Introduction
Upper urinary tract urothelial carcinoma (UTUC) is a
relatively rare malignant tumor, estimated to be approximately 10%
of all renal tumors and only 5% of all urothelial carcinomas
(1,2). This corresponds to an estimated annual
incidence of almost two cases per 100,000 inhabitants in Western
countries.
Radical nephroureterectomy (RNU) with a bladder cuff
excision is the standard treatment for patients with N0M0 UTUC;
however, lymphovascular invasion (LVI) as well as tumor grade and
pathological stage were significantly related to cancer-specific
survival (CSS) and overall survival rates in patients with UTUC who
underwent open or laparoscopic RNU (3). Based on these findings, appropriate
markers to monitor or predict oncologic outcomes for patients with
localized UTUC are necessary.
The prognostic value of many systemic inflammatory
response (SIR) markers as predictors of poor CSS or recurrence-free
survival in several types of carcinoma has been reported (4-6).
SIR was found to be related to shorter time to cancer progression
and cancer-specific death (4-6).
SIR markers include preoperative C-reactive protein (CRP), the
Glasgow Prognostic Score (GPS), neutrophil to lymphocyte ratio
(NLR), and platelet to lymphocyte ratio (PLR) (7-10).
Taken together, several studies have focused on the
relationship between various cancers and hemostatic factors. Among
such hemostatic factors, fibrinogen, an essential hemostatic
factor, is converted to fibrin (a final product of the hemostatic
pathway) by activated thrombin. In addition, elevated plasma
fibrinogen has been reported to correlate with tumor progression in
some types of cancer (11-13).
Moreover, preoperative plasma fibrinogen levels were found to be a
useful predictor of lymph node metastasis in earlier studies
(11,12). As a result, preoperative plasma
fibrinogen levels were also relevant in terms of predicting a poor
prognosis in patients with several types of cancer (14-16).
Based on these studies, we hypothesize that fibrinogen could be one
cause of distant metastasis in patients with UTUC. Furthermore, a
higher plasma fibrinogen level may also be a feasible marker of
premetastatic status such as LVI; therefore, this marker mayenable
us to monitor or predict oncologic outcomes of patients with
localized UTUC, considered in conjunction with other
clinicopathological factors. In the present study, we
retrospectively examined the preoperative plasma fibrinogen levels
of patients with UTUC who underwent RNU and evaluated the
association of clinicopathological risk factors including
preoperative plasma fibrinogen levelswith the presence of LVI and
CSS and ERFS rates to establish a preoperative risk stratification
model.
Patients and methods
Patients
We retrospectively reviewed and analyzed
clinicopathological data from the medical records of 145 patients
who underwent RNU at our institution and who were histologically
diagnosed with UTUC. The study protocol (ID 2734) was approvedon
June 14, 2017, by our institutional ethics committee, and we used
an opt-out approach on the Web page of the National Defense Medical
College instead of collecting written informed consent from all
participants. The patients included 109 men and 36 women. The
median follow-up period after nephroureterectomy was 54.2 months
(range: 3.4 to 209.2 months). We present additional
clinicopathological data in Table
I.
 | Table IClinicopathological features. |
Table I
Clinicopathological features.
| Parameters | Patients (n) |
|---|
| Age (median 70) | |
|
≥71 | 71 |
|
≤70 | 74 |
| Sex | |
|
Male | 109 |
|
Female | 36 |
| Urine cytology | |
|
Positive | 88 |
|
Negative | 57 |
| Histology | |
|
UC with
other components | 29 |
|
UC
alone | 116 |
| Pathological T
stage | |
|
≥T3 | 71 |
|
≤T2 | 74 |
| Tumor grade | |
|
High | 101 |
|
PUNLMP/Low | 44 |
| Lymph node
metastasis | |
|
Positive | 9 |
|
Negative | 136 |
| Ureteral
involvement | |
|
Positive | 81 |
|
Negative | 64 |
| Surgical margins | |
|
Positive | 19 |
|
Negative | 126 |
| Lymphovascular
invasion | |
|
Positive | 51 |
|
Negative | 94 |
| Carcinoma in
situ | |
|
Present | 17 |
|
Absent | 128 |
| Hydronephrosis | |
|
Positive | 86 |
|
Negative | 59 |
| CRP (≥0.4 or
≤0.3) | |
|
≥0.4 | 34 |
|
≤0.3 | 111 |
| Albumin (≤3.7 or
≥3.8) | |
|
≤3.7 | 17 |
|
≥3.8 | 128 |
| NLR (≥1.652 or
<1.652) | |
|
≥1.652 | 114 |
|
<1.652 | 31 |
| PLR (≥154.122 or
<154.122) | |
|
≥154.122 | 60 |
|
<154.122 | 85 |
| GPS | |
|
≥1 | 15 |
|
0 | 130 |
| Fibrinogen (≥400 or
<400) | |
|
≥400 | 23 |
|
<400 | 122 |
Extraurothelial recurrence after nephroureterectomy
indicates tumor recurrence outside the bladder or distant
metastasis. In this study, we defined recurrence-free survival as
extraurothelial recurrence-free survival (ERFS). No patients had
distant metastasis at diagnosis. Extraurothelialor intravesical
recurrence was monitored for each patient every 3-6 months for the
first 5 years after nephroureterectomy and 6-12 months thereafter.
All surgical specimens were processed according to standard
pathological procedures and were histologically confirmed to be
urothelial carcinoma with or without other tumor cell types. The
pathological staging of the primary tumor was determined according
to the American Joint Committee on Cancer TNM Classification
(17), whereas tumor grading was
determined according to the 2004 World Health Organization (WHO)
classification of urothelial tumors (18). Tumor specimens were evaluated by two
pathologists, and the patients were categorized into two groups on
the basis of the 2004 WHO classification system for tumor
grading.
Inflammatory indices
Inflammatory indices were evaluated by laboratory
tests. The neutrophil to lymphocyte ratio (NLR) was calculated by
dividing the absolute neutrophil count by the absolute lymphocyte
count (19). The platelet to
lymphocyte ratio (PLR) was calculated by dividing the absolute
platelet count by the absolute lymphocyte count (19). The Glasgow prognostic score (GPS)
was evaluated by using the CRP and Alb values. Patients with
elevated CRP levels (>1 mg/dl) in combination with
hypoalbuminemia (<3.5 g/dl) were allocated 2 points. Patients
with elevated CRP alone or hypoalbuminemia alone were allocated 1
point, whereas patients exhibiting these parameters within normal
limits were allocated 0 points (10). In the presents study, normal limits
of serum albumin, CRP levels and plasma fibrinogen levels were
designated according to analyzing kits used at our institution
based on the methods shown in earlier reports (11-13,20).
Statistical analysis
Cox proportional hazards model was used in the
examination of independent factors for worse CSS and ERFS rates in
multivariate analysis. A receiver operator characteristic (ROC)
analysis was performed to determine the cut-off values of NLR and
PLR according to the method shown in previous reports (21,22).
Fisher's exact probability test was performed to examine the
relationship between clinicopathological factors and the presence
of LVI. Multiple logistic regression analysis was performed to
detect predictors of LVI. The effect of independent markers for
worse CSS and ERFS rates was evaluated with Kaplan-Meier plots and
the log-rank test. The statistical analysis was undertaken using
JMP version 14 (SAS Institute, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
Independent factors for poor CSS rate
and ERFS rate
The Cox proportional hazards model indicated that
tumor histology, pathological T stage, tumor grade, lymph node
metastasis, positive surgical margins, LVI, and the presence of
hydronephrosis were independent factors for poor CSS and ERFS
ratesin univariate analysis, and positive surgical margins and LVI
were independent predictors of worse CSS (P=0.035, P=0.001,
respectively) and ERFS (P<0.001, P=0.003, respectively) rates in
multivariate analysis among pathological factors (Tables II and III). Finally, cancer-specific mortality
and extraurothelial recurrence were found in 37 and 41 patients,
respectively.
 | Table IIUnivariate and multivariate analyses
of independent factors for cancer-specific survival. |
Table II
Univariate and multivariate analyses
of independent factors for cancer-specific survival.
| | Univariate | Multivariate |
|---|
| Pathological
parameters | HR | P-value | HR | 95% CI | P-value |
|---|
| Age (≥71 or
≤70) | 1.436 | 0.272 | | | | | |
| Sex (male or
female) | 0.836 | 0.623 | | | | | |
| Urine cytology
(positive or negative) | 1.416 | 0.313 | | | | | |
| Tumor histology (UC
with other components or UC alone) | 3.099 | 0.003 | 1.762 | 0.738 | - | 3.969 | 0.195 |
| Pathological T
stage (≥T3 or ≤T2) | 4.275 | <0.001 | 1.624 | 0.710 | - | 4.017 | 0.258 |
| Tumor grade (high
or PUNLMP/low) | 4.824 | <0.001 | 2.549 | 0.926 | - | 9.012 | 0.072 |
| Lymph node
metastasis (positive or negative) | 4.320 | 0.006 | 0.720 | 0.230 | - | 2.029 | 0.544 |
| Ureter involvement
(positive or negative) | 1.652 | 0.143 | | | | | |
| Surgical margins
(positive or negative) | 3.281 | 0.003 | 2.388 | 1.064 | - | 5.083 | 0.035 |
| Lymphovascular
invasion (positive or negative) | 7.570 | <0.001 | 3.965 | 1.688 | - | 9.903 | 0.001 |
| Carcinoma in
situ (positive or negative) | 0.582 | 0.333 | | | | | |
| Hydronephrosis
(positive or negative) | 2.144 | 0.035 | 1.227 | 0.562 | - | 2.903 | 0.618 |
 | Table IIIUnivariate and multivariate analyses
of independent factors for recurrence-free survival. |
Table III
Univariate and multivariate analyses
of independent factors for recurrence-free survival.
| | Univariate | Multivariate |
|---|
| Pathological
parameters | HR | P-value | HR | 95% CI | P-value |
|---|
| Age (≥71 or
≤70) | 1.499 | 0.197 | | | | | |
| Sex (men or
women) | 0.698 | 0.295 | | | | | |
| Urine cytology
(positive or negative) | 1.150 | 0.664 | | | | | |
| Tumor histology (UC
with other components or UC alone) | 3.625 | <0.001 | 2.178 | 0.993 | - | 4.643 | 0.052 |
| Pathological T
stage (≥T3 or ≤T2) | 4.461 | <0.001 | 2.093 | 0.965 | - | 4.859 | 0.062 |
| Tumor grade (high
or PUNLMP/low) | 5.426 | <0.001 | 3.182 | 1.162 | - | 11.223 | 0.023 |
| Lymph node
metastasis (positive or negative) | 6.713 | <0.001 | 1.338 | 0.495 | - | 3.283 | 0.547 |
| Ureter involvement
(positive or negative) | 1.751 | 0.086 | | | | | |
| Surgical margins
(positive or negative) | 3776 | <0.001 | 5.102 | 2.318 | - | 10.833 | <0.001 |
| Lymphovascular
invasion (positive or negative) | 6.822 | <0.001 | 3.147 | 1.480 | - | 7.000 | 0.003 |
| Carcinoma in
situ (positive or negative) | 0.541 | 0.263 | | | | | |
| Hydronephrosis
(positive or negative) | 2.288 | 0.016 | 1.438 | 0.685 | - | 3.240 | 0.345 |
Association between
clinicopathological factors and the presence of LVI
Out of these two pathological factors, LVI was
expected to be more frequently seen in surgical specimens unless
pathological T stage was equal to or higher than 3. Therefore, we
investigated the association between preoperative factors and the
presence of LVI. Positive cytology, the presence of hydronephrosis,
C-reactive protein levels, NLR, PLR, and plasma fibrinogen levels
were significantly associated with the presence of LVI among
preoperative factors in Fisher's exact probability test (Table IV). Among these related factors,
positive cytology, the presence of hydronephrosis, and plasma
fibrinogen levels were significant preoperative predictors of the
presence of LVI (P=0.008, P=0.010, P<0.001, respectively)
(Table V). In fact, patients with
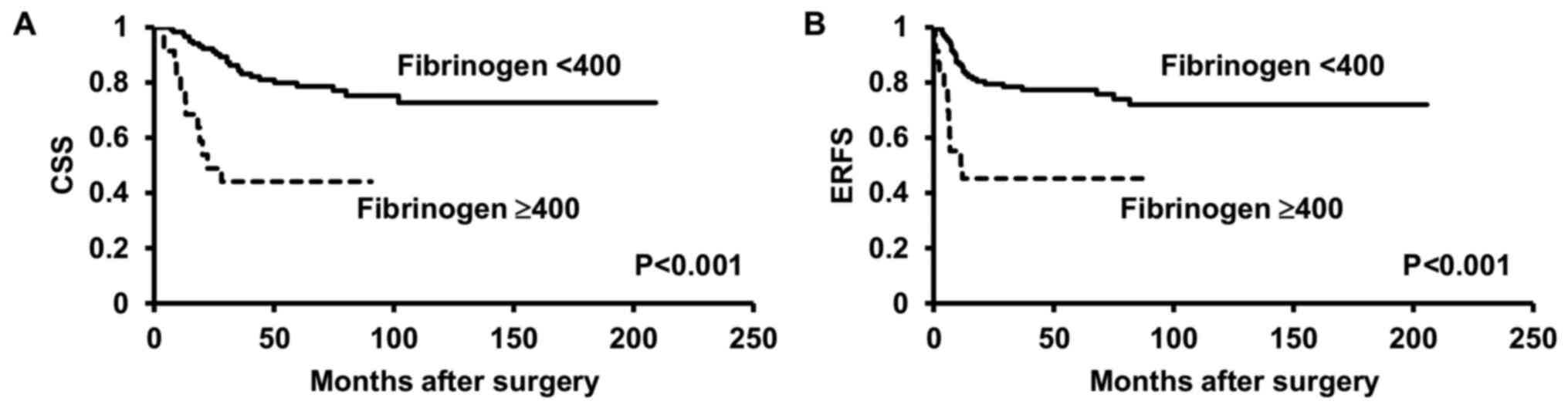
higher fibrinogen levels (≥400 mg/dl) had worse CSS and ERFS rates
(both P<0.001) (Fig. 1).
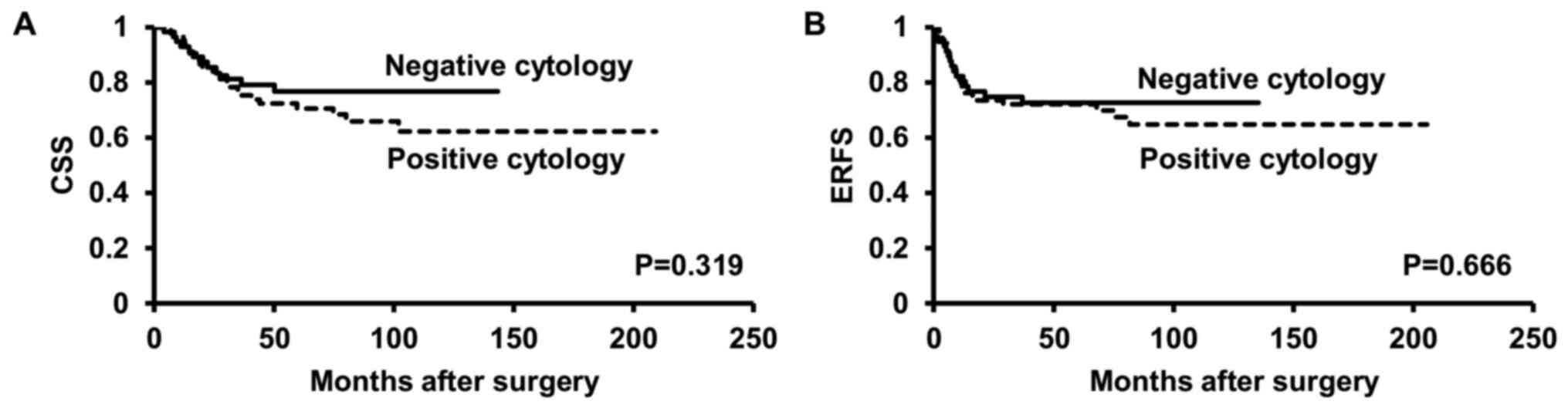
Patients with positive urine cytology did not have poor CSS or ERFS
rates when compared with those with negative cytology (P=0.319,
P=0.666, respectively) (Fig. 2).
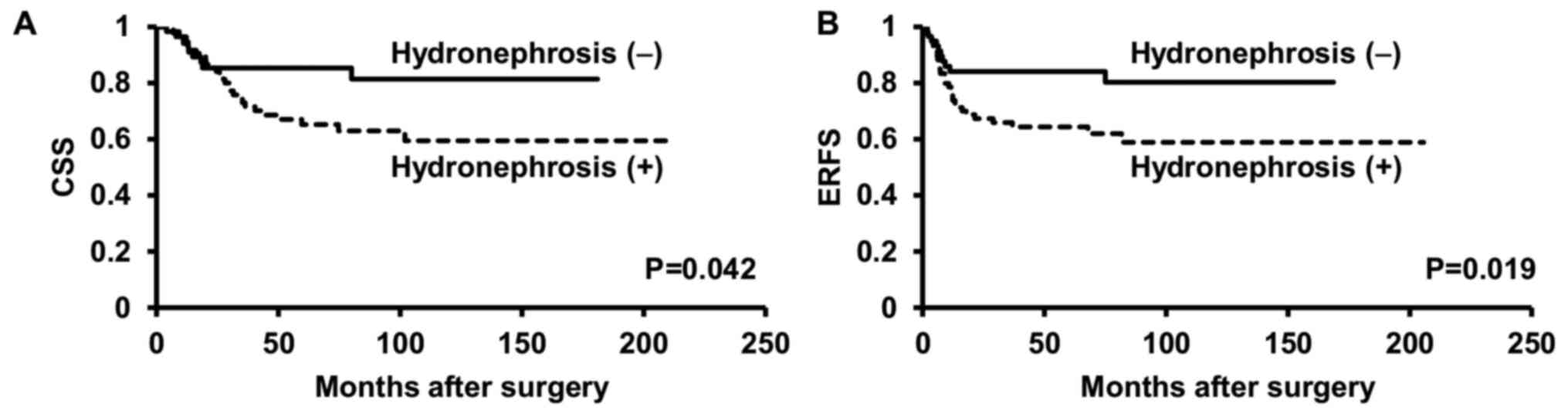
Patients with hydronephrosis had worse CSS and ERFS rates than
those without hydronephrosis (P=0.042, P=0.019, respectively)
(Fig. 3).
 | Table IVAssociation between preoperative
parameters and LVI. |
Table IV
Association between preoperative
parameters and LVI.
| A,
Clinicopathological parameters |
|---|
| | LVI | |
|---|
| Parameter | Total | (%) | Positive | (%) | Negative | (%) | P-value |
|---|
| Age (median
70) | | | | | | | 0.492 |
|
≥71 | 71 | (49.0) | 23 | (32.4) | 48 | (67.6) | |
|
≤70 | 74 | (51.0) | 28 | (37.8) | 46 | (62.2) | |
| Sex | | | | | | | 0.085 |
|
Male | 109 | (75.2) | 34 | (31.2) | 75 | (68.8) | |
|
Female | 36 | (24.8) | 17 | (47.2) | 19 | (52.8) | |
| Urine cytology | | | | | | | 0.029 |
|
Positive | 88 | (60.7) | 37 | (42.1) | 51 | (57.9) | |
|
Negative | 57 | (39.3) | 14 | (24.6) | 43 | (75.4) | |
| Hydronephrosis | | | | | | | 0.005 |
|
Positive | 86 | (59.3) | 38 | (44.2) | 48 | (55.8) | |
|
Negative | 59 | (40.7) | 13 | (22.0) | 46 | (78.0) | |
| B, Laboratory
parameters |
| | LVI | |
| Parameter | Total | (%) | Positive | (%) | Negative | (%) | P-value |
| Laboratory
parameters | | | | | | | |
| CRP (≥0.4 or
≤0.3) | | | | | | | 0.015 |
|
≥0.4 | 34 | (23.5) | 18 | (52.9) | 16 | (47.1) | |
|
≤0.3 | 111 | (76.5) | 33 | (29.7) | 78 | (70.3) | |
| Albumin (≤3.7 or
≥3.8) | | | | | | | 0.110 |
|
≤3.7 | 17 | (11.7) | 9 | (52.9) | 8 | (47.1) | |
|
≥3.8 | 128 | (88.3) | 42 | (32.8) | 86 | (67.2) | |
| NLR (≥1.652 or
<1.652) | | | | | | | <0.001 |
|
≥1.652 | 114 | (78.6) | 48 | (42.1) | 66 | (57.9) | |
|
<1.652 | 31 | (21.4) | 3 | (9.7) | 28 | (90.3) | |
| PLR (≥154.122 or
<154.122) | | | | | | | 0.005 |
|
≥154.122 | 60 | (41.4) | 29 | (48.3) | 31 | (51.7) | |
|
<154.122 | 85 | (58.6) | 22 | (25.9) | 63 | (74.1) | |
| GPS (≥1 or 0) | | | | | | | 0.128 |
|
≥1 | 15 | (10.3) | 8 | (53.3) | 7 | (46.7) | |
|
0 | 130 | (89.7) | 43 | (33.1) | 87 | (66.9) | |
| Fibrinogen (≥400 or
<400) | | | | | | | <0.001 |
|
≥400 | 23 | (15.9) | 19 | (82.6) | 4 | (17.4) | |
|
<400 | 122 | (84.1) | 32 | (26.2) | 90 | (73.8) | |
 | Table VUnivariate and multivariate analyses
of preoperative factors for the presence of LVI. |
Table V
Univariate and multivariate analyses
of preoperative factors for the presence of LVI.
| | Univariate | Multivariate |
|---|
| Preoperative
parameters | OR | P-value | OR | 95% CI | P-value |
|---|
| Urine cytology
(positive or negative) | 2.228 | 0.033 | 3.439 | 1.373 | - | 8.613 | 0.008 |
| Hydronephrosis
(present or absent) | 2.801 | 0.007 | 3.398 | 1.334 | - | 8.654 | 0.010 |
| CRP (≥0.4 or
≤0.3) | 2.659 | 0.015 | 0.842 | 0.286 | - | 2.479 | 0.756 |
| Albumin (≤3.7 or
≥3.8) | 2.304 | 0.110 | | | | | |
| NLR (≥1.652 or
<1.652) | 6.788 | 0.003 | 4.158 | 0.971 | - | 17.794 | 0.055 |
| PLR (≥154.122 or
<154.122) | 2.679 | 0.006 | 1.131 | 0.479 | - | 2.673 | 0.778 |
| GPS (≥1 or 0) | 2.312 | 0.128 | | | | | |
| Fibrinogen (≥400 or
<400) | 13.359 | <0.001 | 14.702 | 3.799 | - | 56.900 | <0.001 |
Preoperative risk stratification using
plasma fibrinogen levels and other risk factors to predict worse
CSS and ERFS rates
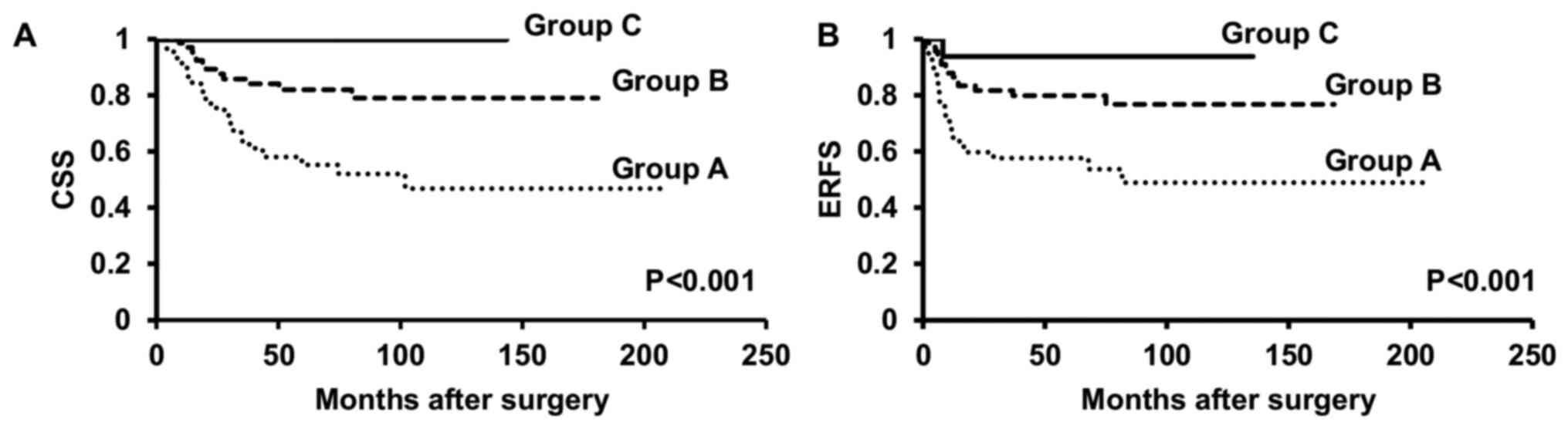
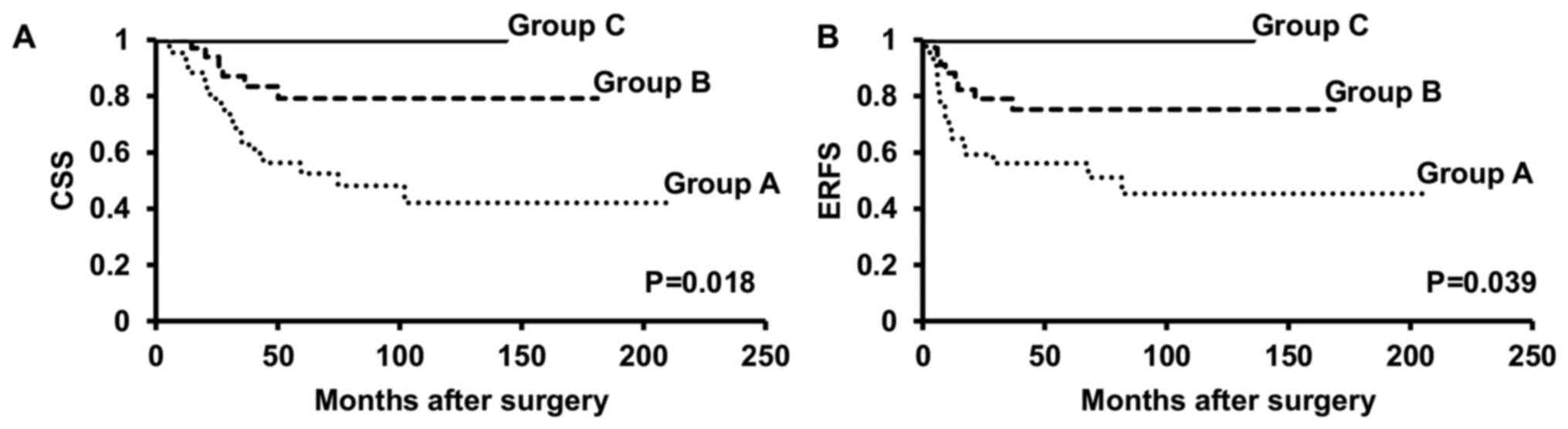
Based on these findings, we stratified patients into
three groups. Group A was comprised by patients exhibiting two or
more of higher fibrinogen levels (≥400 mg/dl), positive urine
cytology, and the presence of hydronephrosis. Group B consisted of
patients with only one of those factors, and Group C consisted of
those with none of the three factors. Group A patients showed worse
CSS and ERFS rates than those in Groups B and C (both P<0.001,
Fig. 4). Moreover, we further
divided patients into those with renal pelvic cancer alone and
those with renal pelvic and/or ureteral cancer. Kaplan-Meier curves
revealed that in both patients with renal pelvic cancer alone and
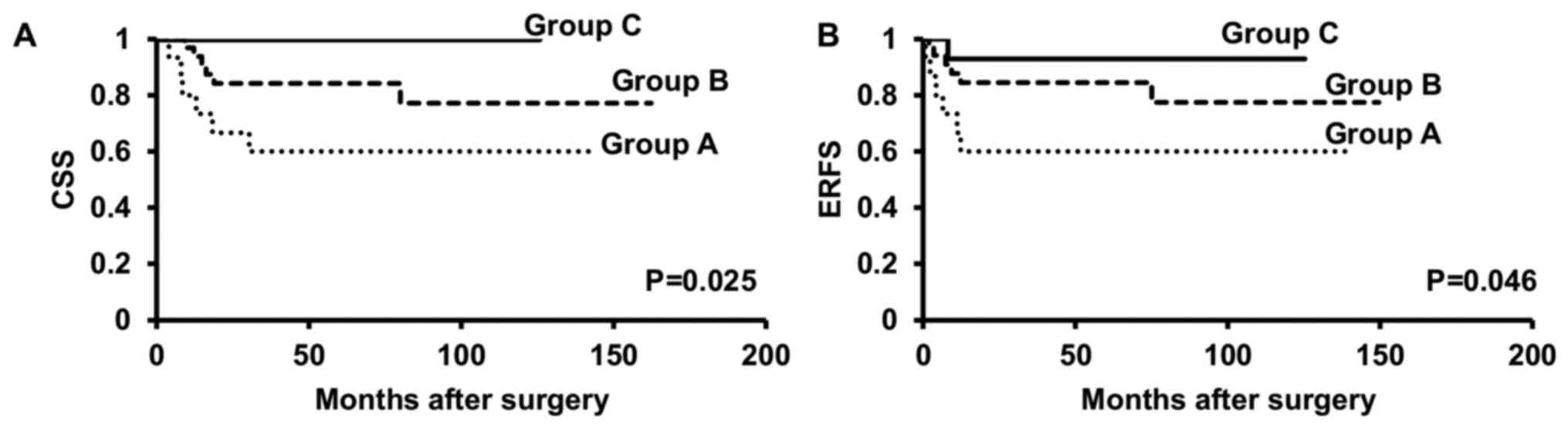
those with renal pelvic and/or ureteral cancer, patients of Group A
had significantly worse CSS (P=0.025, P=0.018, respectively) and
ERFS (P=0.046, P=0.039, respectively) rates than those in Groups B
and C (Figs. 5 and 6).
Discussion
In this study, a Cox proportional hazards model
demonstrated that positive surgical margins and LVI were
independent predictors of shorter CSS and ERFS time (Tables II and III). In addition, we found that
fibrinogen levels, positive urine cytology, and the presence of
hydronephrosis were significant factors associated with the
presence of LVI (Tables IV and
V). In fact, patients exhibiting
two or more of these factors showed worse CSS or ERFS rates than
those exhibiting only one factor or those exhibiting none of the
three factors (Fig. 4). These
results were also consistent in patients with renal pelvic cancer
alone and those with renal pelvic and/or ureteral canceras seen in
all patients (Figs. 5 and 6). In addition, plasma fibrinogen levels
are associated with other diseases (e.g., atherosclerosis,
hypertension, and stroke). However, histories of such severe
diseases were not found in patients enrolled in the present study.
It is speculated that plasma fibrinogen levels were only predictors
of worse CSS and ERFS in this study.
Several studies have indicated the association
between elevated preoperative plasma fibrinogen levels and poor
prognosis in a number of cancers by using meta-analyses (23-25).
Earlier studies have also revealed that the metastatic status can
potentiallyoccur in the environment of circulating tumor cells
which may be caused by high plasma fibrinogen levels (26). Furthermore, preoperative plasma
fibrinogen levels may berelated to tumor volume and may provide
favorable conditions for circulating tumor cells to metastasize
through the lymphatic drainage orvascular flow (12,23). A
preliminary study showed that mice lacking fibrinogen had a
remarkably decreased level of lymphatic and hematogenous metastases
compared to that observed in wild-type mice. They suggested that an
increase in plasma fibrinogen levels can play an important role in
cancer metastasis (26). Tanaka
et al especially showed that patients with malignant tumors
and preoperative high plasma fibrinogen levels have a higher risk
for a worse prognosis than those with low fibrinogen levels in
patients with UTUC (20). Hence,
the results of the present study concur with the results reported
by Tanaka et al and others.
The prognostic value of chronic kidney disease and
positive surgical margins in UTUC patients undergoing RNU was
previously reported (27). However,
preoperative plasma fibrinogen levels were not independent
predictors of worse CSS or ERFS because the patient group of the
previous study was different from that of the present study. In
addition, the cut-off value of plasma fibrinogen levels also
differed from that used in the present study (27).
Questions remain on how fibrinogen can influence the
progression and metastatic possibility ofmalignant tumors. Several
tentative theories have been reported on this. First, fibrinogen
may aggregate around cancer cells to work as a structure for cancer
cell proliferation. Fibrinogen can also serve as a basement to
sustain some growth factors, such as vascular endothelial growth
factor and fibroblast growth factor, to promote tumor angiogenesis
(25). Second, tumor cells retain
fibrinogen receptors, which play a role in bridging fibrinogen
molecules to tumor cells and thus enhance the endothelial adhesion
of tumor cell emboli in a target organ's vasculature. This
mechanism appears to enable metastasis and successively increase
the survival rates of metastatic cells (24). Third, fibrinogen can enhance the
adhesion of platelets to tumor cells, mediated by β3-integrin which
was expressed by tumor cells. The aggregation of fibrinogen can be
proceeded by platelet migration to the tumor cell environment; a
thick layer of fibrin may then be formed to provide protection of
the tumor cells against natural killer cell cytotoxicity, resulting
in a higher possibility of distant metastasis (28). Moreover, fibrinogen can be
integrated by cancer cells endogenously. This event can lead to the
proliferation of cancer cells and angiogenesis, caused by
fibroblast growth factor-2, which induces cancer progression and
metastasis (29).
Positive urine cytology and the presence of
hydronephrosis have been associated with the presence of LVI.
Sakano et al showed that positive urine cytology and
hydronephrosis were significantly associated with the presence of
LVI (30). Ito et al also
indicated that a higher hydronephrosis grade had a significant
correlation with the presence of ureteral tumors, a higher pT
stage, and the presence of LVI (31). Another study also showed that
patients with positive voided urine cytology had higher incidences
of high-grade tumors and positive LVI in UTUC patients treated with
RNU (32). These findings are
consistent with those of the present study, although neither
positive urine cytology nor hydronephrosis was found as an
independent factor for worse CSS or ERFS rates. However, using
plasma fibrinogen levels in conjunction with these
clinicopathological factors couldmore accurately predict LVI and a
poorer prognosis in UTUC, as described in an earlier study
evaluating the effectiveness of a two-factor risk stratification
model (33).
This study had some limitations. First, it was
retrospective in nature, and our sample size was relatively small;
however, the median follow-up period after RNU was more than four
years, and so our observation time to cancer-specific death and
extraurothelial recurrence was long enough to support our findings.
Second, the underlying mechanism explaining the association of
positive urine cytology or hydronephrosis with the presence of LVI
remains unclear. As to hydronephrosis, its presence may imply
tumoral extension to the outer urothelial layers, which may be an
indicator of cancer cells spreading to the reginal nodes or distant
organs. Considering that the urothelial lumen under hydronephrosis
is thinner than normal lumen, the spreading of urothelial carcinoma
cells beyond the urothelial layers is possible. The increased
pressure in the urothelial lumen may also lead to counterflow in
lymphatics and blood vessels, increasing the possibility of cancer
cell migration (34). Furthermore,
we could not validate the efficacy of positive urine cytology as an
independent predictor of the presence of LVI, although several
reports suggest a significant relationship between positive urine
cytology and the presence of LVI (30,32).
Despite these limitations, our data suggests that a high plasma
fibrinogen level can be an independent prognosticator for worse CSS
and ERFS in patients with UTUC undergoing RNU.
In conclusion, stratifying preoperative risk using
fibrinogen levels, positive urine cytology, and the presence of
hydronephrosis before RNU can provide additional information
aboutthe possibility of worse CSS and ERFS rates in patients with
localized UTUC. This finding can serve as a basis for selecting
candidates for additional therapy before and/or after RNU in
patients with UTUC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KK, ST, JA, AH and KI were involved in the
conception and design of the study. KK collected and analyzed the
data. KK drafted the manuscript. The authenticity of all the raw
data was assessed by KK and KI. KK and KI reviewed and edited the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed in this study were approved
bythe National Defense Medical College (Saitama, Japan; approval
no. 2734). All procedures were conducted in accordance with the
1964 Declaration of Helsinki and its later amendments.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Roupret M, Babjuk M, Comperat E, Zigeuner
R, Sylvester RJ, Burger M, Cowan NC, Böhle A, Van Rhijn BW,
Kaasinen E, et al: European association of urology guidelines on
upper urinary tract urothelial cell carcinoma: 2015 update. Eur
Urol. 68:868–879. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Liu JY, Dai YB, Zhou FJ, Long Z, Li YH,
Xie D, Liu B, Tang J, Tan J, Yao K and He LY: Laparoscopic versus
open nephroureterectomy to treat localized and/or locally advanced
upper tract urothelial carcinoma: Oncological outcomes from a
multicenter study. BMC Surg. 17(17)2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Templeton AJ, Ace O, McNamara MG,
Al-Mubarak M, Vera-Badillo FE, Hermanns T, Seruga B, Ocaña A,
Tannock IF and Amir E: Prognostic role of platelet to lymphocyte
ratio in solid tumors: A systematic review and meta-analysis.
Cancer Epidemiol Biomarkers Prev. 23:1204–1212. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Teng JJ, Zhang J, Zhang TY, Zhang S and Li
BS: Prognostic value of peripheral blood lymphocyte-to-monocyte
ratio in patients with solid tumors: A meta-analysis. Onco Targets
Ther. 9:37–47. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Koh CH, Bhoo-Pathy N, Ng KL, Jabir RS, Tan
GH, See MH, Jamaris S and Taib NA: Utility of pre-treatment
neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as
prognostic factors in breast cancer. Br J Cancer. 113:150–158.
2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gunduz S, Mutlu H, Tural D, Yildiz O,
Uysal M, Coskun HS and Bozcuk H: Platelet to lymphocyte ratio as a
new prognostic for patients with metastatic renal cell cancer. Asia
Pac J Clin Oncol. 11:288–292. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Allin KH and Nordestgaard BG: Elevated
C-reactive protein in the diagnosis, prognosis, and cause of
cancer. Crit Rev Clin Lab Sci. 48:155–170. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kang M, Jeong CW, Kwak C, Kim HH and Ku
JH: Preoperative neutrophil-lymphocyte ratio can significantly
predict mortality outcomes in patients with non-muscle invasive
bladder cancer undergoing transurethral resection of bladder tumor.
Oncotarget. 8:12891–12901. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yuksel OH, Akan S, Uukmez A, Yildirim C,
Sahin A and Verit A: Preoperative Glasgow prognostic score as a
predictor of primary bladder cancer recurrence. Mol Clin Oncol.
5:201–206. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yamashita H, Kitayama J, Kanno N, Yatomi Y
and Nagawa H: Hyperfibrinogenemia is associated with lymphatic as
well as hematogenous metastasis and worse clinical outcome in T2
gastric cancer. BMC Cancer. 6(147)2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yamashita H, Kitayama J and Nagawa H:
Hyperfibrinogenemia is a useful predictor for lymphatic metastasis
in human gastric cancer. Jpn J Clin Oncol. 35:595–600.
2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Palumbo JS, Potter JM, Kaplan LS, Talmage
K, Jackson DG and Degen JL: Spontaneous hematogenous and lymphatic
metastasis, but not primary tumor growth or angiogenesis, is
diminished in fibrinogen-deficient mice. Cancer Res. 62:6966–6972.
2002.PubMed/NCBI
|
|
14
|
Fan S, Guan Y, Zhao G and An G:
Association between plasma fibrinogen and survival in patients with
small-cell lung carcinoma. Thorac Cancer. 9:146–151.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pichler M, Hutterer GC, Stojakovic T,
Mannweiler S, Pummer K and Zigeuner R: High plasma fibrinogen level
represents an independent negative prognostic factor regarding
cancer-specific, metastasis-free, as well as overall survival in a
European cohort of non-metastatic renal cell carcinoma patients. Br
J Cancer. 109:1123–1129. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Erdem S, Amasyali AS, Aytac O, Onem K,
Issever H and Sanli O: Increased preoperative levels of plasma
fibrinogen and d dimer in patients with renal cell carcinoma is
associated with poor survival and adverse tumor characteristics.
Urol Oncol. 32:1031–1040. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sobin LH, Gospodarowicz MK and Wittekind
Ch (eds): TNM Classification of Malignant Tumours, 7th Edition.
Wiley-Blackwell, Hoboken, NJ, 2009.
|
|
18
|
The International Agency for Research on
Cancer (IARC): Pathology and Genetics of Tumours of the Urinary
System and Male Genital Organs (IARC WHO Classification of Tumours)
1st Edition. Eble J, Epstein J, Sesterhenn I and Sauter G (eds).
World Health Organization, Geneva, 2004.
|
|
19
|
Qu J, Qu X, Li Z, Zhang Jd, Liu J, Teng
Ye, Jin B, Zhao Mf, Yu P, Shi J, et al: Prognostic model based on
systemic inflammatory response and clinicopathological factors to
predict outcome of patients with node-negative gastric cancer. PLoS
One. 10(0128540)2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tanaka N, Kikuchi E, Matsumoto K, Hayakawa
N, Ide H, Miyajima A, Nakamura S and Oya M: Prognostic value of
plasma fibrinogen levels in patients with localized upper tract
urothelial carcinoma. BJU Int. 111:857–864. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kasuga J, Kawahara T, Takamoto D, Fukui S,
Tokita T, Tadenuma T, Narahara M, Fusayasu S, Terao H, Izumi K, et
al: Increased neutrophil-to-lymphocyte ratio is associated with
disease-specific mortality in patients with penile cancer. BMC
Cancer. 16(396)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Uemura K, Kawahara T, Yamashita D, Jikuya
R, Abe K, Tatenuma T, Yokomizo Y, Izumi K, Teranishi JI, Makiyama
K, et al: Neutrophil-to-lymphocyte ratio predicts prognosis in
castration-resistant prostate cancer patients who received
cabazitaxel chemotherapy. Biomed Res Int.
2017(7538647)2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lee JH, Ryu KW, Kim S and Bae JM:
Preoperative plasma fibrinogen levels in gastric cancer patients
correlate with extent of tumor. Hepatogastroenterology.
51:1860–1863. 2004.PubMed/NCBI
|
|
24
|
HKitayama JY, Hatano K, Tsuno N, Osada T,
Watanabe T, Tsuruo T, Muto T and Nagawa H: Clustered cancer cells
show a distinct adhesion behavior from single cell form under
physiological shear conditions. J Exp Clin Cancer Res. 20:407–412.
2001.PubMed/NCBI
|
|
25
|
Çalışkan S and Sungur M: Fibrinogen and
D-dimer levels in prostate cancer: Preliminary results. Prostate
Int. 5:110–112. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Palumbo JS, Kombrinck KW, Drew AF, Grimes
TS, Kiser JH, Degen JL and Bugge TH: Fibrinogen is an important
determinant of the metastatic potential of circulating tumor cells.
Blood. 96:3302–3309. 2000.PubMed/NCBI
|
|
27
|
Kuroda K, Asakuma J, Horiguchi A,
Kawaguchi M, Shinchi M, Masunaga A, Tasaki S, Sato A and Ito K:
Chronic kidney disease and positive surgical margins as
prognosticators for upper urinary tract urothelial carcinoma
patients undergoing radical nephroureterectomy. Mol Clin Oncol.
10:547–554. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bagoly Z: Cancer and thrombosis: A fresh
look at an old story. Thromb Res. 136:1–2. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Thurner EM, Krenn-Pilko S, Langsenlehner
U, Stojakovic T, Pichler M, Gerger A, Kapp KS and Langsenlehner T:
The association of an elevated plasma fibrinogen level with
cancer-specific and overall survival in prostate cancer patients.
World J Urol. 33:1467–1473. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sakano S, Inamoto T, Inoue R, Matsumoto H,
Nagao K, Yamamoto Y, Azuma H and Matsuyama H: Positive voided urine
cytology predicts worse pathological findings of nephroureterectomy
specimens in patients with upper tract urothelial carcinoma: Does
selective ureteral cytology have an additional efficacy? Jpn J Clin
Oncol. 45:968–972. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ito Y, Kikuchi E, Tanaka N, Miyajima A,
Mikami S, Jinzaki M and Oya M: Preoperative hydronephrosis grade
independently predicts worse pathological outcomes in patients
undergoing nephroureterectomy for upper tract urothelial carcinoma.
J Urol. 185:1621–1626. 2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tanaka N, Kikuchi E, Kanao K, Matsumoto K,
Shirotake S, Kobayashi H, Miyazaki Y, Ide H, Obata J, Hoshino K, et
al: The predictive value of positive urine cytology for outcomes
following radical nephroureterectomy in patients with primary upper
tract urothelial carcinoma: A multi-institutional study. Urol
Oncol. 32:e19–e26. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kohada Y, Hayashi T, Goto K, Kobatake K,
Abdi H, Honda Y, Sentani K, Inoue S, Teishima J, Awai K, et al:
Preoperative risk classification using neutrophil-lymphocyte ratio
and hydronephrosis for upper tract urothelial carcinoma. Jpn J Clin
Oncol. 48:841–850. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chung PH, Krabbe LM, Darwish OM, Westerman
ME, Bagrodia A, Gayed BA, Haddad AQ, Kapur P, Sagalowsky AI, Lotan
Y and Margulis V: Degree of hydronephrosis predicts adverse
pathological features and worse oncologic outcomes in patients with
high-grade urothelial carcinoma of the upper urinary tract. Urol
Oncol Semin Orig Investig. 32:981–988. 2014.PubMed/NCBI View Article : Google Scholar
|