Introduction
Neuroendocrine neoplasms (NENs) are considered to be
composed of cells with characteristics similar to those of the
normal diffuse neuroendocrine system, which consists of cells that
are a combination of hormone-producing endocrine and nerve cells,
scattered throughout the body (for example gastroenteropancreatic,
the enteroendocrine hormonal signaling system; respiratory and
urogenital systems; paraganglia; adrenal medulla; skin; thymus;
heart; middle ear; and other tissues). NENs are a wide
heterogeneous family of neoplasms in which neuroendocrine
differentiation is demonstrated by the presence of neurosecretory
granules in the cytoplasmic component in electron microscopy
analysis and in immunohistochemistry for positivity to
neuroendocrine markers, including synaptophysin, chromogranin, CD56
and NSE (1). In humans, NENs are
typically located in the gastrointestinal tract, the pancreas, and
the lungs and are subdivided into well-differentiated and poorly
differentiated NENs (2).
In 1996, the College of American Pathologists and
the National Cancer Institute suggested reduction in the number of
terms used to describe neuroendocrine tumors of the cervix,
introducing a classification system of 4 categories: Typical
(classical) carcinoid tumor, atypical carcinoid tumor, large cell
neuroendocrine carcinoma and small (oat) cell carcinoma (3).
The current WHO Classification of neuroendocrine
tumors of the female genital originating in the cervix suggests a
terminology similar to that used for gastro-entero-pancreatic
neuroendocrine tumors. Thus, according to this classification,
tumors originating in the female genital tract can be classified
as: Low grade neuroendocrine tumors (grade 1 and grade 2) and high
grade neuroendocrine carcinoma, with small and large cells
(4). Small Cell Neuroendocrine
carcinoma (SCNEC) can be found anywhere in the gynecological tract,
but is most commonly observed in the cervix (4). The causative agent underlying the
development of this malignancy is Human Papilloma Virus (HPV)
(5-7).
Merkel cell carcinoma (MCC) is a rare primary
cutaneous neuroendocrine carcinoma (8). Factors involved in the development of
MCC include the Merkel cell polyoma virus (MCPyV), a weakened
immune system and exposure to ultraviolet radiation (9,10).
MCPyV has also been observed in respiratory tract secretions
(11-14)
and it has been demonstrated that it can be transmitted during
sexual activity. In addition, MCPyV has been detected in the oral
and ano-genital mucosa of human immunodeficiency virus-positive
patients (15,16). Moreover, this virus has been found
in a series of the most common squamous cervical carcinomas in
Japanese patients (17).
These findings prompted the present study into the
investigation of the co-presence of MCPyV and HPV in three cases of
SCNEC of the cervix, using both immunohistochemical and molecular
analyses.
Materials and methods
Patients
Three cases of SCNEC of the cervix were identified
in the pathology archives of Parma University (Italy).
Neuroendocrine differentiation was recognized by
morphological analysis and immunohistochemical findings.
In all cases, the diagnosis had been made prior to a
small cervical biopsy and then, in two of these cases, on
macroscopic and microscopic examination of the hysterectomy with
bilateral annessiectomy specimens and in the remaining case on a
conization specimen.
The surgical specimens were fixed in 10% neutral
buffered formalin for a routine light microscopic examination.
Sections of neoplasms were submitted for histological examination
and the samples were embedded in paraffin. Then 3-µm sections were
cut and stained with hematoxylin-eosin.
Immunohistochemical analysis
For immunohistochemical analysis, after
deparaffinization and rehydration, sections were treated with 3%
hydrogen peroxidase for 5 min. For antigen retrieval, sections were
treated with pH9 Tris-EDTA buffer for 30 min in a water-bath at
98˚C. The following primary antibodies were used: MCPyV large
T-antigen (clone CM2B4; mouse monoclonal antibody, 1/50; Santa Cruz
Biotechnology. Inc.) Ki67 (clone MIB-1; mouse monoclonal antibody,
1/100; Dako: Agilent Technologies, Inch.) P63 (clone 4A4, mouse
monoclonal antibody; ready to use; Ventana Medical System, Inc.)
CD56 (clone MRQ-42; rabbit monoclonal primary antibody, ready to
use; Ventana Medical System, Inc.), TTF-1 (clone 8G7G3; mouse
monoclonal antibody; ready to use, Ventana Medical System, Inc.),
P40 (clone SPBC28; mouse monoclonal antibody; ready to use; Ventana
Medical System, Inc.), P53 (clone DO-7; mouse monoclonal antibody;
ready to use, Ventana Medical System, Inc.), cytokeratin 20 (clone
SP33; rabbit monoclonal antibody; ready to use; Ventana Medical
System, Inc.), cytokeratin CAM 5.2 (clone CAM 5.2; mouse monoclonal
antibody; ready to use; Ventana Medical System, Inc.), chromogranin
A (clone LK2H10; mouse monoclonal antibody; ready to use; Ventana
Medical System, Inc.), synaptophysin (clone SP11; rabbit monoclonal
antibody; ready to use, Ventana Medical System, Inc.).
All sections were immunostained with automatic
immunostaining Benchmark Ultra-Roche Diagnostics.
For incubation with antibodies MCPyV, TTF-1, P40,
synaptophysin an HRP Polymer-Optiview DAB Detection kit, was used
for detection (Ventana Medical System, Inc.), whereas an HRP
Polymer-Ultraview Universal DAB Detection kit was used for
detection of binding of all other antibodies, both according to the
manufacturer's protocol.
Diaminobenzidine was used for development of
staining, and the sections were counterstained with hematoxylin.
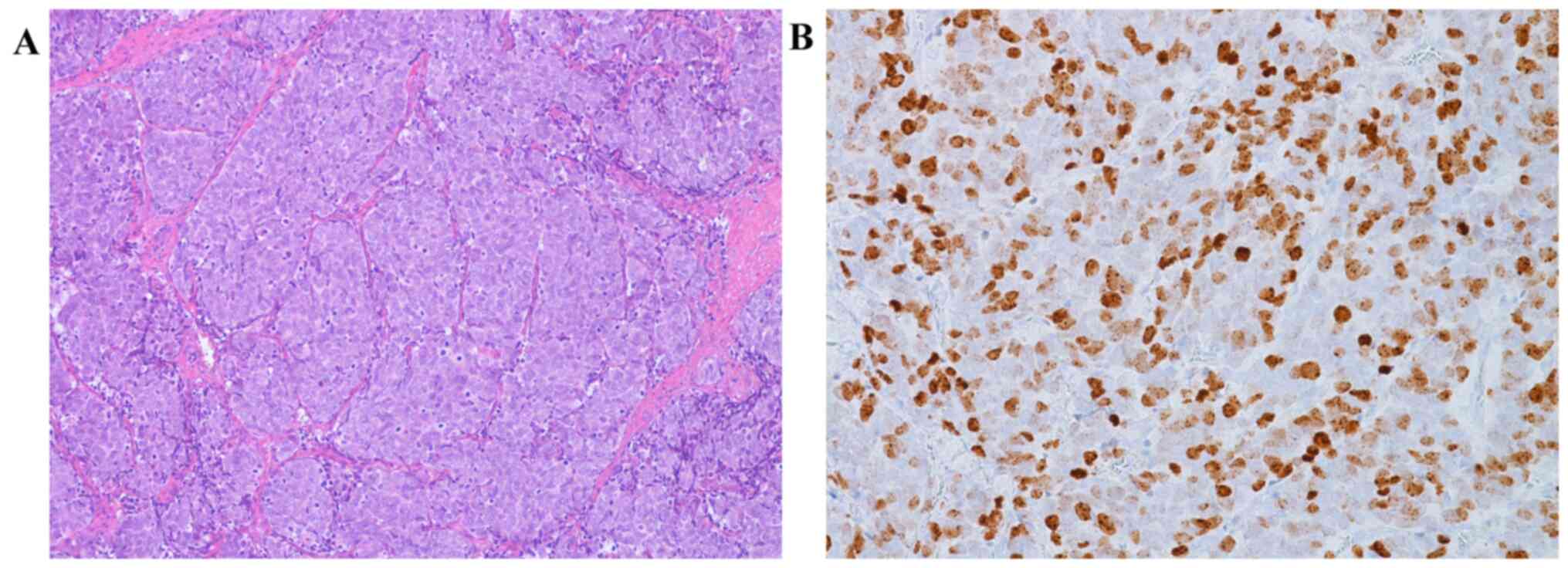
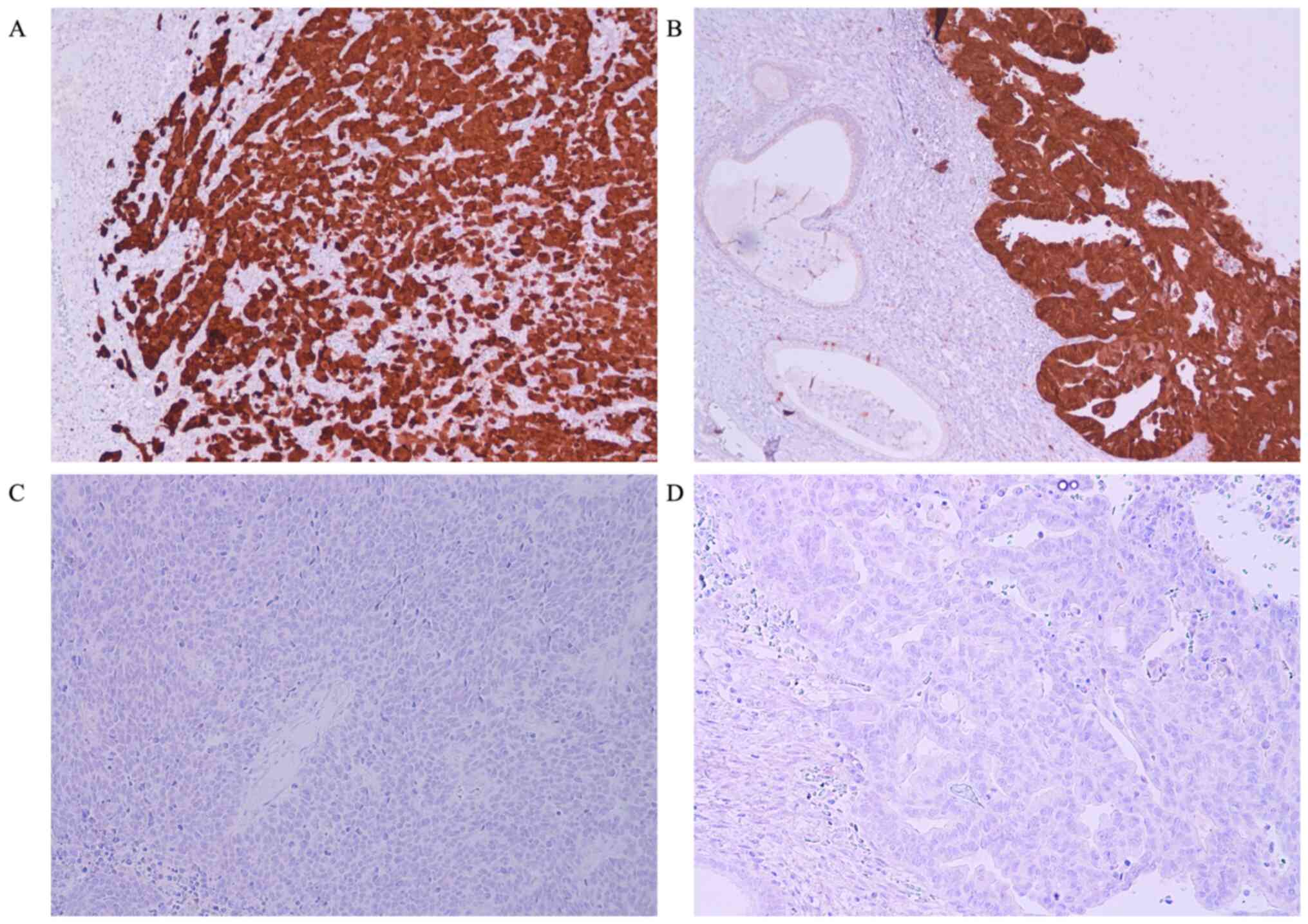
For immunohistochemical study a histological section of cutaneous
Merkel cell carcinoma of the neck from a male 71 year old man and a
histological section of adenocarcinoma of large bowell from female
61 years old patient, identified in the pathology archives of Parma
University (Italy), were used respectively as external positive
(Fig. 1A and B) and negative controls, to verify the
presence of MCPyV in our neoplasms.
Molecular analysis
PCR was used to evaluate the presence of HPV and
MCPyV DNA in the neoplasms. For DNA extraction, 4-µm histological
sections were stained with hematoxylin and examined under a
stereomicroscope. Neoplastic areas were manually microdissected
using sterile scalpels, then suspended in a buffer for tissue lysis
(Tris-HCl 50 mM, pH 9, 1 mM EDTA pH 8.0, 0.5% Tween-20, 5% Chelex
100), and incubated overnight with Proteinase K (0.4 mg/ml) at
55˚C. After enzyme inactivation by 10-min of boiling, the DNA
extracted was directly used in the PCR mix without further
purification. As described by Li et al (18), extracted DNA was PCR amplified using
a set of general primers (L1C1/L1C2+C2M) designed to match the L1
region of the conserved region amongst the different HPV genotypes,
in order to detect a broad spectrum of genital HPV DNAs (including
HPV types 6, 11, 16, 18, 31, 33, 39, 45, 51, 52, 56, 58 and 59)
(Table I). Conventional qualitative
PCR analysis was performed as follows: 95˚C for 7 min, 94˚C for 1
min, 40-47˚C (1˚C increase each cycle) for 1 min and 72˚C for 1 min
for 8 cycles; then 94˚C for 1 min, 48˚C for 1 min and 72˚C for 1
min for 35 cycles; and a final extension step at 72˚C for 7 min. A
negative (sterile water) and a positive [DNA of a high-grade
squamous intraepithelial lesion (H-SIL) HPV-related] controls were
included in the amplification run. The presence of PCR products of
the correct size (243-262 bp) was confirmed by 2% agarose gel
electrophoresis.
 | Table IPrimer sequences used for HPV and
MCPyV PCR amplification. |
Table I
Primer sequences used for HPV and
MCPyV PCR amplification.
| A, HPV, amplicon
size 243-262 bp, Li et al (18) |
|---|
| Primer | Sequence
(5'-3') |
|---|
| L1C1 |
CGTAAACGTTTTCCCTATTTTTTT |
| L1C2 |
TACCCTAAATACTCTGTATTG |
| LiC2M |
TACCCTAAATACCCTATATTG |
| B, MCPyV, amplicon
size 153 bp, Alvarez-Argüelles et al (19) |
| MCPyV-1 |
CAACAGAGGGCTTTGGGTAAA |
| MCPyV-2 |
AAGTGTCAGGCCAACCTATGGAA |
DNA extracted from the neoplastic tissues were
tested for PCR amplification of MCPyV DNA using a specific primer
set, as described previously (19)
(Table I). Conventional qualitative
PCR was performed as follows: 35 cycles of 1 min at 94˚C, 1 min at
56˚C and 1 min at 72˚C, followed a final extension step at 72˚C for
10 min. A negative (sterile water) and a positive (DNA of a MCC
MCPyV-positive, of the neck from a male year old man) controls were
included in the amplification run. The presence of PCR products of
the correct size (153 bp) was verified by 2% agarose gel
electrophoresis.
Case reports
Case 1
A 42-year-old Caucasian woman, para 3, gravida 3,
with a history of abnormal cervical-vaginal cytology due to the
presence of cervical intraepithelial neoplasia (CIN 3, H-SIL) and
cervical glandular intra-epithelial neoplasia, underwent
conization. On the histological examination, the conization
specimen showed an infiltrating, well-differentiated cervical
adenocarcinoma (Fig. 2A), H-SIL and
a neoplasm characterized by a trabecular and nodular growth
pattern, presenting with focal necrosis, accounting for 1% of
neoplastic mass. Peri-tumoral and intra-tumoral flogistic reaction
was absent.
The neoplastic cells were round with ovoid nuclei,
finely granular chromatin and clearly nucleoli (Fig. 2B). Immunohistochemical analysis
revealed that the small cell component was positive for
neuroendocrine markers, such as NSE, synaptophysin (Fig. 2C), chromogranin A (Fig. 2D) and for CAM5.2 (epithelial marker)
(Fig. 2E), but they were negative
for TFF1, p63 and p40 both. Well-differentiated cervical
adenocarcinoma instead was negative for both all neuroendocrine
markers, and for TFF1, p63 and p40 (data not shown).
The mitotic index was high [20 mitoses/10
(high-power fields) (HPF)] and >25% intra-nuclear positivity
expression for the proliferation marker ki 67 was observed.
In addition, p16 immunoreactivity and PCR analysis
demonstrated that all components of the neoplasm were associated
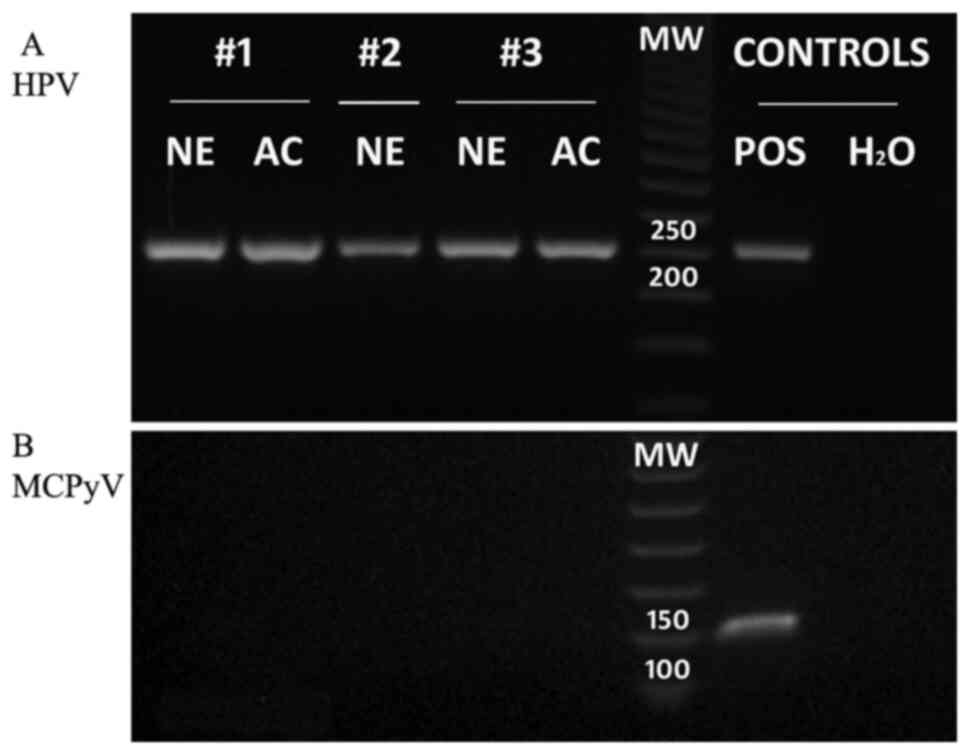
with an HPV infection (Fig. 3A).
Thus, the microscopic characteristics, in association with the
immunohistochemical and molecular findings, were conclusive of a
final histological diagnosis of SCNEC of the cervix, associated
with H-SIL, a well-differentiated cervical adenocarcinoma and HPV
infection. Conversely, immunohistochemical expression for CM2B4 was
negative, and MCPyV DNA was not found in PCR analysis (Fig. 3B) in either the neuroendocrine or
adenocarcinomatous components. A total hysterectomy with bilateral
salpingo-oophorectomy and pelvic lymphadenectomy was performed to
establish the stage of the neoplasm. On macroscopic and microscopic
analyses, all the specimens were unremarkable and free from
neoplasms.
In addition, abdominal ultrasound, chest X-ray,
thoracic and abdominal computed tomography and bone scans were
unremarkable.
Therefore, the final diagnosis was primary cervical
SCNEC with pT1a N0 M0 stage of at diagnosis.
Following radical hysterectomy with lymph-nodes
dissection, the patient received chemotherapy (80 mg/m2
and etoposide 100 mg/m2 repeated for two cycles of
treatment), followed by the administration of carboplatin (area
under the carboplatin plasma concentration vs. time curve=5), Taxol
175 mg/m2 for two cycles and pelvis radiation of 50 Gy
and of the lymph nodes (45 Gy) to L1.
A total of 2 years after diagnosis, surgery,
chemotherapy and radiotherapy, the patient is free of the
disease.
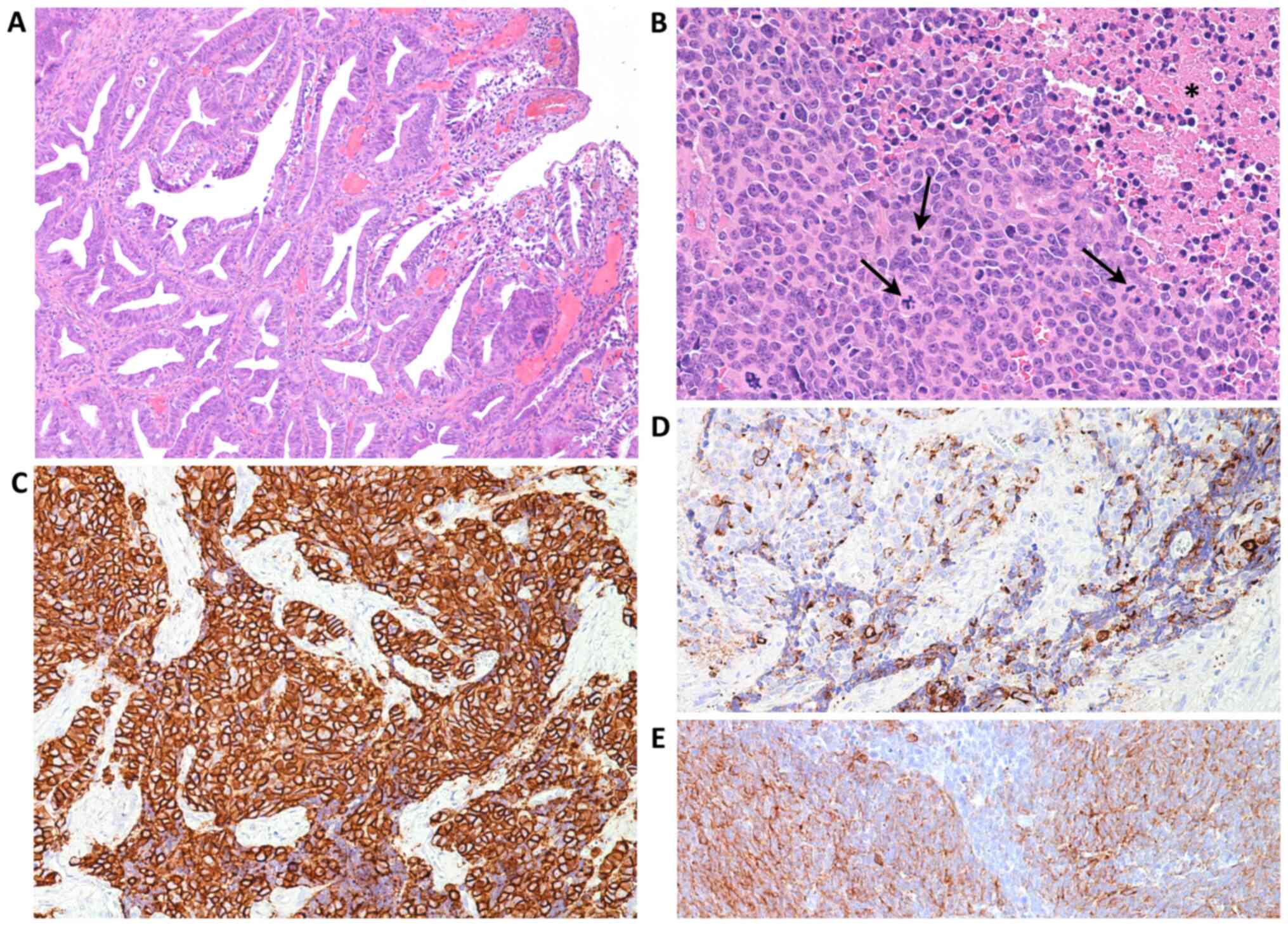
Case 2
An 81-year-old Caucasian woman, para 2, gravida 2,
was referred for postmenopausal bleeding which had lasted 7
months'. Gynecological examination, trans-vaginal echography and
colposcopy revealed an exophytic fungating red to tan mass that
obscured the cervical os. On histological examination, the lesion
was composed of nests and cords of small blue neoplastic cells with
round or ovoid nuclei (Fig. 4A),
finely granular chromatin, small nucleoli and a high mitotic rate
(Fig. 4B), accounting for 18
mitoses/10 HPF with>20% intranuclear expression of Ki-67.
Peri-tumoral and intra-tumoral flogistic reaction was absent. On
immunohistochemical analysis, the neoplastic cells expressed
synaptophysin (Fig. 4C), CD 56
(Fig. 4D), chromogranin A and
CAM5.2 (Fig. 4E), and p16, but were
negative for TFF1, p63 and p40.
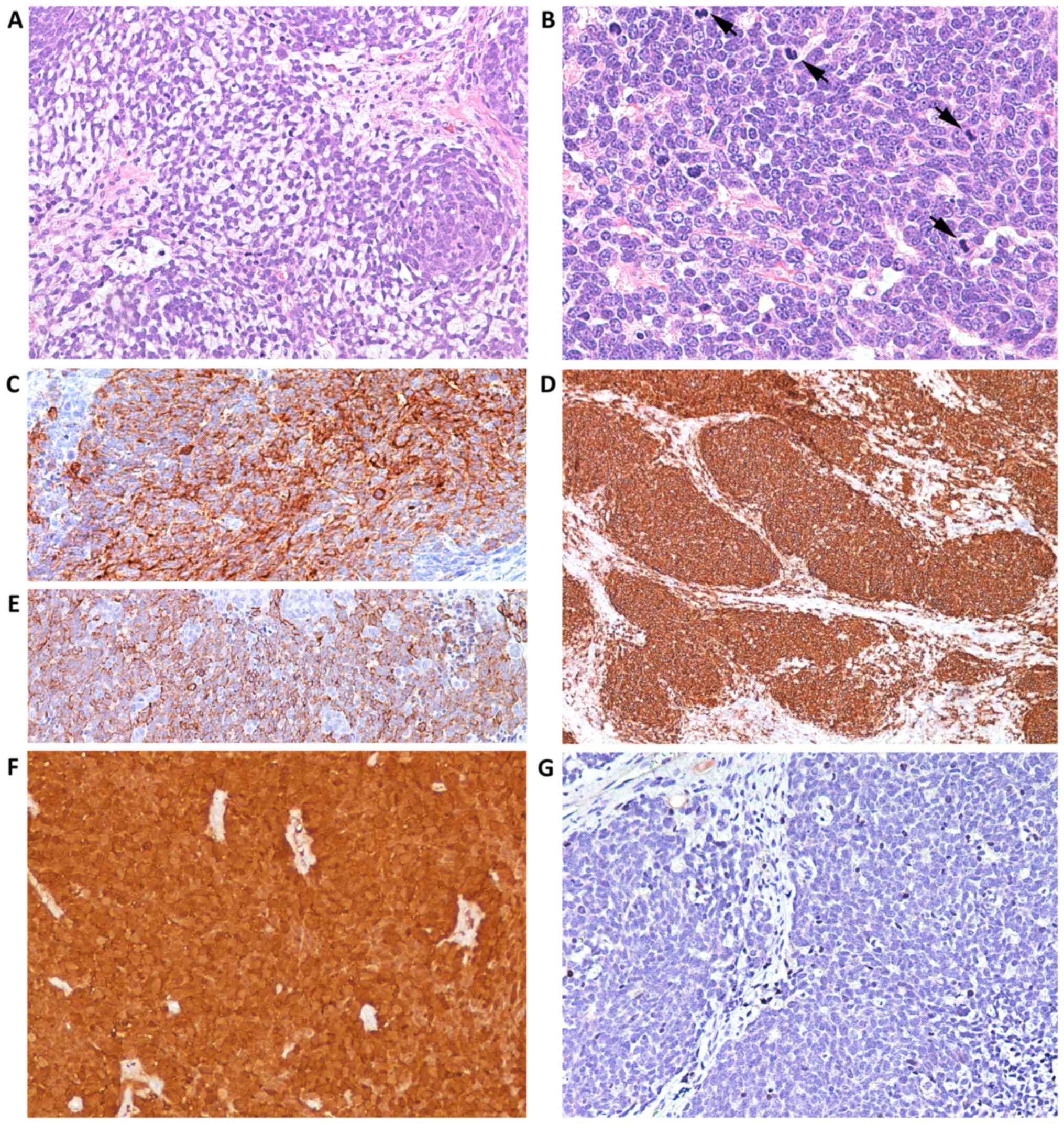 | Figure 4Case 2. Following histologic
examination, the lesion exhibited (A) nests and cords of small blue
neoplastic cells (magnification, x200), with (B) round or ovoid
nuclei, finely granular chromatin, small nucleoli and a high
mitotic rate (arrows) (magnification, x400). (C) Neoplastic cells
exhibited membrane immunoreactivity for synaptophysin
(magnification, x400). (D) CD56 (magnification, x100) and (E)
CAM5.2 (magnification, x200) staining confirmed the lesion was
neuroendocrine in nature. Immunohistochemical analysis revealed (F)
diffuse cytoplasmic and nuclear over-expression of p16
(magnification, x100) and (G) negativity for CM2B4 antibody
(magnification, x200), which was indicative of merkel cell
poliomavirus virus negativity. |
Pre-opertaive contrast-enhanced computed tomography
(CT) of the abdomen and pelvis revealed the presence of a large
ulcerated exophytic cervical lesion, measuring 4 cm in its lagest
diameter. Magnetic resonance imaging (MRI) of the abdomen confirmed
these findings, identifying large uterine cervical neoplasm. There
was no evidence of mass or metastatic disease in the chest, bone or
brain on CT scan. The pre-operative Positron Emission Tomography
(PET) was not performed.
The patient underwent surgery and total radical
hysterectomy with bilateral salpingo-oophorectomy, pelvic and
para-aortic lymph node dissection. Macroscopic examination of the
surgical specimen revealed the presence of a cervical large
exophytic lesion, that was partially ulcerated, measuring 4.7x4 cm,
which entirely obscured the cervical os. On sectioning, the lesion
involved the endocervix and had replaced its wall. The endometrium,
fallopian tubes and ovaries were grossly unremarkable in both
specimens. Histopathological examination confirmed the
pre-operative diagnosis of SCNEC, with numerous vascular tumor
emboli [(stage pT1b2, LVI (+)]. In addition, the neoplastic cells
exhibited over-expression of p16 (Fig.
4F) and revealed the presence of HPV DNA (Fig. 3A). Conversely, the neoplastic
elements did not express TTF-1, estrogen, progesterone receptors or
MCPyV (Fig. 4G). Furthermore, PCR
amplification did not show the presence of MCPyV DNA (Fig. 3B). These findings confirmed the
diagnosis of primary SCNEC of the cervix associated with HPV with
pT1 b2 N0 M0, with numerous vascular tumor emboli.
Conversely, post-operative PET showed many hepatic
metastatic lesions.
The patient received carboplatin (area under the
carboplatin plasma concentration vs. time curve=4) and Taxol (60
mg/m2) for two cycles. At 6 months after hysterectomy
with salpingo-oophorectomy and chemotherapy, the succumbed to the
disease due to the development of diffuse metastatic lesions.
Case 3
An 83-year-old Caucasian woman, para 3, gravida 3,
was referred for postmenopausal bleeding which had lasted 8 months'
duration. Gynecological examination, colposcopy and transvaginal
echography revealed a hemorrhagic cervical mass, measuring 5 cm,
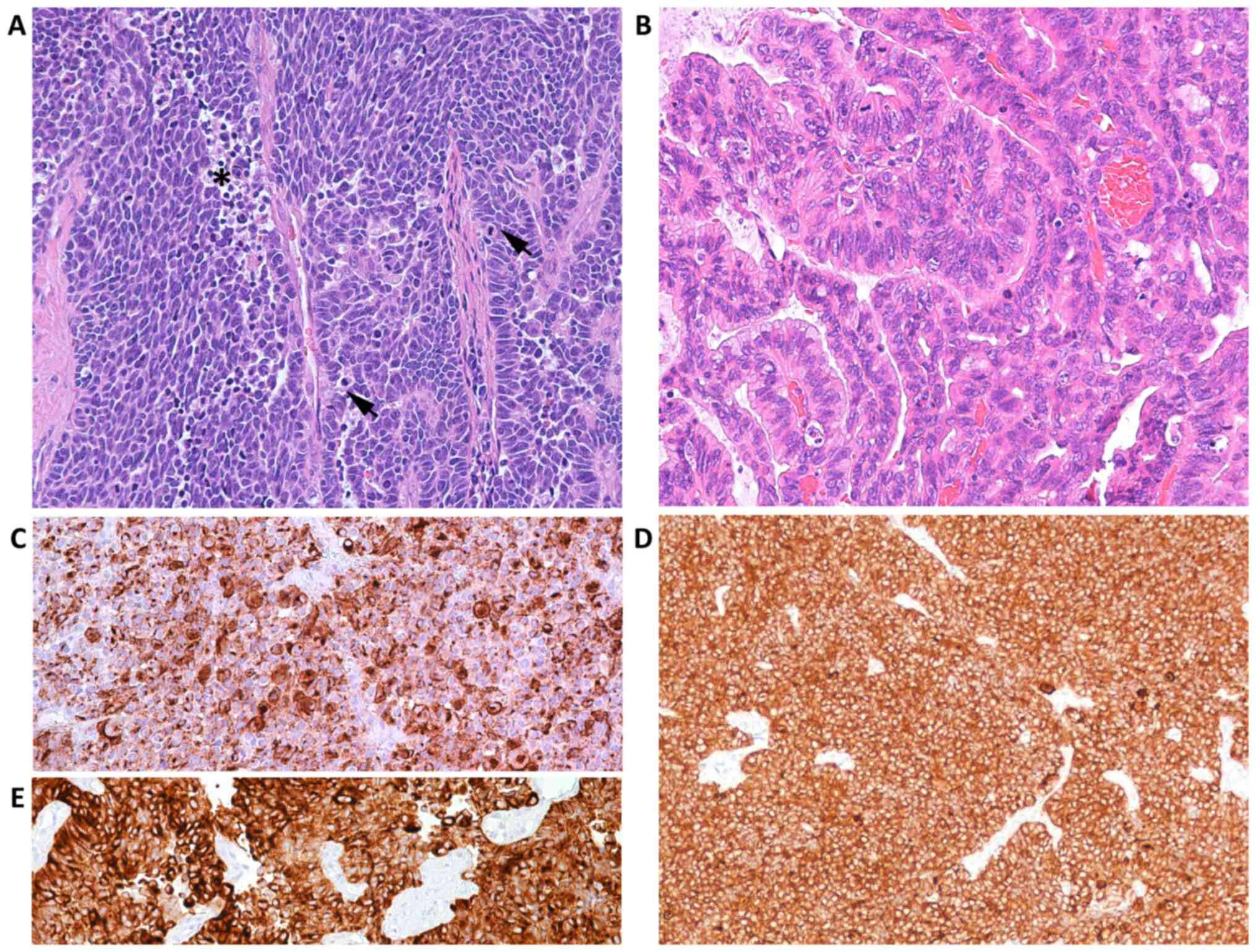
which was biopsied. Histopathology al examination revealed the
presence of a malignant epithelial tumor composed of cords and
trabecula. Peri-tumoral and intra-tumoral flogistic reaction was
absent. The neoplastic elements were oval to spindle-shaped with
hyperchromatic nuclei. The cytoplasm was scant (Fig. 5A). The mitotic index was high and
accounting for 16 mitoses/10 HPF and >20% intranuclear
expression of Ki-67. Individual cell necrosis with karyorrhexis and
karyolysis was visible, but not particularly extensive (Fig. 5A).
In addition, there was a well-differentiated
cervical adenocarcinomatous component (Fig. 5B). Immunohistochemistry revealed
positivity for chromogranin A (Fig.
5C), synaptophysin (Fig. 5D),
and CAM 5.2 (Fig. 5E). Diffuse and
marked positivity for P16 protein was observed in both the
neuroendocrine (Fig. 6A) and in the
adenocarcinomatous component (Fig.
6B). The tumor cells did not express TTF-1, estrogen,
progesterone receptors, p63, p40 (data not shown) and in either the
neuroendocrine MCPyV (Fig. 6C) or
adenocarcinomatous components (Fig.
6D). Moreover, the adenocarcinomatous component was negative
for all neuroendocrine markers.
In addition, PCR amplification did not reveal the
presence of MCPyV DNA (Fig. 2B).
These findings confirmed the diagnosis of a primary SCNEC that was
not associated with MCPyV. Based on the PCR analysis, the
neoplastic element cells exhibited the presence of HPV DNA, in both
the neuroendocrine and adenocarcinomatous components (Fig. 3A). Pre-operative contrast-enhanced
CT of the abdomen and pelvis revealed the presence of a large
exophytic uterine cervical lesion, measuring 5.5 cm in its larger
diameter. MRI of the abdomen confirmed these findings and revealed
a large uterine cervical neoplasm. There was no evidence of mass or
metastatic disease to the chest, bone, or brain on CT scan.
Pre-operative Positron Emission Tomography (PET) was not
performed.
A total hysterectomy with bilateral
salpingo-oophorectomy and pelvic lymphadenectomy were performed.
Macroscopic examination of the surgical specimens identified a
large ulcerated exophytic cervical lesion, measuring 5.5x3.5 cm
that partially obscured the cervical os. On sectioning, the lesions
involved the endocervix and had replaced its wall.
The endometrium, fallopian tubes and ovaries were
grossly unremarkable. On histological examination, the neoplasm was
composed of a SCNEC with oval to spindle-shaped hyperchromatic
nuclei and a differentiated cervical adenocarcinomatous component.
All lymph nodes were free from metastases. These findings confirmed
a diagnosis of primary SCNEC of the cervix associated with HPV,
stage pT1-b2-N0-M0, with prominent intravascular and perineural
invasion (LVI+). Post-opertative PET showed many metastatic
abnormal hypermetabolic lesions in the liver and lungs.
The patient did not receive post-operative treatment
and succumbed to diffuse metastatic lesions 1 month after.
Discussion
Neuroendocrine tumors arise from the
hormone-producing cells of the body's neuroendocrine system, which
consists of cells that are a combination of hormone-producing
endocrine and nerve cells. These neoplasms can most commonly be
found in the gastrointestinal tract, pancreas (20,21)
and lungs (22). Less frequent
locations of neuroendocrine tumors include the urinary system, male
genital organs, female genital organs, head, neck and breast
(23).
SCNECs are rare malignancies accounting 1-3% of all
cervical tumors (24,25). In line with small cell lung cancer,
SCNEC of the cervix is associated with an aggressive and often
fatal clinical course (26), which
is considerably worse compared with that of squamous carcinoma and
adenocarcinoma (27). This
malignancy metastasizes early to the lymph nodes and then to
distant organs. Widespread dissemination may involve the bones,
liver, lung and other soft tissues (27). Moreover, brain metastases have been
reported to be associated with lung metastases (28).
SCNECs are composed of a monotonous population of
small round or fusiform cells with scant cytoplasm and
hyperchromatic nuclei, finely granular chromatin and non-prominent
nucleoli, growing in diffuse or more rarely, nested or trabecular
pattern. Single cell infiltration of the stroma may also observed.
Nuclear molding and crush artifacts are common and are helpful for
diagnosis. Necrosis and apoptosis are a common and lymphatic
invasion is frequently extensive. SCNECs may coexist with other
more typical cervical carcinomas, such as squamous cell carcinomas
or adenocarcinomas, adenocarcinoma in situ and cervical
intraepithelial neoplasia (29,30).
Several studies have reported that the presence of
other subtypes of cervical carcinoma in SCNEC does not improve the
prognosis, which remains significantly worse compared with pure
cervical squamous cell carcinomas and adenocarcinomas (31). These data are in agreement with two
of these cases in the present report (cases 2 and 3) in which both
patients underwent hysterectomy with salpingo-oophorectomy but
developed diffuse metastatic lesions and succumbed to the disease
within a few months after surgery. Moreover, prominent
lympho-vascular invasion was observed in both cases in the
histological examination of radical hysterectomy with bilateral
salpingo-oophorectomy specimen, explaining the rapid demise after a
few of diagnosis.
According to previous studies, the tumor stage, at
diagnosis, in these patients would have been more advanced (pT1b2,
M1, LVI +) than in a patient (case 1, pt1a) who was still alive 2
years after diagnosis (32,33). SCNECs should be distinguished from
primary and metastatic small blue-cell tumors, such as poorly
differentiated squamous cell carcinomas, undifferentiated
carcinomas of the lower uterine segment, primary lymphomas of the
cervix and neuroendocrine metastatic tumors of a different primary
origin (such as the lung), to the cervix. For all three cases of
the present work, poorly differentiated squamous cell carcinomas
can be distinguished from small cell neuroendocrine carcinomas due
to the fact that they lack certain morphological characteristcs on
microscopic examination that typically characterize small cell
cervical cancer, including nuclear molding and non-diagnostic (or
crush) artifacts. In addition, immunoreactivity to p63 and p40 may
be useful in differentiating SCNEC from squamous cell carcinoma
since these markers are consistently expressed in squamous cell
carcinomas, but are negative in SCNEC. Moreover, whilst p63
expression may be observed in SCNEC (34), negativity to p40 excludes the
diagnosis of squamous cell carcinoma, as p40 is consistently
negative in neuroendocrine neoplasms at any site (35,36).
Neuroendocrine markers with morphological evaluation may assist in
the differential diagnosis of SCNEC and non-keratinizing squamous
cell HPV-related carcinomas.
For case 2, that exhibited only neuroendocrine
component, undifferentiated carcinoma of the lower uterine segment
(LUS), which accounts for 6% of all endometrial cancers (37), was excluded by MRI, by the presence
of HPV DNA and p16 immunoreactivity, demonstrating that the
neoplasm does not originate from this segment of the uterus.
Conversely, neuroendocrine markers may not be useful for the
differential diagnosis, in this case, since undifferentiated
carcinomas of the LUS may occasionally display focal positivity for
neuroendocrine markers (38).
As well as for case 2, differential the diagnosis of
SCNEC and embryonal rhabdomyosarcoma can be made because, under
normal circumstances, this do not stain positive for cytokeratin
and neuroendocrine markers (39,40).
In case 2, primary lymphoma of the cervix, which is
an exceedingly rare neoplasm (41)
and typically consist of diffuse large B-cell lymphoma (DLBCL)
(42) was excluded for
morphological analysis and immunophenotyping. In fact, tumor cells
in DLBCL generally express pan B-cell antigens (CD19, CD20, CD22
and CD79a) and are negative for neuroendocrine markers (42).
The possibility of SCNEC developing as a metastasis
from other sites, such as the lungs and gastro-enteropancreatic
tract must be ruled out. That said, isolated metastatic involvement
of the cervix does appear to be exceedingly rare. For example, the
most common sites of distant metastases from small cell carcinomas
of the lung are the liver, adrenal glands, bones and brain.
Moreover, the identification of a synchronous cervical neoplasia,
such as H-SIL and cervical adenocarcinoma in close proximity to the
neuroendocrine tumor, may also be useful to exclude the metastatic
nature of neuroendocrine neoplasms arising in the cervix. In the
present report, foci of H-SIL and foci of invasive cervical
adenocarcinoma of usual type in case 1 and case 3 were
observed.
SCNEC is frequently associated with HPV infections
(5,6). Given that MCPyV has been observed in a
series of squamous cervical carcinomas and cervical adenocarcinomas
in Japanese patients (17), as well
as in certain neuroendocrine neoplasms, such as MCC of the skin,
the present study employed immunohistochemical and PCR analysis,
and the presence of MCPyV in all 3 cases of SCNEC of the cervix was
assessed to determine whether this virus can coexist with HPV in
this rare neoplasm. In the present study, HPV DNA was demonstrated
in all three neuroendocrine carcinomas investigated and in the
adenocarcinomatous component in the two mixed cases by PCR
analysis. However, the lack of sequencing of the amplicon obtained
by PCR in either neoplastic tissue or positive control represents a
limitation of the present study. As well as, it may be a
limtitation does not know viral load in these patients. Conversely,
the positivity for p16 observed in all these cases and all
components of the neoplasms, allows to establish that HPV detected
corresponded to high-risk HPV (43).
In addition to the immunohistochemical study, in
order to detect the presence of MCPyV DNA, PCR analysis, using a
primer set was performed (19), as
Moshiri et al (44)
demonstrated that the immunohistochemistry alone was insufficient
to classify a tumor as positive for virus (44). In fact, they evaluated the presence
of MCPyV in a large series of MCC, using immunohistochemistry with
two distinct antibodies and MCPyV DNA by PCR analysis, and
concluded that a neoplasm can be considered MCPyV-positive when two
or more of these three assays indicated the presence of this
virus.
In the present study the immunoreactivity to
cytokeratin 20 (CK20), which is expressed in more typical MCC
(34), was verified. As CK20
immunoreactivity was negative, the present cases may be considered
as non-Merkel neuroendocrine neoplasms. This finding may also
explain the negativity to the CM2B4 monoclonal antibody in line
with many cases reported by Glenn McCluggage et al (34) and in accordance with absence of
MCPyV DNA as revealed by the combined use of immunohistochemical
and PCR analysis.
In addition to the negativity for CK20 and CM2B4, in
all three cases of the present study, we did not observe
peri-tumoral inflammatory infiltrate, with CD8 positive cells,
abnormal p53 expression and positivity for TTF1, which may be
related with stage of neoplasms and prognosis, as reported by
Kervarrec et al in many case of MCC (45). In fact, in case 1 the stage of
neoplams was low and patient was alive and free of disease.
Whilist, in cases 2 and case 3 the patients succumbed after a few
months of diagnosis with diffuse metastatic disease.
Conclusions and future goals
In conclusion, these cases of SCNECs could suggest
that they are non-Merkel neuroendocrine neoplasms and likely not
associated with MCPyV, as revealed by the combined
immunohistochemical and PCR analyses. However, in the cases of the
present study, the absence of MCPyV may due to a genotype that was
not detectable using the primers utilized for PCR analysis and the
antibody used for immunohistochemimistry. Several studies,
investigating the presence of MCPyV in MCC in different countries,
have suggested that there are different MCPyV genotypes (46,47).
In fact, Martel-Jantin et al (46), using molecular analysis,
demonstrated the existence of 5 major geographically related MCPyV
genotypes (Europe/North America, Africa [sub-Saharan], Oceania,
South America, and Asia/Japan] (44). Additionally, Matsushita et al
(47) have suggested that MCPyV
strains in Japanese MCC are distinct from MCPyVs genotypes
associated with Caucasian populations (47).
As well as the lack of large T antigen expression in
the present case series may reflect the presence of MCPyV microRNA
that inhibits LT expression (48).
Additional studies with larger cohorts cases and more advanced
molecular biology techniques may be useful in evaluating the
presence of MCPyV. Using more advanced molecular techniques, it may
be possible to detecte MCPyV-associated microRNAs (miRNAs), which
have the ability to autoregulate viral gene expression (48) allowing for improved identification
in MCPyV-positive and MCPyV-negative SCNECs, and these techniques
may allow for identification of a subset of miRNAs associated with
tumor metastasis and specific survival, similarly to cases of MCCs
(49).
As well as, further studies with larger cohorts
cases of SCNEC of the cervix, may confirm that the expression for
CK20, positivity for TTF1, abnormal expression for p53, negativity
for CM2B4, and absence of inflammatory infiltrate, with CD 8
positive cells may be related with advanced stage and poor
prognosis of the neoplams, similarly to many cases of MCC (45).
Acknowledgements
The authors would like to thank Mrs Gabriella Becchi
(Department of Medicine and Surgery, Pathology Unit, University of
Parma, Italy) for her technical assistance.
Funding
The present study was funded by FIL Parma University.
Availability of data and materials
The data sets used and/or data analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
GGi and TDA conceived the study and wrote the
manuscript. SP and NC performed molecular and immunohistochemical
studies. GGa provided the clinical data and RB performed the
surgery. GGi and RB declare the authenticity of the all raw data.
All authors have read and approved the final manuscript.
Ethics approval and consent participate
The present study was approved by Ethics Committee
of the Parma University Hospital (approval no. 26600).
Patient consent for publication
The informed consent was obtained from all
individual participants except for the cases 2 and 3 due to their
death and the inability to contact their relatives who do not
reside in Italy.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dikmen Y, Kazandi M, Zekioglu O, Ozsaran
A, Terek MC and Erhan Y: Large cell neuroendocrine carcinoma of the
uterine cervix: A report of a case and review of the literature.
Arch Gynecol Obstet. 270:185–188. 2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kim JY, Hong SM and Ro JY: Recent updates
on grading and classification of neuroendocrine tumors. Ann Diagn
Pathol. 29:11–16. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Albores-Saavedra J, Gersell D, Gilk CB,
Henson DE, Lindberg G, Santiago H, Scully RE, Silva E, Sobin LH,
Tavassoli FJ, et al: Terminology of endocrine tumors of the uterine
cervix: Results of a work-shop sponsored by College of American
Pathologists and the National Cancer Institute. Arch Lab.
121:34–39. 1997.PubMed/NCBI
|
|
4
|
Cree IA, Malpica A and McCluggage WG:
Neuroendocrine neoplasia in WHO classification of femal genital
Tumours 5th edition. Lyon, IARC, pp452-458, 2019.
|
|
5
|
Ambros RA, Park JS, Shah KV and Kurman RJ:
Evaluation of histologic, morphometric, and immunohistochemical
criteria in the differential diagnosis of small cell carcinomas of
the cervix with particular reference to human papillomavirus types
16 and 18. Mod Pathol. 4:586–593. 1991.PubMed/NCBI
|
|
6
|
Stoler MH, Mills SE, Gersell DJ and Walker
AN: Small-cell neuroendocrine carcinoma of the cervix. A human
papillomavirus type 18-associated cancer. Am J Surg Pathol.
15:28–32. 1991.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li P, Ma J, Zhang X, Guo Y, Liu Y, Li X,
Zhao D and Wang Z: Cervical small cell carcinoma frequently
presented in multiple high risk HPV infection and often associated
with other type of epithelial tumors. Diagn Pathol. 13:31–40.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bolognia J, Jorizzo JL, Rapini RP, Callen
JP, Horn TD, Mancini AJ, Salasche SJ, Schaffer JV, Schwarz T,
Stingl G and Stone MS: Dermatology (2nd edition). St. Louis, MO,
Mosby/Elsevier, 2008.
|
|
9
|
Feng H, Shuda M, Chang Y and Moore PS:
Clonal integration of a polyomavirus in human Merkel cell
carcinoma. Science. 319:1096–1100. 2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kervarrec T, Samimi M, Guyétant S, Sarma
B, Chéret J, Blanchard E, Berthon P, Schrama D, Houben R and Touzé
A: Histogenesis of merkel cell carcinoma: A comprehensive review.
Front Oncol. 9(451)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bialasiewicz S, Lambert SB, Whiley DM,
Nissen MD and Sloots TP: Merkel cell polyomavirus DNA in
respiratory specimens from children and adults. Emerg Infect Dis.
15:492–494. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Goh S, Lindau C, Tiveljung-Lindell A and
Allander T: Merkel cell polyomavirus in respiratory tract
secretions. Emerg Infect Dis. 15:489–491. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Babakir-Mina M, Ciccozzi M, Lo Presti A,
Greco F, Perno CF and Ciotti M: Identification of Merkel cell
polyomavirus in the lower respiratory tract of Italian patients. J
Med Virol. 82:505–509. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Abedi Kiasari B, Vallely PJ and Klapper
PE: Merkel cell polyomavirus DNA in immunocompetent and
immunocompromised patients with respiratory disease. J Med Virol.
83:2220–2224. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wieland U, Mauch C, Kreuter A, Krieg T and
Pfister H: Merkel cell polyomavirus DNA in persons without Merkel
cell carcinoma. Emerg Infect Dis. 15:489–491. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wieland U and Kreuter A: Merkel cell
polyomavirus infection and Merkel cell carcinoma in HIV-positive
individuals. Curr Opin Oncol. 23:488–493. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Imajoh M, Hashida Y, Nemoto Y, Oguri H,
Maeda N, Furihata M, Fukaya T and Daibata M: Detection of Merkel
cell polyomavirus in cervical squamous cell carcinomas and
adenocarcinomas from Japanese patients. Virol J.
9(154)2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li J, Gerhard DS, Zhang Z, Huettner PC,
Wright J, Nguyen L, Lu D and Rader JS: Denaturing high-performance
liquid chromatography for detecting and typing genital human
papillomavirus. J Clin Microbiol. 41:5563–5571. 2003.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Alvarez-Argüelles ME, Melón S, Rojo S,
Fernandez-Blázquez A, Boga JA, Palacio A, Vivanco B and de Oña M:
Detection and quantification of Merkel cell polyomavirus. Analysis
of Merkel cell carcinoma cases from 1977 to 2015. J Med Virol.
89:2224–2229. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kloppel G, Perren A and Heitz PU: The
gastroenteropancreatic neuroendocrine cell system and its tumors:
The WHO classification. Ann N Y Acad Sci. 1014:13–27.
2004.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Plöckinger U, Rindi G, Arnold R, Eriksson
B, Krenning EP, de Werder WW, Goede A, Caplin M, Oberg K, Reubi JC,
et al: Guidelines for the diagnosis and treatment of neuroendocrine
gastrointestinal tumours. A consensus statement on behalf of the
European Neuroendocrine Tumour Society (ENETS). Neuroendocrinology.
80:394–424. 2004.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yeh YC and Chou TY: Pulmonary
neuroendocrine tumors: Study of 90 cases focusing on
clinicopathological characteristics, immunophenotype, preoperative
biopsy, and frozen section diagnoses. J Surg Oncol. 109:280–286.
2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Guadagno E, De Rosa G and Del Basso De
Caro M: Neuroendocrine tumours in rare sites: Differences in
nomenclature and diagnostics-a rare and ubiquitous histotype. J
Clin Pathol. 69:563–574. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
van Nagell JR Jr, Powell DE, Gallion HH,
Elliott DG, Donaldson ES, Carpenter AE, Higgins RV, Kryscio R and
Pavlik EJ: Small cell carcinoma of the uterine cervix. Cancer.
62:1586–1593. 1988.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sevin BU, Method MW, Nadji M, Lu Y and
Averette HA: Efficacy of radical hysterectomy as treatment for
patients with small cell carcinoma of the cervix. Cancer.
77:1489–1493. 1996.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Conner MG, Richter H, Moran CA, Hameed A
and Albores-Saavedra J: Small cell carcinoma of the cervix: A
clinicopathologic and immunohistochemical study of 23 cases. Ann
Diagn Pathol. 6:345–348. 2002.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chen J, Macdonald OK and Gaffney DK:
Incidence, mortality, and prognostic factors of small cell
carcinoma of the cervix. Obstet Gynecol. 111:1394–1402.
2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Viswanathan AN, Deavers MT, Jhingran A,
Ramirez PT, Levenback C and Eifel PJ: Small cell neuroendocrine
carcinoma of the cervix: outcome and patterns of recurrence.
Gynecol Oncol. 93:27–33. 2004.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Abeler VM, Holm R, Nesland JM and Kjørstad
KE: Small cell carcinoma of the cervix. A clinicopathologic study
of 26 patients. Cancer. 73:672–677. 1994.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Alphandery C, Dagrada G, Frattini M,
Perrone F and Pilotti S: Neuroendocrine small cell carcinoma of the
cervix associated with endocervical adenocarcinoma: A case report.
Acta Cytol. 51:589–593. 2007.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhou J, Wu SG, Sun JY, Tang LY, Lin HX, Li
FY, Chen QH, Jin X and He ZY: Clinicopathological features of small
cell carcinoma of the uterine cervix in the surveillance,
epidemiology, and results database. Oncotarget. 8:40425–40433.
2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chan JK, Loizzi V, Burger RA, Rutgers J
and Monk BJ: Prognostic factors in neuroendocrine small cell
cervical carcinoma, a multivariate analysis. Cancer. 97:568–574.
2003.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chang TC, Lai CH, Tseng CJ, Hsueh S, Huang
KG and Chou HH: Prognostic factors in surgically treated small cell
cervical carcinoma followed by adjuvant chemotherapy. Cancer.
83:712–718. 1998.PubMed/NCBI View Article : Google Scholar
|
|
34
|
McCluggage WG, Kennedy K and Busam KJ: An
immunohistochemical study of cervical neuroendocrine carcinomas:
Neoplasms that are commonly TTF1 positive and which may express
CK20 and P63. Am J Surg Pathol. 34:525–532. 2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhang C, Schmidt LA, Hatanaka K, Thomas D,
Lagstein A and Myers JL: Evaluation of napsin, A; TTF-1, p63, p40,
and CK5/6 immunohistochemical stains in pulmonary neuroendocrine
tumors. Am J Clin Pathol. 142:320–324. 2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tatsumori T, Tsuta K, Masai K, Kinno T,
Taniyama T, Yoshida A, Suzuki K and Tsuda H: p40 is the best marker
for diagnosing pulmonary squamous cell carcinoma: Comparison with
p63, cytokeratin 5/6, desmocollin-3, and sox2. Appl Immunohistochem
Mol Morphol. 22:377–382. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Masuda K, Banno K, Yanokura M, Kobayashi
Y, Kisu I, Ueki A, Ono A, Nomura H, Hirasawa A, Susumu N and Aoki
D: Carcinoma of the lower uterine segment (LUS):
Clinicopathological characteristics and association with Lynch
syndrome. Curr Genomics. 12:25–29. 2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Rabban JT and Zaloudek CJ: Neuroendocrine
differentiation in endometrial tumors: A practical diagnostic
approach. Pathol Case Rev. 16:119–125. 2011.
|
|
39
|
Sebire NJ and Malone M: Myogenin and MyoD1
expression in paediatric rhabdomyosarcomas. J Clin Pathol.
56:412–416. 2003.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Dehner LP, Jarzembowski JA and Hill DA:
Embryonal rhabdomyosarcoma of the uterine cervix: A report of 14
cases and a discussion of its unusual clinicopathological
associations. Mod Pathol. 25:602–614. 2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Upanal N and Enjeti A: Primary lymphoma of
the uterus and cervix: Two case reports and review of the
literature. Aust N Z J Obstet Gynaecol. 51:559–562. 2011.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Cubo AM, Soto ZM, Cruz MÁ, Doyague MJ,
Sancho V, Fraino A, Blanco Ó, Puig N, Alcoceba M, González M and
Sayagués JM: Primary diffuse large B cell lymphoma of the uterine
cervix successfully treated by combined chemotherapy alone: A case
report. Medicine (Baltimore). 96(e6846)2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kalof AN and Cooper K: p16INK4a
immunoexpression: Surrogate marker of high-risk HPV and high-grade
cervical intraepithelial neoplasia. Adv Anat Pathol. 13:190–194.
2006.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Moshiri AS, Doumani R, Yelistratova L,
Blom A, Lachance K, Shinohara MM, Delaney M, Chang O, McArdle S,
Thomas H, et al: Polyomavirus-negative Merkel cell carcinoma: A
more aggressive subtype based on analysis of 282 cases using
multimodal tumor virus detection. J Invest Dermatol. 137:819–827.
2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Kervarrec T, Samimi M, Gaboriaud P, Gheit
T, Beby-Defaux A, Houben R, Schrama D, Fromont G, Tommasino M, Le
Corre Y, et al: Detection of the Merkel cell polyomavirus in the
neuroendocrine component of combined Merkel cell carcinoma.
Virchows Arch. 472:825–837. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Martel-Jantin C, Filippone C, Tortevoye P,
Afonso PV, Betsem E, Descorps-Declere S, Nicol JT, Touzé A,
Coursaget P, Crouzat M, et al: Molecular epidemiology of merkel
cell polyomavirus: Evidence for geographically related variant
genotypes. J Clin Microbiol. 52:1687–90. 2014.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Matsushita M, Iwasaki T, Kuwamoto S, Kato
M, Nagata K, Murakami I, Kitamura Y and Hayashi K: Merkel cell
polyomavirus (MCPyV) strains in Japanese merkel cell carcinomas
(MCC) are distinct from Caucasian type MCPyVs: Genetic variability
and phylogeny of MCPyV genomes obtained from Japanese
MCPyV-infected MCCs. Virus Genes. 48:233–242. 2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Seo GJ, Chen CJ and Sullivan CS: Merkel
cell polyomavirus encodes a microRNA with the ability to
autoregulate viral gene expression. Virology. 383:183–18.
2009.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Xie H, Caramuta S, Höög A, Browaldh N,
Björnhagen V, Larsson C and Lui WO: MicroRNA expression patterns
related to merkel cell polyomavirus infection in human merkel cell
carcinoma. J Invest Dermatol. 134:507–517. 2014.PubMed/NCBI View Article : Google Scholar
|