immune mechanisms, or TILs, are
thought to be hot tumors and are more responsive to ICB therapy. An
analysis of samples from clinical trials of ICB-treated malignant
melanoma and renal-cell carcinoma cases reported that the maturity
of B cells in TLSs was associated with the treatment response
(12). We previously reported the
presence of TLSs in gastric cancer tissue, the correlation of
peri-tumor TLSs with TILs, and the favorable prognosis of TLSs
observed more frequently in patients with gastric cancer (11). In addition, we showed that an
analysis of B cells in TLSs was able to induce CTLs in TLSs and
TLSs may be involved in cellular immunity (52). TLSs can also be associated with
humoral immunity (53). These
results suggest that TLSs plays an important role in the induction
of local tumor immunity. Moreover, in this study, we suggested a
potential association between the NLR and TLSs in the tumor
microenvironment.
The present findings suggest that the NLR may be
useful for evaluating the immunologic status of the tumor
microenvironment. Importantly, the NLR can be easily calculated
from peripheral blood counts, eliminating the need for invasive
procedures, such as a tissue biopsy, to evaluate the tumor
microenvironment. Thus, the NLR in the peripheral blood might serve
as an easy and useful marker for evaluating the immunoreactivity in
the tumor microenvironment.
The present study was associated with some
limitations. First, this was a retrospective, single-center study
with a relatively small number of patients. Thus, the results may
be biased. Second, the correlation between the preoperative NLR and
TLS density did not reach statistical significance. Further
studies, including prospective studies with a larger number of
patients, should be performed to confirm our findings.
In conclusion, the preoperative NLR appears to be
correlated with the TLS density in the primary tumor and to be a
useful prognostic factor. Our results suggested that the local
immune response might be related to systemic neutrophilic
induction. Therefore, the preoperative NLR and TLSs surrounding the
tumor may be a predictive biomarker with applications in cancer
immunotherapy against gastric cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed in the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YY acquired, analyzed and interpreted the data,
confirmed the authenticity of the data and drafted the manuscript.
HT made substantial contributions to the conception and design of
the study, interpreted the data, confirmed the authenticity of the
data and revised the manuscript critically. CS, TM, SD, MY, TTa,
TTo, SL and KM acquired and analyzed the data. KH and MO
contributed to the conception and design of the study, and revised
the manuscript critically. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All experimental procedures after 2013 were approved
by the Osaka City University Ethics Committee (approval no. 3138;
Osaka, Japan), and all patients provided written informed consent
for the collection and analysis of the specimens. All patients who
were managed prior to 2013 provided their written informed consent
for sample collection and were allowed to withdraw from the study
by signing an opt-out form approved by the Osaka City University
Ethics Committee (approval no. 4092).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Figueroa-Protti L, Soto-Molinari R,
Calderon-Osorno M, Mora J and Alpizar-Alpizar W: Gastric cancer in
the Era of immune checkpoint blockade. J Oncol.
2019(1079710)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Al-Shibli KI, Donnem T, Al-Saad S, Persson
M, Bremnes RM and Busund LT: Prognostic effect of epithelial and
stromal lymphocyte infiltration in non-small cell lung cancer. Clin
Cancer Res. 14:5220–5227. 2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sudo T, Nishida R, Kawahara A, Saisho K,
Mimori K, Yamada A, Mizoguchi A, Kadoya K, Matono S, Mori N, et al:
Clinical impact of tumor-infiltrating lymphocytes in esophageal
squamous cell carcinoma. Ann Surg Oncol. 24:3763–3770.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kang BW, Seo AN, Yoon S, Bae HI, Jeon SW,
Kwon OK, Chung HY, Yu W, Kang H and Kim JG: Prognostic value of
tumor-infiltrating lymphocytes in Epstein-Barr virus-associated
gastric cancer. Ann Oncol. 27:494–501. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kong JC, Guerra GR, Pham T, Mitchell C,
Lynch AC, Warrier SK, Ramsay RG and Heriot AG: Prognostic impact of
tumor-infiltrating lymphocytes in primary and metastatic colorectal
cancer: A systematic review and meta-analysis. Dis Colon Rectum.
62:498–508. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Stanton SE and Disis ML: Clinical
significance of tumor-infiltrating lymphocytes in breast cancer. J
Immunother Cancer. 4(59)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Germain C, Gnjatic S, Tamzalit F,
Knockaert S, Remark R, Goc J, Lepelley A, Becht E, Katsahian S,
Bizouard G, et al: Presence of B cells in tertiary lymphoid
structures is associated with a protective immunity in patients
with lung cancer. Am J Respir Crit Care Med. 189:832–844.
2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hennequin A, Derangere V, Boidot R, Apetoh
L, Vincent J, Orry D, Fraisse J, Causeret S, Martin F, Arnould L,
et al: Tumor infiltration by Tbet+ effector T cells and
CD20+ B cells is associated with survival in gastric
cancer patients. Oncoimmunology. 5(e1054598)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Schweiger T, Berghoff AS, Glogner C,
Glueck O, Rajky O, Traxler D, Birner P, Preusser M, Klepetko W and
Hoetzenecker K: Tumor-infiltrating lymphocyte subsets and tertiary
lymphoid structures in pulmonary metastases from colorectal cancer.
Clin Exp Metastasis. 33:727–739. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Figenschau SL, Fismen S, Fenton KA, Fenton
C and Mortensen ES: Tertiary lymphoid structures are associated
with higher tumor grade in primary operable breast cancer patients.
BMC Cancer. 15(101)2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sakimura C, Tanaka H, Okuno T, Hiramatsu
S, Muguruma K, Hirakawa K, Wanibuchi H and Ohira M: B cells in
tertiary lymphoid structures are associated with favorable
prognosis in gastric cancer. J Surg Res. 215:74–82. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Helmink BA, Reddy SM, Gao J, Zhang S,
Basar R, Thakur R, Yizhak K, Sade-Feldman M, Blando J, Han G, et
al: B cells and tertiary lymphoid structures promote immunotherapy
response. Nature. 577:549–555. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Cabrita R, Lauss M, Sanna A, Donia M,
Skaarup Larsen M, Mitra S, Johansson I, Phung B, Harbst K,
Vallon-Christersson J, et al: Tertiary lymphoid structures improve
immunotherapy and survival in melanoma. Nature. 577:561–565.
2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sautès-Fridman C, Lawand M, Giraldo NA,
Kaplon H, Germain C, Fridman WH and Dieu-Nosjean MC: Tertiary
lymphoid structures in cancers: Prognostic value, regulation, and
manipulation for therapeutic intervention. Front Immunol.
7(407)2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pimenta EM and Barnes BJ: Role of tertiary
lymphoid structures (TLS) in anti-tumor immunity: Potential
tumor-induced cytokines/chemokines that regulate TLS formation in
epithelial-derived cancers. Cancers (Basel). 6:969–997.
2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Carragher DM, Rangel-Moreno J and Randall
TD: Ectopic lymphoid tissues and local immunity. Semin Immunol.
20:26–42. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nagtegaal ID, Marijnen CA, Kranenbarg EK,
Mulder-Stapel A, Hermans J, van de Velde CJ and van Krieken JH:
Local and distant recurrences in rectal cancer patients are
predicted by the nonspecific immune response; specific immune
response has only a systemic effect-a histopathological and
immunohistochemical study. BMC Cancer. 1(7)2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Guner A and Kim HI: Biomarkers for
evaluating the inflammation status in patients with cancer. J
Gastric Cancer. 19:254–277. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kosumi K, Baba Y, Ishimoto T, Harada K,
Nakamura K, Ohuchi M, Kiyozumi Y, Izumi D, Tokunaga R, Taki K, et
al: Neutrophil/lymphocyte ratio predicts the prognosis in
esophageal squamous cell carcinoma patients. Surg Today.
46:405–413. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang X, Zhang W and Feng LJ: Prognostic
significance of neutrophil lymphocyte ratio in patients with
gastric cancer: A meta-analysis. PLoS One.
9(e111906)2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Haram A, Boland MR, Kelly ME, Bolger JC,
Waldron RM and Kerin MJ: The prognostic value of
neutrophil-to-lymphocyte ratio in colorectal cancer: A systematic
review. J Surg Oncol. 115:470–479. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gao F, Li X, Geng M, Ye X, Liu H, Liu Y,
Wan G and Wang X: Pretreatment neutrophil-lymphocyte ratio: An
independent predictor of survival in patients with hepatocellular
carcinoma. Medicine (Baltimore). 94(e639)2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Mowbray NG, Griffith D, Hammoda M,
Shingler G, Kambal A and Al-Sarireh B: A meta-analysis of the
utility of the neutrophil-to-lymphocyte ratio in predicting
survival after pancreatic cancer resection. HPB (Oxford).
20:379–384. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Aktas G, Sit M, Dikbas O, Erkol H,
Altinordu R, Erkus E and Savli H: Elevated neutrophil-to-lymphocyte
ratio in the diagnosis of Hashimoto's thyroiditis. Rev Assoc Med
Bras (1992). 63:1065–1068. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sit M, Aktas G, Erkol H, Yaman S, Keyif F
and Savli H: Neutrophil to lymphocyte ratio is useful in
differentiation of malign and benign thyroid nodules. P R Health
Sci J. 38:60–63. 2019.PubMed/NCBI
|
|
26
|
Duman TT, Aktas G, Atak BM, Kocak MZ,
Erkus E and Savli H: Neutrophil to lymphocyte ratio as an
indicative of diabetic control level in type 2 diabetes mellitus.
African Health Sci. 19:1602–1606. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bilgin S, Aktas G, Zahid Kocak M, Atak BM,
Kurtkulagi O, Duman TT and Savli H: Association between novel
inflammatory markers derived from hemogram indices and metabolic
parameters in type 2 diabetic men. Aging Male. 1–5. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Jeong Y, Jeon SR, Kim HG, Moon JR, Lee TH,
Jang JY, Cho JH, Park JS, Park H, Lee KH, et al: The role of
platelet to lymphocyte ratio and neutrophil to lymphocyte ratio in
ulcerative colitis. Intest Res. 19:62–70. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Aktas G, Duman T, Atak B, Kurtkulagi O,
Bilgin S, Basaran E, Demirkol ME and Kosekli MA: Irritable bowel
syndrome is associated with novel inflammatory markers derived from
hemogram parameters. Fam Med Prim Care Rev. 22:107–110. 2020.
|
|
30
|
Khoury T, Mari A, Nseir W, Kadah A, Sbeit
W and Mahamid M: Neutrophil-to-lymphocyte ratio is independently
associated with inflammatory activity and fibrosis grade in
nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol.
31:1110–1115. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Fridlender ZG, Sun J, Kim S, Kapoor V,
Cheng G, Ling L, Worthen GS and Albelda SM: Polarization of
tumor-associated neutrophil phenotype by TGF-beta: ‘N1’ versus ‘N2’
TAN. Cancer Cell. 16:183–194. 2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Mishalian I, Bayuh R, Levy L, Zolotarov L,
Michaeli J and Fridlender ZG: Tumor-associated neutrophils (TAN)
develop pro-tumorigenic properties during tumor progression. Cancer
Immunol Immunother. 62:1745–1756. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Hurt B, Schulick R, Edil B, El Kasmi KC
and Barnett C Jr: Cancer-promoting mechanisms of tumor-associated
neutrophils. Am J Surg. 214:938–944. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tokumoto M, Tanaka H, Ohira M, Go Y, Okita
Y, Sakurai K, Toyokawa T, Kubo N, Muguruma K, Maeda K, et al: A
positive correlation between neutrophils in regional lymph nodes
and progression of gastric cancer. Anticancer Res. 34:7129–7136.
2014.PubMed/NCBI
|
|
35
|
Hiramatsu S, Tanaka H, Nishimura J,
Yamakoshi Y, Sakimura C, Tamura T, Toyokawa T, Muguruma K, Yashiro
M, Hirakawa K and Ohira M: Gastric cancer cells alter the
immunosuppressive function of neutrophils. Oncol Rep. 43:251–259.
2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Nakamura K, Yoshida N, Baba Y, Kosumi K,
Uchihara T, Kiyozumi Y, Ohuchi M, Ishimoto T, Iwatsuki M, Sakamoto
Y, et al: Elevated preoperative neutrophil-to-lymphocytes ratio
predicts poor prognosis after esophagectomy in T1 esophageal
cancer. Int J Clin Oncol. 22:469–475. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Song Y, Yang Y, Gao P, Chen X, Yu D, Xu Y,
Zhao J and Wang Z: The preoperative neutrophil to lymphocyte ratio
is a superior indicator of prognosis compared with other
inflammatory biomarkers in resectable colorectal cancer. BMC
Cancer. 17(744)2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Fu Y, Liu W, OuYang D, Yang A and Zhang Q:
Preoperative neutrophil-to-lymphocyte ratio predicts long-term
survival in patients undergoing total laryngectomy with advanced
laryngeal squamous cell carcinoma: A single-center retrospective
study. Medicine (Baltimore). 95(e2689)2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Feng Z, Wen H, Bi R, Ju X, Chen X, Yang W
and Wu X: Preoperative neutrophil-to-lymphocyte ratio as a
predictive and prognostic factor for high-grade serous ovarian
cancer. PLoS One. 11(e0156101)2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Nayak A, McDowell DT, Kellie SJ and
Karpelowsky J: Elevated preoperative neutrophil-lymphocyte ratio is
predictive of a poorer prognosis for pediatric patients with solid
tumors. Ann Surg Oncol. 24:3456–3462. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Mori M, Shuto K, Kosugi C, Narushima K,
Hayashi H, Matsubara H and Koda K: An increase in the
neutrophil-to-lymphocyte ratio during adjuvant chemotherapy
indicates a poor prognosis in patients with stage II or III gastric
cancer. BMC Cancer. 18(1261)2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Pang W, Lou N, Jin C, Hu C, Arvine C, Zhu
G and Shen X: Combination of preoperative platelet/lymphocyte and
neutrophil/lymphocyte rates and tumor-related factors to predict
lymph node metastasis in patients with gastric cancer. Eur J
Gastroenterol Hepatol. 28:493–502. 2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Nakayama Y, Gotohda N, Shibasaki H, Nomura
S, Kinoshita T and Hayashi R: Usefulness of the
neutrophil/lymphocyte ratio measured preoperatively as a predictor
of peritoneal metastasis in patients with advanced gastric cancer.
Surg Today. 44:2146–2152. 2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Tanaka H, Tamura T, Toyokawa T, Muguruma
K, Miki Y, Kubo N, Sakurai K, Hirakawa K and Ohira M: Clinical
relevance of postoperative neutrophil-lymphocyte ratio (NLR) to
recurrence after adjuvant chemotherapy of S-1 for gastric cancer.
Anticancer Res. 38:3745–3751. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Tanaka H, Muguruma K, Toyokawa T, Kubo N,
Ohira M and Hirakawa K: Differential impact of the
neutrophil-lymphocyte ratio on the survival of patients with stage
IV gastric cancer. Dig Surg. 31:327–333. 2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Hiramatsu S, Tanaka H, Nishimura J,
Sakimura C, Tamura T, Toyokawa T, Muguruma K, Yashiro M, Hirakawa K
and Ohira M: Neutrophils in primary gastric tumors are correlated
with neutrophil infiltration in tumor-draining lymph nodes and the
systemic inflammatory response. BMC Immunol. 19(13)2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Choi Y, Kim JW, Nam KH, Han SH, Kim JW,
Ahn SH, Park DJ, Lee KW, Lee HS and Kim HH: Systemic inflammation
is associated with the density of immune cells in the tumor
microenvironment of gastric cancer. Gastric Cancer. 20:602–611.
2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Tanaka R, Kimura K, Eguchi S, Tauchi J,
Shibutani M, Shinkawa H, Ohira GO, Yamazoe S, Tanaka S, Amano R, et
al: Preoperative neutrophil-to-lymphocyte ratio predicts
tumor-infiltrating CD8(+) T cells in biliary tract cancer.
Anticancer Res. 40:2881–2887. 2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Ha SY, Choi S, Park S, Kim JM, Choi GS,
Joh JW and Park CK: Prognostic effect of preoperative
neutrophil-lymphocyte ratio is related with tumor necrosis and
tumor-infiltrating lymphocytes in hepatocellular carcinoma.
Virchows Archiv. 477:807–816. 2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Han S, Liu Y, Li Q, Li Z, Hou H and Wu A:
Pre-treatment neutrophil-to-lymphocyte ratio is associated with
neutrophil and T-cell infiltration and predicts clinical outcome in
patients with glioblastoma. BMC Cancer. 15(617)2015.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Kang YK, Boku N, Satoh T, Ryu MH, Chao Y,
Kato K, Chung HC, Chen JS, Muro K, Kang WK, et al: Nivolumab in
patients with advanced gastric or gastro-oesophageal junction
cancer refractory to, or intolerant of, at least two previous
chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet.
390:2461–2471. 2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Yamakoshi Y, Tanaka H, Sakimura C, Deguchi
S, Mori T, Tamura T, Toyokawa T, Muguruma K, Hirakawa K and Ohira
M: Immunological potential of tertiary lymphoid structures
surrounding the primary tumor in gastric cancer. Int J Oncol.
57:171–182. 2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Sautès-Fridman C, Petitprez F, Calderaro J
and Fridman WH: Tertiary lymphoid structures in the era of cancer
immunotherapy. Nat Rev Cancer. 19:307–325. 2019.PubMed/NCBI View Article : Google Scholar
|