Introduction
Hepatocellular carcinoma (HCC) is a common
malignancy and one of the major leading causes of cancer deaths
worldwide (1) Patients with
unresectable HCC show poor prognoses (2) Systemic chemotherapy is a treatment
strategy for unresectable HCC. Molecular targeted agents (MTAs),
such as sorafenib (SORA), regorafenib (REGO), and lenvatinib (LEN),
are available for the treatment of unresectable HCC (3-5).
Ramucirumab (RAM), an MTA, has recently been approved for
second-line treatment of unresectable HCC patients with a baseline
α-fetoprotein (AFP) concentration ≥400 ng/ml (6).
RAM is a human immunoglobulin G1 (IgG1) monoclonal
antibody that inhibits ligand activation of vascular endothelial
growth factor (VEGF) receptor 2. In a phase 3 trial (REACH-2 trial)
involving unresectable HCC patients, RAM significantly improved
progression-free survival (PFS) and overall survival relative to
placebo (6) However, in the REACH-1
trial, more than 5% of the patients treated with RAM suffered grade
≥3 treatment-emergent adverse events (AEs), including ascites.
Moreover, according to the REACH-2 trial, patients with Child-Pugh
scores of 7 and 8 showed a higher incidence of RAM-related grade 3
ascites than those with Child-Pugh scores of 5 or 6(7).
The development of ascites in patients with HCC is
associated with various factors, including malnutrition (8) The controlling nutritional status
(CONUT) score is a nutritional index consisting of three
parameters: Serum albumin level, total cholesterol level, and
lymphocyte count (9) CONUT is
superior than other nutritional assessment tools such as the
Nutrition Risk Screening-2002 and subjective global assessment to
predict infectious complication in patients with digestive diseases
(10). In addition, the CONUT score
was reported to predict the development of ascites in patients with
HCC (11) While, intramuscular
adipose tissue (IMAT) content is a method for the quantification of
fatty infiltration in skeletal muscles (12) IMAT is reported to be an independent
risk factor for mortality in patients with HCC (13) IMAT was also reported to be
significantly associated with liver dysfunction and the prognosis
of HCC patients who underwent hepatectomy (14).
The aim of this study was to investigate patient
profiles, including the CONUT score and IMAT content, associated
with the development of RAM-related ascites in patients with
HCC.
Materials and methods
Study design
This retrospective study was conducted at the Kurume
University Hospital and Omuta City Hospital. The protocol conformed
to the ethical guidelines of the 1975 Declaration of Helsinki and
was approved by the ethics committees of the Kurume University
School of Medicine (approval no. 19203). An opt-out approach was
employed to obtain informed consent from the patients, and personal
information was protected during data collection.
Inclusion and exclusion criteria
The patient inclusion criteria for this study were
as follows: i) diagnosis of unresectable HCC according to the
Barcelona Clinic Liver Cancer (BCLC) staging system (15); ii) age ≥18 years; iii) Eastern
Cooperative Oncology Group performance status 0; iv) history of
pretreatment with MTAs; and v) completion of follow-up until death
or study cessation (August 30, 2020). The patient exclusion
criteria were as follows: i) history of a malignant tumor other
than HCC in the 5 years preceding the study; ii) participation in
any clinical trial; iii) Child-Pugh class C; iv) creatinine >1.5
mg/dl; v) chronic heart failure; vi) infiltrative HCC; vii)
presence of ascites; viii) esophageal varices with a high risk of
rupture; and ix) history of liver transplantation.
Patients
A total of 16 consecutive HCC patients who received
RAM treatment between September 19, 2019 and July 31, 2020 were
registered. The data cutoff date for this analysis was August 31,
2020. Patients meeting any of the exclusion criteria were excluded
from the analysis (n=2). Thus, a total of 14 patients were enrolled
in the study.
Assessment of nutritional status and
liver function
CONUT score, used to assess nutritional status, was
calculated from serum albumin level, total cholesterol level, and
lymphocyte count, as previously described (9) Albumin concentrations of ≥3.5,
3.0-3.49, 2.5-2.99, and <2.5 g/dl were scored as 0, 2, 4 and 6
points, respectively. Total lymphocyte counts of ≥1,600,
1,200-1,599, 800-1,199, and <800/µl were scored as 0, 1, 2 and 3
points, respectively. Total cholesterol concentrations of ≥180,
140-179, 100-139 and <100 mg/dl were scored as 0, 1, 2 and 3
points, respectively. Liver function was evaluated using the
albumin-bilirubin (ALBI) score, as previously described (16) It based on serum albumin and total
bilirubin levels; ALBI-score = [log10 bilirubin (µmol/l) x 0.66] +
[albumin (g/l) x -0.085], and was graded as following: ≤-2.60 =
ALBI grade 1, >-2.60 to ≤-1.39 = ALBI grade 2, >-1.39 = ALBI
grade 3).
Evaluation of skeletal muscle
index
The skeletal muscle index (SMI) was evaluated at the
third lumbar vertebra level on computed tomography (CT) scans,
which were obtained as part of the HCC assessment (17) SMI were calculated by normalizing the
L3 skeletal muscle areas by the square of the height
(m2) (18). The targets
of measurement were psoas, erector spinae, quadratus lumborum,
transversus abdominis, external and internal obliques, and rectus
abdominis. The measurement was performed by two
government-certified physical therapists (S.K. and K.H.) who were
blinded to the patients' information. This analysis was performed
using diagnostic software ImageJ Version 1.50 software (National
Institutes of Health) (19).
Evaluation of visceral fat area
(VFA)
We measured VFA using diagnostic CT scans at the
umbilical level, as previously described (20) CT scanning was performed for HCC
evaluation. The measurement was performed by two
government-certified physical therapists (S.K. and K.H.) who were
blinded to the patients' information. The VFA was also measured by
the diagnostic software ImageJ Version 1.50 software (National
Institutes of Health) (19).
Evaluation of IMAT
Muscle quality was evaluated by measuring the IMAT
content. The IMAT was calculated as the psoas
muscle-to-subcutaneous fat attenuation ratio using diagnostic CT
scans at the umbilical level as previously described (21) A higher IMAT indicates a greater
amount of adipose tissue within the skeletal muscle (22) The SMI, VFA, and IMAT were measured
by two government-certified physical therapists (S.K. and K.H.) who
were blinded to patient information.
Diagnosis of HCC
HCC was diagnosed using a combination of tests for
serum tumor markers, such as AFP and des-γ-carboxy prothrombin
(DCP), and imaging procedures, such as ultrasonography, computed
tomography (CT), and magnetic resonance imaging (MRI). HCC was
classified using the BCLC staging system (15).
Treatment with RAM and evaluation of
therapeutic response
RAM was intravenously injected at a dose of 8 mg/kg
once every 2 weeks. Therapeutic response was evaluated according to
the modified Response Evaluation Criteria in Solid Tumors (mRECIST)
(23), using dynamic CT or magnetic
resonance imaging. The evaluation was conducted 4-6 weeks after
initiation of treatment with RAM, and thereafter, at intervals of
2-3 months until death or study cessation.
Assessment of AEs and ascites
AEs and ascites were assessed every month after the
initiation of RAM treatment. AEs were assessed according to the
National Cancer Institute Common Terminology Criteria for Adverse
Events, version 4.0.
Definition of severe ascites
Ascites were assessed by ultrasonography and CT scan
images. Severe ascites were defined as diuretic-resistant
ascites.
Statistical analysis
All data are expressed as frequency or median
(range). All statistical analyses were performed using JMP Pro,
version 14 (SAS Institute Inc.). PFS was calculated using the
Kaplan-Meier method and analyzed using the log-rank test. We also
performed decision tree analysis to identify factors associated
with severe ascites, as previously described (24).
Results
Patient characteristics
Patient profiles are summarized in Table I. The median patient age was 72.5
years, and 28.5% of the patients were female. The median body mass
index (BMI) was 20.7 kg/m2, and 78.6% of patients
(11/14) showed Child-Pugh class A. ALBI grade 2 was observed in
92.3% of the patients, and ALBI 2a was observed in 42.9%. The
frequencies of CONUT scores of 0-1 (normal nutrition), 2-4 (mild
malnutrition), 5-8 (moderate malnutrition), and ≥9 (severe
malnutrition) were 21.4, 42.9, 35.7 and 0.0%, respectively
(Table I). BCLC stage B HCC was
seen in 42.8% of the patients. The median IMAT and VFA were -0.51
and 79.92 cm2, respectively. Prior treatment with
sorafenib (SORA), SORA+LEN, and LEN was seen in 28.6 (4/14), 35.7
(5/14), and 35.7% (5/14) of the patients, respectively. The median
observation period was 4.5 months (1.3-11.5 months) (Table I).
 | Table IClinicopathological characteristics
of patients with hepatocellular carcinoma (n=14). |
Table I
Clinicopathological characteristics
of patients with hepatocellular carcinoma (n=14).
| Characteristic | Value |
|---|
| Median age (range),
years | 72.5 (36-87) |
| Sex,
female/male | 4/10 |
| Median body mass
index (range) | 20.7
(16.4-24.2) |
| Etiology,
HBV/HCV/others | 4/5/5 |
| Child-Pugh class,
A/B | 11/3 |
| Median total
cholesterol (range), mg/dl | 166 (135-254) |
| Median ALBI score
(range) | -2.19 (-2.74 -
-1.65) |
| Modified ALBI
grade, 1/2a/2b/3 | 1/6/7/0 |
| Controlling
nutritional status score | |
|
0-1
(normal) | 3 |
|
2-4
(mild) | 6 |
|
5-8
(moderate) | 5 |
|
≥9
(severe) | 0 |
| Median tumor
diameter (range), mm | 47.8
(11.0-132.0) |
| Number of tumors,
<5/≥5 | 1/13 |
| Barcelona clinic
liver cancer stage, B/C | 6/8 |
| Macroscopic portal
vein invasion, yes/no | 2/12 |
| Extrahepatic
metastasis, yes/no | 8/6 |
| Median
α-fetoprotein (range), ng/ml | 5,917
(906-612,770) |
| Median
des-γ-carboxy prothrombin (range), mAU/ml | 2,035
(22-292,689) |
| Intramuscular
adipose tissue | -0.51 (-0.72 -
-0.38) |
| Median visceral fat
mass (range), cm2 | 79.92
(24.5-162.3) |
| Muscle atrophy,
yes/no | 11/5 |
| Prior treatment
with molecular targeted agents, SORA/SORA+LEN/LEN | 4/5/5 |
| Diuretic, +/- | 8/6 |
| Median follow-up
duration (range), months | 4.5 (1.3-11.5) |
Evaluation of treatment response and
PFS
The therapeutic responses to RAM are shown in
Table II. Complete response,
partial response, stable disease, and progressive disease were
observed in 0, 14, 36 and 50% of the patients, respectively
(Table II). The overall objective
response rate and disease control rate were 14 and 50%,
respectively (Table II). The
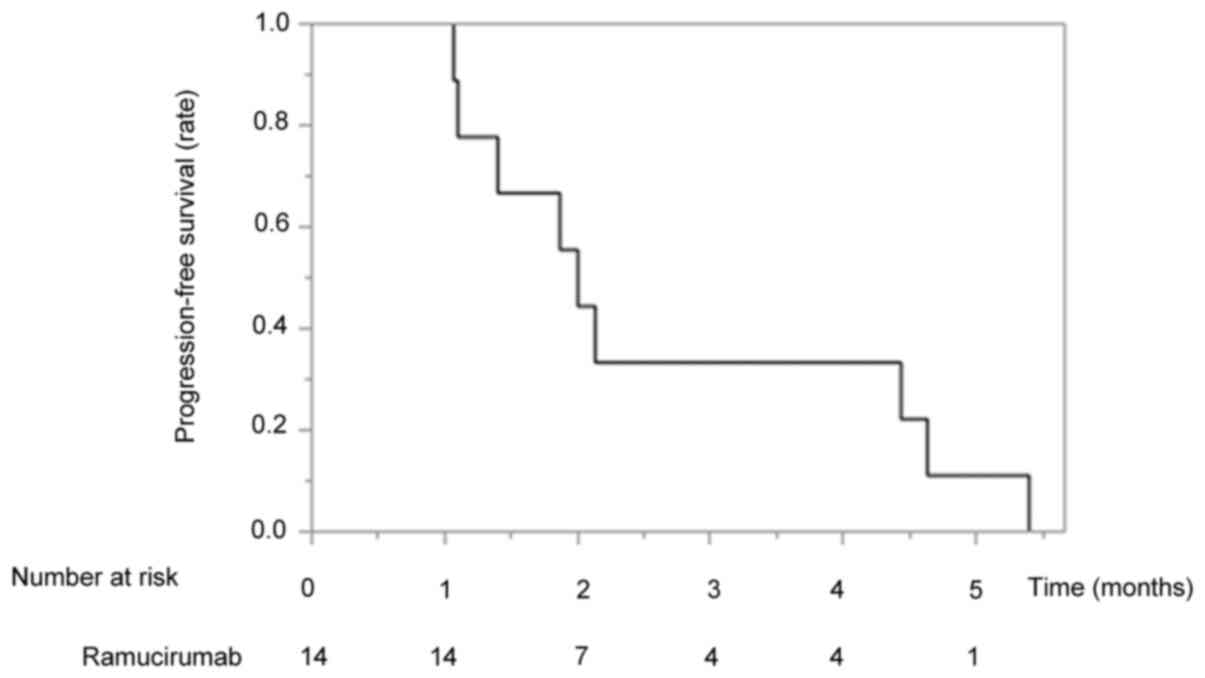
median PFS time was 2.1 months (Fig.
1).
 | Table IITreatment response rate to
ramucirumab in patients with hepatocellular carcinoma (n=14). |
Table II
Treatment response rate to
ramucirumab in patients with hepatocellular carcinoma (n=14).
| Response | N (%) |
|---|
| Complete
response | 0 (0) |
| Partial
response | 2(14) |
| Stable disease | 5(36) |
| Progressive
disease | 7(50) |
| Objective response
rate | 2(14) |
| Disease control
rate | 7(50) |
AEs of RAM
AEs determined by the attending physician are shown
in Table III. Ascites was the
most frequent AE and was seen in 57.1% (8/14). Appetite loss and
fatigue were seen in 50 (7/14) and 50% (7/14), respectively. The
infusion reaction was seen in 7.2% (1/14).
 | Table IIIAdverse events associated with
ramucirumab treatment in patients with hepatocellular carcinoma
(n=14). |
Table III
Adverse events associated with
ramucirumab treatment in patients with hepatocellular carcinoma
(n=14).
| Adverse event | Any, n (%) | Grade 1, n (%) | Grade 2, n (%) | Grade ≥3, n
(%) |
|---|
| Ascites | 8 (57.1) | 0 (0.0) | 2 (14.2) | 6 (42.9) |
| Appetite loss | 7 (50.0) | 3 (21.4) | 3 (21.4) | 1 (7.2) |
| Fatigue | 7 (50.0) | 4 (28.6) | 3 (21.4) | 0 (0.0) |
| Hypertension | 5 (35.6) | 3 (21.4) | 2 (14.2) | 0 (0.0) |
| Diarrhea | 5 (35.6) | 3 (21.4) | 2 (14.2) | 0 (0.0) |
| Proteinuria | 4 (28.6) | 1 (7.2) | 3 (21.4) | 0 (0.0) |
|
Hand-foot-skin-reaction | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Infusion
reaction | 1 (7.2) | 0 (0.0) | 1 (7.2) | 0 (0.0) |
Prevalence of RAM-related severe
ascites
RAM-related severe ascites developed in 57.1% (8/14)
of the patients, and the median onset was 37.5 days (14-61 days)
after RAM treatment initiation. Although ascites was treated with
diuretics, RAM was discontinued in 87.5% (7/8) of patients who
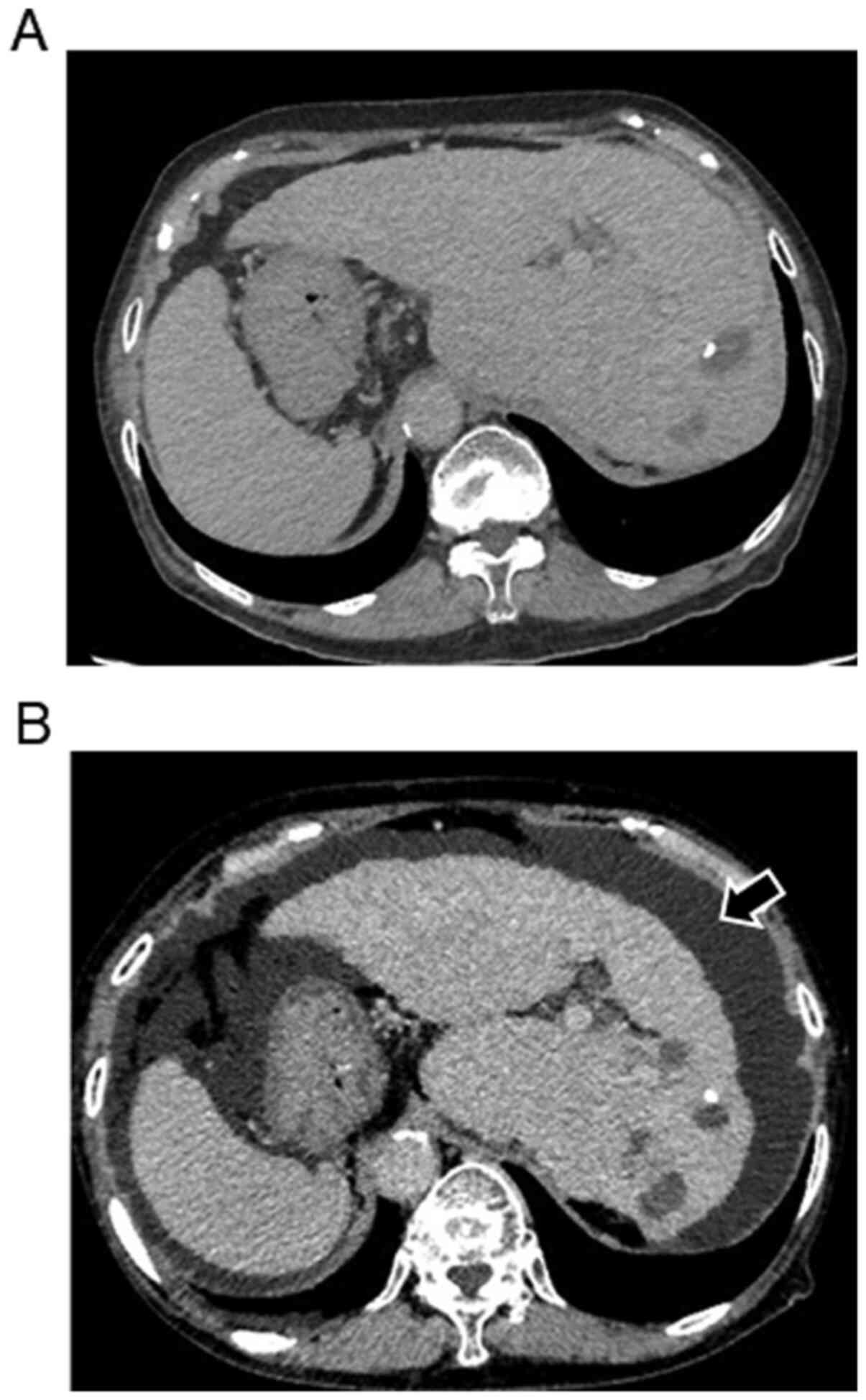
developed severe ascites. A representative case of the development
of severe ascites treated with RAM is shown in Fig. 2A and B. Visceral inversion was seen in this
case. No ascites was seen when multiple recurrent hepatic nodules
developed after LEN treatment (Fig.
2A). Severe ascites developed 27 days after the initiation of
treatment with RAM (Fig. 2B).
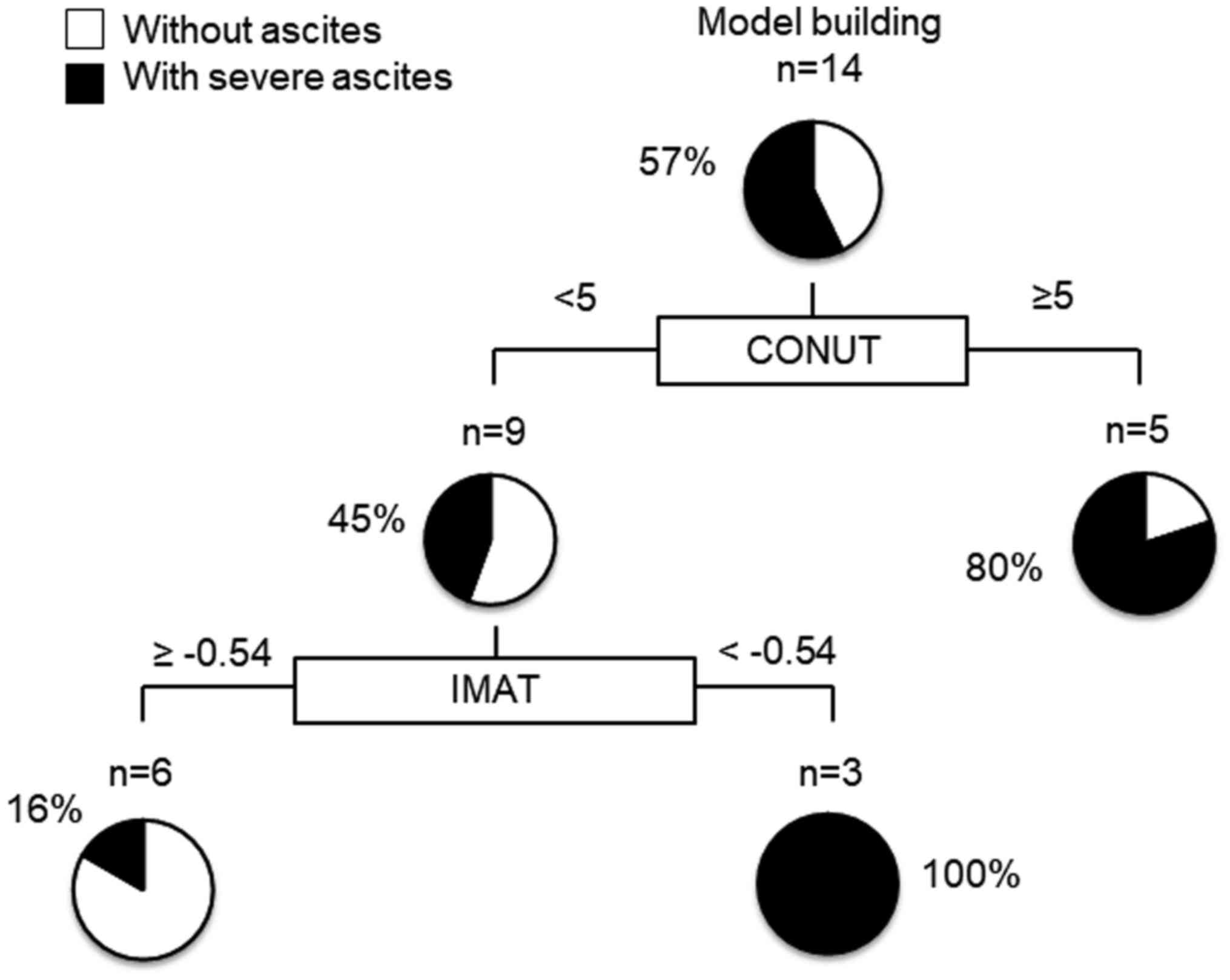
Decision-tree analysis for RAM-related
severe ascites
In this study, severe ascites developed in 57% of
all the subjects by the time of study cessation. To determine the
profiles associated with RAM-related severe ascites, decision tree
analysis was performed. We revealed that the CONUT score was the
first splitting variable for the development of RMA-related severe
ascites. In patients with a CONUT score <5, the second splitting
variable was the IMAT (Fig. 2).
Although severe ascites developed in all patients with an IMAT
<-0.54 and a CONUT score <5, severe ascites developed in only
16% of patients with an IMAT ≥-0.54 and a CONUT score <5
(Fig. 3).
Discussion
In this study, we found a high incidence of severe
ascites in patients with HCC treated with RAM. Moreover, we
revealed that the development of RAM-related severe ascites was
associated with a profile characterized by a CONUT score ≥5 and an
IMAT <-0.54.
Our results showed a high incidence of severe
ascites after treatment with RAM, even in patients with preserved
liver function. RAM inhibits ligand activation of VEGF receptor 2,
which promotes blood vessel permeability (25) Thus, RAM is theoretically supposed to
inhibit the development of severe ascites. In fact, severe ascites
has not been reported in patients with advanced gastric cancer or
colon cancer treated with RAM (26,27)
Moreover, RAM had acceptable tolerability and safety in patients
with preserved liver function in phase 3 clinical trial. Thus, our
findings were different from those of previous studies. The
mechanism underlying the development of severe ascites in HCC
patients treated with RAM remains unclear.
In this study, the CONUT score was identified as the
initial spread variable for the development of RAM-related severe
ascites. A CONUT score ≥5 was associated with the development of
RAM-related severe ascites. A CONUT score ≥5 is classified as
moderate to severe malnutrition (28) Malnutrition has been associated with
the development of severe ascites (29) The CONUT score is based on the total
lymphocyte count, total cholesterol level, and serum albumin level.
All three parameters have been reported to be associated with
ascites (30,31) Therefore, the CONUT score may be
associated with the development of severe ascites by reflecting
malnutritional status.
In patients with a CONUT score <5, IMAT content
was selected as the variable for the second split associated with
the development of RAM-related severe ascites. The prevalence of
severe ascites was higher in patients with lower IMAT content. The
findings suggest that the development of severe ascites was
associated with low-fat infiltration of muscles. Our findings were
different from a previous report that suggested that low-fat
infiltration of muscles was an independent negative predictor of
postoperative complications in patients with HCC (32) The reason for the association of
low-fat muscle infiltration with RAM-related severe ascites in this
study remains unclear. However, Addison et al reported that
lower IMAT content was related to a decrease in muscle
capillarization in older adults (33) Additionally, Solomon et al
reported that impairment of muscle capillarization was associated
with high plasma nitric oxide levels in older adults (34) Higher plasma nitric oxide levels are
known to be important for the development of ascites (35,36)
Therefore, a lower IMAT content may be associated with the
development of severe ascites through the impairment of muscle
capillarization and the subsequent increase in nitric oxide
production.
This study has several limitations. First, this was
a retrospective study with small sample size. Second, the
observational period was short. Third, no information was available
on factors associated with nutritional status and IMAT content,
including energy intake and physical activity. Thus, further
multicenter prospective studies with large sample sizes, longer
observational periods, and including lifestyle information are
warranted.
In conclusion, a high incidence of severe ascites
was seen in patients treated with RAM. Moreover, the development of
severe ascites was associated with a CONUT score ≥5 and IMAT
<-0.54. Accordingly, we must be cautious of severe ascites in
patients with HCC treated with RAM, in particular patients with
malnutrition and muscle fatty infiltration.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NK and SS participated in the conception and design
of the study, acquisition and interpretation of data, and drafting
of the manuscript. KH, SK, HI, TN, TS, MN and RH participated in
the acquisition of data. TK participated in the analysis and
interpretation of data, and drafting of the manuscript. HM, KN, HK
and TT participated in the conception, design and critical revision
of the study. NK and SS confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Committee of Kurume University School of Medicine (approval no.
19203; Kurume, Japan), and an opt-out approach was used to obtain
informed consent from the patients.
Patient consent for publication
An opt-out approach was used to obtain patient
informed consent for publication.
Competing interests
TK received an honorarium (lecture fee) from
Mitsubishi Tanabe Pharma Corporation and Otsuka Pharmaceutical Co.,
Ltd. All other authors declare that they have no competing
interests.
References
|
1
|
Global Burden of Disease Liver Cancer C.
Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C,
Al-Raddadi R, Alvis-Guzman N, Amoako Y, et al: The burden of
primary liver cancer and underlying etiologies from 1990 to 2015 at
the Global, Regional, and National Level: Results from the global
burden of disease study 2015. JAMA Oncol. 3:1683–1691.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
European Association for the study of the
liver. Electronic address simpleeasloffice@easloffice.eu;
European association for the study of the liver. EASL clinical
practice guidelines: Management of hepatocellular carcinoma. J
Hepatol. 69:182–236. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kudo M, Finn RS, Qin S, Han KH, Ikeda K,
Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al: Lenvatinib
versus sorafenib in first-line treatment of patients with
unresectable hepatocellular carcinoma: A randomised phase 3
non-inferiority trial. Lancet. 391:1163–1173. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bruix J, Qin S, Merle P, Granito A, Huang
YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, et al:
Regorafenib for patients with hepatocellular carcinoma who
progressed on sorafenib treatment (RESORCE): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet. 389:56–66.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhu AX, Kang YK, Yen CJ, Finn RS, Galle
PR, Llovet JM, Assenat E, Brandi G, Pracht M, Lim HY, et al:
Ramucirumab after sorafenib in patients with advanced
hepatocellular carcinoma and increased α-fetoprotein concentrations
(REACH-2): A randomised, double-blind, placebo-controlled, phase 3
trial. Lancet Oncol. 20:282–296. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhu AX, Baron AD, Malfertheiner P, Kudo M,
Kawazoe S, Pezet D, Weissinger F, Brandi G, Barone CA, Okusaka T,
et al: Ramucirumab as second-line treatment in patients with
advanced hepatocellular carcinoma: Analysis of REACH trial results
by child-pugh score. JAMA Oncol. 3:235–243. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cheung K, Lee SS and Raman M: Prevalence
and mechanisms of malnutrition in patients with advanced liver
disease, and nutrition management strategies. Clin Gastroenterol
Hepatol. 10:117–125. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Shimose S, Kawaguchi T, Iwamoto H, Tanaka
M, Miyazaki K, Ono M, Niizeki T, Shirono T, Okamura S, Nakano M, et
al: Controlling nutritional status (CONUT) score is associated with
overall survival in patients with unresectable hepatocellular
carcinoma treated with lenvatinib: A multicenter cohort study.
Nutrients. 12(1076)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chavez-Tostado M, Cervantes-Guevara G,
Lopez-Alvarado SE, Cervantes-Pérez G, Barbosa-Camacho FJ,
Fuentes-Orozco C, Hernández-Corona DM, González-Heredia T,
Cervantes-Cardona GA and González-Ojeda A: Comparison of
nutritional screening tools to assess nutritional risk and predict
clinical outcomes in Mexican patients with digestive diseases. BMC
Gastroenterol. 20(79)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Takagi K, Umeda Y, Yoshida R, Nobuoka D,
Kuise T, Fushimi T, Fujiwara T and Yagi T: Preoperative controlling
nutritional status score predicts mortality after hepatectomy for
hepatocellular carcinoma. Dig Surg. 36:226–232. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ogawa M, Lester R, Akima H and Gorgey AS:
Quantification of intermuscular and intramuscular adipose tissue
using magnetic resonance imaging after neurodegenerative disorders.
Neural Regen Res. 12:2100–2105. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Fujiwara N, Nakagawa H, Kudo Y, Tateishi
R, Taguri M, Watadani T, Nakagomi R, Kondo M, Nakatsuka T, Minami
T, et al: Sarcopenia, intramuscular fat deposition, and visceral
adiposity independently predict the outcomes of hepatocellular
carcinoma. J Hepatol. 63:131–140. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Harimoto N, Hoshino H, Muranushi R,
Hagiwara K, Yamanaka T, Ishii N, Tsukagoshi M, Igarashi T, Watanabe
A, Kubo N, et al: Skeletal muscle volume and intramuscular adipose
tissue are prognostic predictors of postoperative complications
after hepatic resection. Anticancer Res. 38:4933–4939.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Forner A, Reig M and Bruix J:
Hepatocellular carcinoma. Lancet. 391:1301–1314. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Johnson PJ, Berhane S, Kagebayashi C,
Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A,
Palmer D, et al: Assessment of liver function in patients with
hepatocellular carcinoma: A new evidence-based approach-the ALBI
grade. J Clin Oncol. 33:550–558. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Koya S, Kawaguchi T, Hashida R, Hirota K,
Bekki M, Goto E, Yamada M, Sugimoto M, Hayashi S, Goshima N, et al:
Effects of in-hospital exercise on sarcopenia in hepatoma patients
who underwent transcatheter arterial chemoembolization. J
Gastroenterol Hepatol. 34:580–588. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sinclair M, Gow PJ, Grossmann M and Angus
PW: Review article: Sarcopenia in cirrhosis-aetiology, implications
and potential therapeutic interventions. Aliment Pharmacol Ther.
43:765–777. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Schneider CA, Rasband WS and Eliceiri KW:
NIH Image to ImageJ: 25 years of image analysis. Nat Methods.
9:671–675. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hirota K, Kawaguchi T, Koya S, Nagamatsu
A, Tomita M, Hashida R, Nakano D, Niizeki T, Matsuse H, Shiba N and
Torimura T: Clinical utility of the liver frailty index for
predicting muscle atrophy in chronic liver disease patients with
hepatocellular carcinoma. Hepatol Res. 50:330–341. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kitajima Y, Takahashi H, Akiyama T,
Murayama K, Iwane S, Kuwashiro T, Tanaka K, Kawazoe S, Ono N,
Eguchi T, et al: Supplementation with branched-chain amino acids
ameliorates hypoalbuminemia, prevents sarcopenia, and reduces fat
accumulation in the skeletal muscles of patients with liver
cirrhosis. J Gastroenterol. 53:427–437. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hamaguchi Y, Kaido T, Okumura S, Kobayashi
A, Shirai H, Yao S, Yagi S, Kamo N, Seo S, Taura K, et al:
Preoperative visceral adiposity and muscularity predict poor
outcomes after hepatectomy for hepatocellular carcinoma. Liver
Cancer. 8:92–109. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lencioni R and Llovet JM: Modified RECIST
(mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis.
30:52–60. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Shimose S, Tanaka M, Iwamoto H, Niizeki T,
Shirono T, Aino H, Noda Y, Kamachi N, Okamura S, Nakano M, et al:
Prognostic impact of transcatheter arterial chemoembolization
(TACE) combined with radiofrequency ablation in patients with
unresectable hepatocellular carcinoma: Comparison with TACE alone
using decision-tree analysis after propensity score matching.
Hepatol Res. 49:919–928. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yoshiji H, Kuriyama S, Hicklin DJ, Huber
J, Yoshii J, Ikenaka Y, Noguchi R, Nakatani T, Tsujinoue H and
Fukui H: The vascular endothelial growth factor receptor KDR/Flk-1
is a major regulator of malignant ascites formation in the mouse
hepatocellular carcinoma model. Hepatology. 33:841–847.
2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tabernero J, Takayuki Y and Cohn AL:
Correction to Lancet Oncol 2015; 16: 499-508. Ramucirumab versus
placebo in combination with second-line FOLFIRI in patients with
metastatic colorectal carcinoma that progressed during or after
first-line therapy with bevacizumab, oxaliplatin, and a
fluoropyrimidine (RAISE): A randomised, double-blind, multicentre,
phase 3 study. Lancet Oncol. 16(e262)2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wilke H, Muro K, Van Cutsem E, Oh SC,
Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, et
al: Ramucirumab plus paclitaxel versus placebo plus paclitaxel in
patients with previously treated advanced gastric or
gastro-oesophageal junction adenocarcinoma (RAINBOW): A
double-blind, randomised phase 3 trial. Lancet Oncol. 15:1224–1235.
2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Saito A, Amiya E, Hatano M, Shiraishi Y,
Nitta D, Minatsuki S, Maki H, Hosoya Y, Tsuji M, Bujo C, et al:
Controlling nutritional status score as a predictive marker for
patients with implantable left ventricular assist device. ASAIO J.
66:166–172. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Vidot H, Bowen DG, Carey S, McCaughan GW,
Allman-Farinelli M and Shackel NA: Aggressive nutrition
intervention reduces ascites and frequency of paracentesis in
malnourished patients with cirrhosis and ascites. JGH Open.
1:92–97. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Bernardi M, Angeli P, Claria J, Moreau R,
Gines P, Jalan R, Caraceni P, Fernandez J, Gerbes AL, O'Brien AJ,
et al: Albumin in decompensated cirrhosis: New concepts and
perspectives. Gut. 69:1127–1138. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhao LY, Yang DD, Ma XK, Liu MM, Wu DH,
Zhang XP, Ruan DY, Lin JX, Wen JY, Chen J, et al: The prognostic
value of aspartate aminotransferase to lymphocyte ratio and
systemic immune-inflammation index for overall survival of
hepatocellular carcinoma patients treated with palliative
treatments. J Cancer. 10:2299–2311. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hamaguchi Y, Kaido T, Okumura S, Kobayashi
A, Fujimoto Y, Ogawa K, Mori A, Hammad A, Hatano E and Uemoto S:
Muscle steatosis is an independent predictor of postoperative
complications in patients with hepatocellular carcinoma. World J
Surg. 40:1959–1968. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Addison O, Ryan AS, Blumenthal J and Prior
SJ: Increased intramuscular adipose tissue is related to increased
capillarization in older adults. J Frailty Aging. 9:134–138.
2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Solomon TP, Haus JM, Li Y and Kirwan JP:
Progressive hyperglycemia across the glucose tolerance continuum in
older obese adults is related to skeletal muscle capillarization
and nitric oxide bioavailability. J Clin Endocrinol Metab.
96:1377–1384. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ferguson JW, Dover AR, Chia S, Cruden NL,
Hayes PC and Newby DE: Inducible nitric oxide synthase activity
contributes to the regulation of peripheral vascular tone in
patients with cirrhosis and ascites. Gut. 55:542–546.
2006.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Angeli P, Fernandez-Varo G, Dalla Libera
V, Fasolato S, Galioto A, Arroyo V, Sticca A, Guarda S, Gatta A and
Jiménez W: The role of nitric oxide in the pathogenesis of systemic
and splanchnic vasodilation in cirrhotic rats before and after the
onset of ascites. Liver Int. 25:429–437. 2005.PubMed/NCBI View Article : Google Scholar
|