Introduction
Lenvatinib is a multi-kinase inhibitor that inhibits
signaling via vascular endothelial growth factor (VEGF) receptors
1-3, fibroblast growth factor receptors 1-4, platelet-derived
growth factor receptor-α, and the ret and KIT proto-oncogene
receptor tyrosine kinases; its overall antitumor effect relates to
its capacity to inhibit angiogenesis (1,2). In a
phase III study of radioiodine-refractory differentiated thyroid
carcinoma (DTC, SELECT study), lenvatinib treatment significantly
improved the progression-free survival of patients compared with
placebo (3). In a further
subanalysis of the SELECT study, the overall survival was improved
in age of over 65 years group (4).
More recently, in a limited series of patients with stage ⅣC
anaplastic thyroid cancer (ATC), the lenvatinib group had a better
survival benefit than the palliative therapy group (5).
According to the real-world data of lenvatinib
treatment for radioiodine-refractory DTC in Italy, 26 out of 82
patients experienced serious adverse events (SAEs); among the SAEs,
five were drug related, including tracheal bleeding, pulmonary
embolism, tracheoesophageal fistula, wound healing impairment, and
cardiac flutter (6). In real-world
data of France, in which 75 patients were analyzed, the incidence
of treatment-related adverse events in all grades was 95%, whereas
that in grade 3 or higher was 48%. SAEs were observed in 12
patients (16%); 2 patients developed pneumothorax, 1 died, and the
others required continued chest tube drainage (7).
Moreover, complications such as upper airway fistula
formation and bleeding resulted in death in a small cohort of
patients (8,9). Of particular note, ongoing treatment
with anti-VEGF inhibitors frequently results in wound complications
due to inhibition of angiogenesis (10,11).
Lenvatinib therapy is often prolonged in patients with
radioiodine-refractory DTC; a subset of these patients may
ultimately experience medical conditions or complications require
invasive procedures. There have been no studies that focus on the
safety of invasive procedures in patients treated with lenvatinib.
As such, we designed a study to address this issue among patients
undergoing lenvatinib treatment at our hospital.
Patients and methods
Patients' selection and
characteristics
Between June 2015 and August 2019, 82 patients
received lenvatinib for thyroid cancer at Kanagawa Cancer Center.
Among them, 58 had DTC, whereas 24 had ATC. All eligible
participants provided informed consent. This study is approved by
the institutional review board of Kanagawa Cancer Center (IRB
approval number 27-61). Within this cohort, we identified 11
patients who required invasive procedures while undergoing
treatment with lenvatinib. Patients in this cohort included those
involving general or local anesthesia; those reporting minor
procedures including simple sutures or dental procedures were
excluded from the study. Eleven patients (6 males and 5 females)
underwent 14 invasive procedures; the median age of the patients in
this cohort was 72 (range, 59 to 83) years. The histological
thyroid cancer diagnoses included papillary carcinoma (n=8),
follicular carcinoma (n=2), and ATC (n=1). In addition, three
patients had diabetes mellitus, and two were taking
anticoagulants.
Evaluation of the nutrition
status
For evaluating the nutrition status, we calculated
the prognostic nutritional index (PNI): PNI=10 Alb. +0.005 Lymph.
C., where Alb. is the serum albumin level (g/100 ml) and Lymph. C.
is the total lymphocytes count/mm3 of peripheral blood.
When the index is below 40, resection and anastomosis of
gastrointestinal tract may be contraindicated because of the risk
of wound complication (12).
Unfortunately, we have not acquired the data of total cholesterol
level required for CONUT. Hence, we evaluated the nutritional
status instead using the prognostic nutritional index. Four
patients had a PNI score of below 40 (Table I). We examined the postsurgical
course, including lenvatinib dose, preprocedure withdrawal period,
and wound complication reports for each patient.
 | Table IPatient demographics. |
Table I
Patient demographics.
| Characteristic | n=11 |
|---|
| Median age (range),
years | 72 (59-83) |
| Sex, n (%) | |
|
Male | 6 (54.5) |
|
Female | 5 (45.5) |
| Pathological type of
thyroid cancer, n (%) | |
|
Papillary
carcinoma | 8 (72.7) |
|
Follicular
carcinoma | 2 (18.2) |
|
Anaplastic
carcinoma | 1 (9.1) |
| Eastern Cooperative
Oncology Group Performance status, n (%) | |
|
0 | 9 (81.8) |
|
1 | 2 (18.2) |
| Site of metastasis, n
(%) | |
|
Lung | 7 (63.6) |
|
Bone | 4 (36.4) |
|
Lymph
node | 4 (36.4) |
| Wound healing
factors, n (%) | |
|
Diabetes
mellitus | 3 (27.3) |
|
Anti-coagulation
medicine | 2 (18.2) |
| Prognostic
nutritional index, n (%) | |
|
>40 | 7 (63.6) |
|
≤40 | 4 (36.4) |
Results
Invasive procedure
characteristics
Of the 14 invasive procedures performed on patients
with thyroid cancer undergoing treatment with lenvatinib, four were
for thoracic drain placement. Eight of the procedures that were
performed under local anesthesia included construction of a port
for a central venous catheter, cataract surgery, percutaneous
endoscopic gastrostomy, percutaneous coronary angioplasty (PCI),
and the aforementioned thoracic drainage. Six of the procedures
performed under general anesthesia included cerebral aneurysm
clipping, total thyroidectomy with resection of the upper
mediastinal tumor, laparotomy for gastrostomy, radical inguinal
hernia repair, appendectomy, and cholecystectomy. Of all the
procedures, the PCI, appendectomy, and cholecystectomy were
performed on an emergency basis. Meanwhile, total thyroidectomy
with resection of the upper mediastinal tumor was performed in a
75-year-old woman. In this case (13), which we had reported previously, the
tumor had invaded the internal jugular and subclavian veins,
forming a tumor embolus in the right brachiocephalic vein and
reaching the vicinity of the superior vena cava. In addition, for
life-saving purposes, we used lenvatinib. Consequently, the tumor
shrunk after 4 months, and surgery was performed thereafter.
Lenvatinib administration status for
each invasive procedure
The mean period between initiation of lenvatinib
therapy and the performance of an invasive procedure was 393 days
(range, 29 to 1,318 days). Doses taken during the period prior to
surgery included 10, 12, 14, and 20 mg. Three of the patients
(21.4%) underwent a withdrawal period of 1-2 weeks prior to
surgery; eight of MCO-15315-261201_Toda MCO-15315-261201_Toda the
patients (57.1%) remained on lenvatinib while undergoing the
invasive procedure. Lenvatinib therapy had been withdrawn from the
three cases in the first group (21.4%) for unrelated reasons, i.e.,
due to previous adverse events or to facilitate other forms of
treatment. In all cases, lenvatinib treatment was resumed after
confirming appropriate postsurgical wound healing; four of the
patients (28.6%) underwent no withdrawal whatsoever. Among those
who stopped therapy prior to surgery, (42.8%) resumed within 3
weeks, while 7.1% (one case) resumed treatment after more than 3
weeks; lenvatinib therapy was not reintroduced in three of the
patient cases (28.6%; Table
II).
 | Table IILenvatinib administration status for
each invasive procedure. |
Table II
Lenvatinib administration status for
each invasive procedure.
| Lenvatinib
administration status | n (n=14) |
|---|
| Period from start of
lenvatinib to surgical procedure (days) | 393 (range
29-1,318) |
| Dosage of lenvatinib
prior to procedure (mg/per day) | |
|
10 | 3 (21.4) |
|
12 | 2 (14.3) |
|
14 | 4 (28.6) |
|
20 | 5 (35.7) |
| Withdrawal period
prior to procedure (weeks) | |
|
None | 8 (57.1) |
|
1 | 1 (7.1) |
|
2 | 2 (14.3) |
|
≥3 | 3 (21.4) |
| Period from time of
procedure to reintroduction of lenvatinib | |
|
No
withdrawal | 4 (28.6) |
|
Within 7
days | 3 (21.4) |
|
8-21
days | 3 (21.4) |
|
≥22
days | 1 (7.1) |
|
Did not
resume | 3 (21.4) |
Adverse events during invasive
procedures
Most of the patients experienced no postoperative
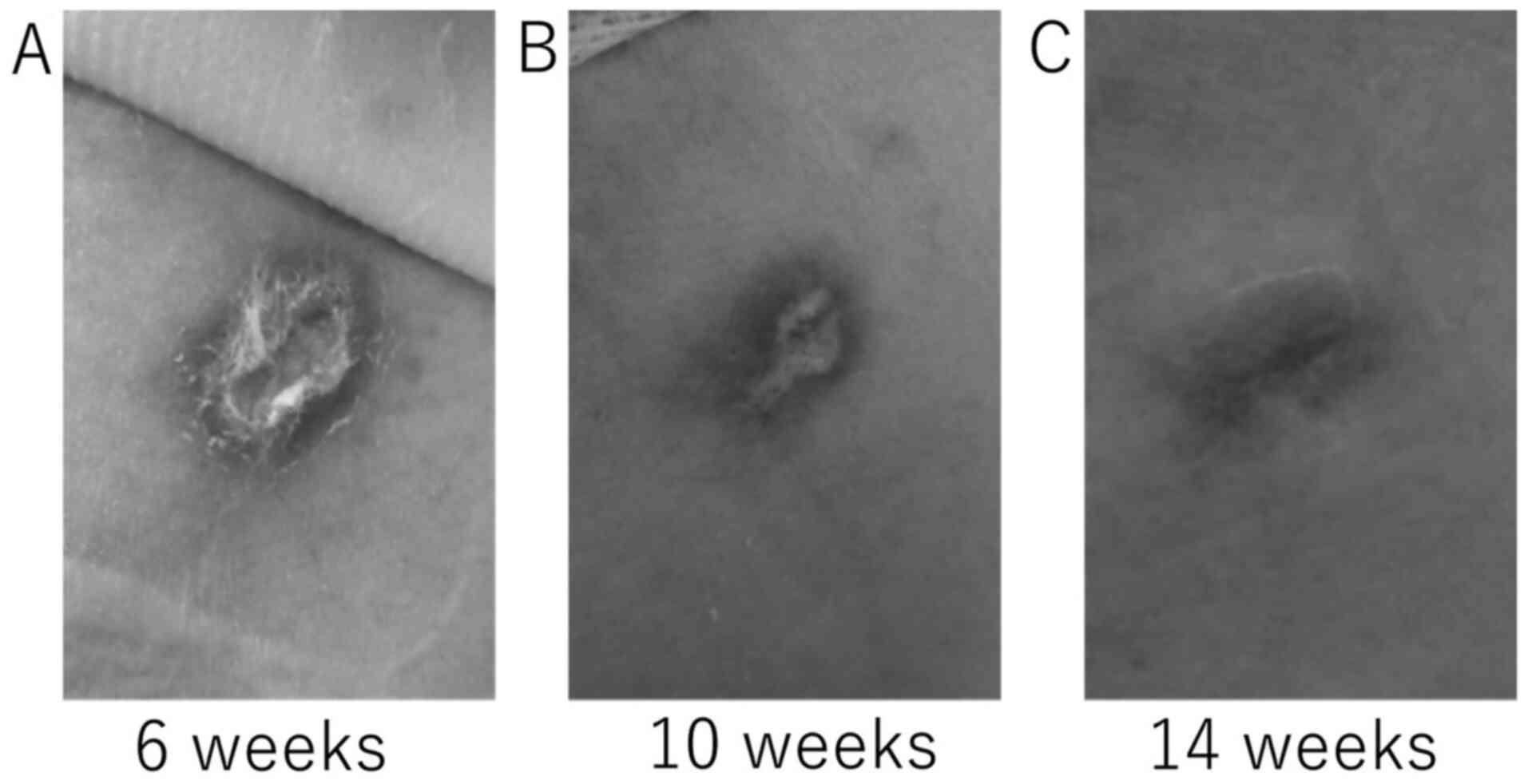
wound complications. We observed delayed wound healing in one
patient secondary to a 20 Fr. trocar catheter thoracic drain placed
for the treatment of a left pneumothorax. In this case, lenvatinib
had been discontinued on the day of the procedure. On day 5
postprocedure, pleurodesis was performed; the catheter was removed
on postoperative day 11. The patient subsequently developed
hypoxemia which may have been related to enlarging lung metastases;
lenvatinib treatment was resumed on day 17. This intervention
resulted in improved oxygenation, although the wound took 14 weeks
to heal (Fig. 1).
No other wound complications were identified in this
patient cohort, although one patient died of disease progression
during the period of lenvatinib withdrawal. He had been undergoing
treatment with lenvatinib for multiple lung metastases and local
recurrences, had undergoing repeated bouts of drug withdrawal and
resumption due to concerns regarding an open leg ulcer. This
patient required a gastrostomy to treat esophageal stenosis due to
local recurrence that caused difficulty with swallowing. He was
withdrawn from lenvatinib after the endoscopic gastrostomy was
performed, but died on postoperative day 8 due to pleural effusion
and general deterioration of his general. The surgical wound was
healing appropriately with no abnormalities.
Discussion
VEGF-targeted inhibitors of angiogenesis have been
associated with an increased risk of wound complications (14). In rats, treatment with the tyrosine
kinase inhibitor, semaxanib, resulted in reduced tissue perfusion
and micro-vessel density, but it did not impair wound healing
(15). Other studies have revealed
that administration of bevacizumab resulted in reduced wound
strength and wound healing rates in a macaque model (16).
VEGF inhibitors include bevacizumab and ramucirumab,
which are humanized monoclonal antibodies that target VEGF and
VEGFR, respectively. Similarly, aflibercept has direct inhibitory
effects on VEGF-A, VEGF-B and placental growth factor (PlGF) and
low molecular weight multi-tyrosine kinase inhibitors (mTKIs),
including sorafenib and lenvatinib, inhibit intracellular tyrosine
kinase activity VEGFR-activated cells. Bevacizumab has a half-life
of 20 days, while the half-life of lenvatinib is 35.4 h; the
half-lives of other TKIs range from 1-3 days, while mTKIs have a
relatively short half-life (Table
III) (17-22).
 | Table IIIHalf-lives of VEGF inhibitors in
vivo. |
Table III
Half-lives of VEGF inhibitors in
vivo.
| Drug | Activity | Half-life
(reference) |
|---|
| Bevacizumab | Antibody against
VEGF-A | 20 days (11) |
| Ramucirumab | Antibody against
VEGFR-2 | 8 days (12) |
| Aflibercept | Inhibitor of VEGF-A,
VEGF-B and PlGF | 5.59 days (13) |
| Sorafenib | Inhibits the tyrosine
kinase activity of VEGFRs | 28.1 h (14) |
| Lenvatinib | Inhibits the tyrosine
kinase activity of VEGFRs | 35.4 h (15) |
| Sunitib | Inhibits the tyrosine
kinase activity of VEGFRs | 41-86 h (16) |
Administration of bevacizumab results in an
increased the risk of postsurgical wound complications. In a phase
III trial (NSABP C-08), adjuvant therapy with bevacizumab that was
initiated at 29-50 days after surgery for colon cancer surgery, was
associated with an increased frequency of wound complications at
grade 3 or higher (10). A
retrospective analysis of cases in which emergency surgery was
required among patients in trials and undergoing treatment with
bevacizumab for stage III colorectal cancer revealed that wound
complications (grade 3 or higher) occurred more frequently in the
bevacizumab group (10/75, or 13%) compared to only 3.4% (1/29) in
the among those receiving standard chemotherapy alone (11).
By contrast, completely different results were
obtained from studies in which preoperative treatment with mTKIs
were evaluated prior to surgical intervention for renal cell
carcinoma. For example, Cowey et al performed laparoscopic
or open nephrectomy on 30 patients that were undergoing treatment
with sorafenib (23). The surgical
complications included only one case of a superficial wound break.
The preoperative withdrawal period among those patients who
underwent sorafenib treatment was 3 days (range 2-14 days); among
the five patients identified with distant metastases, TKI treatment
was reintroduced 4-6 weeks after surgery when wound healing was
confirmed. Similarly, Zhang et al evaluated the impact of
preoperative treatment with sorafenib in 18 patients; only one
patient experienced postoperative bleeding (24). The median preoperative drug
withdrawal period was 12 days (range, 7-30 days) and sorafenib
treatments were resumed within 2-4 weeks after surgery for seven
patients with distant metastases.
In the aforementioned studies, patients had
undergone sorafenib treatment prior to major surgery; the frequency
of wound complications was low in most cases. Lenvatinib is also to
be considered safe for this application, as it has a similar
half-life and mechanism of action. However, due to the small number
of cases that have been reviewed, the decision to perform invasive
treatments should be undertaken carefully.
Wound healing with low-dose lenvatinib treatment has
also been previously reported. Resteghini et al presented a
case of a locally advanced DTC. In this case, lenvatinib was
administered at a standard dose of 24 mg. Although it had a
dramatic effect, it formed an ulcer because of tumor necrosis,
causing s-evere tracheal bleeding. Therefore, lenvatinib was
resumed after a 3-week interval from the last dose. Treatment
dosing and local bleeding control were achieved by progressively
tapering down lenvatinib to 14 mg (25).
We identified one patient in which the overall
condition deteriorated rapidly after lenvatinib was discontinued.
It is clear that TKI withdrawal facilitates tumor growth. VEGF
inhibition with a TKI for a period of 7 days results in 50-60% loss
of the tumor vasculature; however, tumor vessels can regenerate
within 7 days after drug administration has been discontinued
(26). As such, it is critical to
consider this point among patients with DTC when considering acute
withdrawal of lenvatinib from patients who are maintained on
long-term regimens.
Most invasive procedures in patients undergoing
treatment with lenvatinib did not result in postsurgical wound
complications. Current recommendations suggest that patients should
be withdrawn from this drug for a period of 1-2 weeks prior to
scheduled surgical procedures and that therapy can be resumed after
postoperative wound healing has been confirmed. However, this
approach requires caution as it is critical to consider the
possibility of significant disease progression during the course of
an extended drug holiday.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ST, HI, and NS designed the current study. HN
analyzed the data. HI, NS, DM and KM performed the surgery and
provided patient care. DM and KM contributed to patient data
acquisition. ST and HI confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures involving human participants were
conducted in accordance with the ethical standards of the
institutional and/or national research committee and the tenets of
the 1964 Helsinki Declaration and its later amendments or
comparable ethical standards. The current study followed the
‘Ethical Guidelines for Medical Research on Human Subjects’ and
conducted the present study with approval from the institutional
review board of Kanagawa Cancer Center (approval no. 27-61).
Informed consent was obtained from all individual participants
included in the study.
Patient consent for publication
All patients provided a comprehensive consent form
stating that personal data could be used for academic presentation
or paper presentation while ensuring complete anonymity before
receiving the treatment.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Matsui J, Funahashi Y, Uenaka T, Watanabe
T, Tsuruoka A and Asada M: Multi-kinase inhibitor E7080 suppresses
lymph node and lung metastases of human mammary breast tumor
MDA-MB-231 via inhibition of vascular endothelial growth
factor-receptor (VEGF-R) 2 and VEGF- R3 kinase. Clin Cancer Res.
14:5459–5465. 2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Matsui J, Yamamoto Y, Funahashi Y,
Tsuruoka A, Watanabe T, Wakabayashi T, Uenaka T and Asada M: E7080,
a novel inhibitor that targets multiple kinases, has potent
antitumor activities against stem cell factor producing human small
cell lung cancer H146, based on angiogenesis inhibition. Int J
Cancer. 122:664–671. 2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Schlumberger M, Tahara M, Wirth LJ,
Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff
AO, et al: Lenvatinib versus placebo in radioiodine-refractory
thyroid cancer. N Engl J Med. 372:621–630. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Brose MS, Worden FP, Newbold KL, Guo M and
Hurria A: Effect of age on the efficacy and safety of lenvatinib in
radioiodine-refractory differentiated thyroid cancer in the Phase
III SELECT Trial. J Clin Oncol. 35:2692–2699. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Iwasaki H, Toda S, Suganuma N, Murayama D,
Nakayama H and Masudo K: Lenvatinib vs. palliative therapy for
stage IVC anaplastic thyroid cancer. Mol Clin Oncol. 12:138–143.
2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Locati LD, Piovesan A, Durante C, Bregni
M, Castagna MG, Zovato S, Giusti M, Ibrahim T, Puxeddu E, Fedele G,
et al: Real-world efficacy and safety of lenvatinib: data from a
compassionate use in the treatment of radioactive iodine-refractory
differentiated thyroid cancer patients in Italy. Eur J Cancer.
118:35–40. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Berdelou A, Borget I, Godbert Y, Nguyen T,
Garcia ME, Chougnet CN, Ferru A, Buffet C, Chabre O, Huillard O, et
al: Lenvatinib for the treatment of radioiodine-refractory thyroid
cancer in real-life practice. Thyroid. 28:72–78. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Blevins DP, Dadu R, Hu M, Baik C,
Balachandran D, Ross W, Gunn B and Cabanillas ME: Aerodigestive
fistula formation as a rare side effect of antiangiogenic tyrosine
kinase inhibitor therapy for thyroid cancer. Thyroid. 24:918–922.
2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Iwasaki H, Yamazaki H, Takasaki H,
Suganuma N, Nakayama H, Toda S and Masudo K: Lenvatinib as a novel
treatment for anaplastic thyroid cancer: A retrospective study.
Oncol Lett. 16:7271–7277. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Allegra CJ, Yothers G, O'Connell MJ,
Sharif S, Colangelo LH, Lopa SH, Petrelli NJ, Goldberg RM, Atkins
JN, Seay TE, et al: Initial safety report of NSABP C-08: A
randomized phase III study of modified FOLFOX6 with or without
bevacizumab for the adjuvant treatment of patients with stage II or
III colon cancer. J Clin Oncol. 27:3385–3390. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Scappaticci FA, Fehrenbacher L, Cartwright
T, Hainsworth JD, Heim W, Berlin J, Kabbinavar F, Novotny W, Sarkar
S and Hurwitz H: Surgical wound healing complications in metastatic
colorectal cancer patients treated with bevacizumab. J Surg Oncol.
91:173–180. 2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Onodera T, Goseki N and Kosaki G:
Prognostic nutritional index in gastrointestinal surgery of
malnourished cancer patients. Nihon Geka Gakkai Zasshi.
85:1001–1005. 1984.PubMed/NCBI(In Japanese).
|
|
13
|
Iwasaki H, Toda S, Ito H, Nemoto D,
Murayama D, Okubo Y, Hayashi H and Yokose T: A case of unresectable
papillary thyroid carcinoma treated with lenvatinib as neoadjuvant
chemotherapy. Case Rep Endocrinol. 2020(6438352)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chen HX and Cleck JN: Adverse effects of
anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol.
6:465–477. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Roman CD, Choy H, Nanney L, Riordan C,
Parman K, Johnson D and Beauchamp RD: Vascular endothelial growth
factor-mediated angiogenesis inhibition and postoperative wound
healing in rats. J Surg Res. 105:43–47. 2002.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cornacoff JB, Howk K, Pikounis B,
Mendenhall V and Martin P: Development of a method for the
evaluation of wound tensile strength in cynomolgus macaques. J
Pharmacol Toxicol Methods. 57:74–79. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lu JF, Bruno R, Eppler S, Novotny W, Lum B
and Gaudreault J: Clinical pharmacokinetics of bevacizumab in
patients with solid tumors. Cancer Chemother Pharmacol. 62:779–786.
2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yamaguchi K, Fujitani K, Nagashima F,
Omuro Y, Machida N, Nishina T, Koue T, Tsujimoto M, Maeda K and
Satoh T: Ramucirumab for the treatment of metastatic gastric or
gastroesophageal junction adenocarcinoma following disease
progression on first-line platinum- or fluoropyrimidine-containing
combination therapy in Japanese patients: A phase 2, open-label
study. Gastric Cancer. 21:1041–1049. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yoshino T, Yamazaki K, Yamaguchi K, Doi T,
Boku N, Machida N, Onozawa Y, Asayama M, Fujino T and Ohtsu A: A
phase I study of intravenous aflibercept with FOLFIRI in Japanese
patients with previously treated metastatic colorectal cancer.
Invest New Drug;. 31:910–917. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Strumberg D, Richly H, Hilger RA,
Schleucher N, Korfee S, Tewes M, Faghih M, Brendel E, Voliotis D,
Haase CG, et al: Phase I clinical and pharmacokinetic study of the
Novel Raf kinase and vascular endothelial growth factor receptor
inhibitor BAY 43-9006 in patients with advanced refractory solid
tumors. J Clin Oncol. 23:965–972. 2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Dubbelman AC, Rosing H, Nijenhuis C,
Huitema ADR, Mergui-Roelvink M, Gupta A, Verbel D, Thompson G,
Shumaker R, Schellens JH and Beijnen JH: Pharmacokinetics and
excretion of (14) C-lenvatinib in patients with advanced solid
tumors or lymphomas. Invest New Drug. 33:233–240. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Faivre S, Delbaldo C, Vera K, Robert C,
Lozahic S, Lassau N, Bello C, Deprimo S, Brega N, Massimini G, et
al: Safety, pharmacokinetic, and antitumor activity of SU11248, a
novel oral multitarget tyrosine kinase inhibitor, in patients with
cancer. J Clin Oncol. 24:25–35. 2006.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cowey CL, Amin C, Pruthi RS, Wallen EM,
Nielsen ME, Grigson G, Watkins C, Nance KV, Crane J, Jalkut M, et
al: Neoadjuvant clinical trial with sorafenib for patients with
stage II or higher renal cell carcinoma. J Clin Oncol.
28:1502–1507. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang Y, Li Y, Deng J, Ji Z, Yu H and Li
H: Sorafenib neoadjuvant therapy in the treatment of high risk
renal cell carcinoma. PLoS One. 10(E0115896)2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Resteghini C, Locati LD, Bossi P,
Bergamini C, Guzzo M and Licitra L: Do not throw the baby out with
the bathwater: SELECT a personalized, de-escalated lenvatinib
schedule allows response in locally advanced DTC while controlling
major drug-related bleeding. Ann Oncol. 28:2321–2322.
2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mancuso MR, Davis R, Norberg SM, O'Brien
S, Sennino B, Nakahara T, Yao VJ, Inai T, Brooks P, Freimark B, et
al: Rapid vascular regrowth in tumors after reversal of VEGF
inhibition. J Clin Invest. 116:2610–2621. 2006.PubMed/NCBI View
Article : Google Scholar
|