Introduction
For patients with locally advanced and/or recurrent
breast cancer, many multidisciplinary approaches, including
hormonal therapy (1), irradiation
(2), surgery (3), chemotherapy (4) and molecular target treatment (5), are used. However, patients with
advanced breast cancer receiving prolonged periodic standard
treatments may become refractory to many of them, thereby limiting
the application of these therapeutic modalities. Moreover, the
adverse effects of therapy, such as general fatigue, multiple organ
failure (lung, liver, kidney, bone, etc.) and/or pancytopenia
(particularly neutrophil and platelet deficiency) may affect the
general condition of the patient. As a result, it may be difficult
or impossible to continue curative therapy. In case of widespread
metastases, the risk is even higher.
Hyperthermia is one of the conventional forms of
cancer therapy that forces the tumor to become necrotic due to
absorbed heat. The dose is expressed in cumulative equivalent min
for necrotic processes at 43˚C (CEM43˚CTx) (6) and is calibrated for necrotic cell
death at 43˚C in x percentage of the homogeneity of the actually
measured T temperature. The missing technology of the isothermal
heating of the heterogenic tumor mass and the necessity of
performing in-depth temperature measurements of the tumor makes the
treatment complicated, and insufficient results prevent its
widespread acceptance in the clinic (7). Therefore, our hospital conducts
clinical studies using modulated electro-hyperthermia (mEHT) as one
of the new options for the multidisciplinary treatment of advanced
cancer. mEHT (8,9), is a new treatment modality developed
to overcome the problems of traditional hyperthermia; it uses a
precise impedance-matched system and modulated radiofrequency
current flow to malignant tumors (10), which select the malignant cells
based on their biophysical differences, due to their high metabolic
rate, individual (autonomic) behavior and membrane status (11).
mEHT has been successfully used for the treatment of
patients with various stages and forms of breast cancer, including
in a retrospective study with 103 patients (12), a case of primary leiomyosarcoma of
the breast following salvage hyperthermia and pazopanib therapy
(13), and a case of long-term
survival of a breast cancer patient with extensive liver metastases
upon immunotherapy and virotherapy (14).
The aim of this study was to present a clinical
study of patients with advanced metastatic breast cancer who fail
to respond to standard conventional therapies.
Patients and methods
Patients
Ten patients with advanced or recurrent breast
cancer participated in the present study since November 2015. All
patients had undergone conventional therapies following standard
protocols for breast cancer. Patients received hormonal therapy,
external irradiation, surgery, various chemotherapies, targeted
molecular treatment, and other available state of the art therapies
(15). The selected patients were
treated with mEHT coupled with adjuvant therapies (chemotherapy,
hormone therapy or irradiation) when possible (6 cases); in case of
complete failure of conventional methods, monotherapy was used (4
cases). The adjuvant therapies were trastuzumab emtansine (TDM-1; 1
case), mammalian target of rapamycin (mTOR; 3 cases), eribulin (1
case), irradiation (1 case) and fulvestrant (1 case). The study was
approved by the local ethics committee of the University of Toyama
(approval no. 26-13), and the patients provided written consent for
the treatment, as well as for the research and publication of their
data and images.
Procedure of mEHT
mEHT was performed twice a week in 7 patients and
thrice a week in the other 3. The session lasted for ~60 min, with
at least 1 day in between. The treatment was performed using the
EHY2000+ device (Oncotherm Kft.). The electrode used was 30 cm in
diameter. Patients were placed in the supine position on the water
mattress of the treatment bed. A step-up heating protocol was used,
starting with 60 W, which was then increased to 140 W. The average
number of treatments performed per patient was 48.6 (range, 8-90).
The average dose of 374.6 (range, 371-376) kJ was administered.
Procedure and display of the
analytical results
The endpoint of the study was local control
(response rate). A follow-up examination of local control was
conducted via inspection, computed tomography (CT), or magnetic
resonance imaging, and was compared with that at baseline before
the start of the mEHT treatment process. The age, estrogen receptor
(ER)/progesterone receptor (PgR)/human epidermal growth factor
receptor type 2 (HER2) status, actual status of metastases, and
pretreatment for each patient are shown in Table I. The number and duration of mEHT
sessions and the total amount of mEHT energy delivered to each
patient are summarized in Table
II. The complementary therapies and local responses are shown
in Table III. The statistical
analysis results are shown in Table
IV.
 | Table IPatient statistics and metastasis. |
Table I
Patient statistics and metastasis.
| Case | Age, years | Surgery | Stage | ER | PgR | HER2 | Metastasis | CT | HT | RT | Total |
|---|
| 1 | 58 | Bp+Ax | 2A | + | + | + | Skin, lung, LN | 4 | 1 | 1 | 6 |
| 2 | 66 | Bt+Ax | 3A | + | + | - | Lung, liver,
bone | 7 | 2 | 1 | 10 |
| 3 | 63 | Bt+Ax | 2B | + | + | - | Lung, LN | 0 | 2 | 0 | 2 |
| 4 | 45 | Bp+Ax | 2B | + | + | - | Bone, LN | 1 | 2 | 2 | 5 |
| 5 | 74 | Bt+SLN | 1 | + | + | - | Liver, bone,
LN | 2 | 1 | 0 | 3 |
| 6 | 68 | (-) | 4 | + | - | - | Lung, liver,
bone | 0 | 4 | 2 | 6 |
| 7 | 75 | (-) | 4 | + | - | - | Skin, lung | 3 | 0 | 1 | 4 |
| 8 | 49 | Bt+Ax | 4 | + | + | - | Lung | 0 | 2 | 0 | 2 |
| 9 | 66 | Bt+Ax | 2A | + | - | - | Lung, liver,
bone | 4 | 2 | 1 | 7 |
| 10 | 71 | Bt+Ax | 3A | + | + | - | Skin, muscle | 0 | 0 | 0 | 0 |
 | Table IIStatistics of the mEHT
treatments. |
Table II
Statistics of the mEHT
treatments.
| Case | Total mEHT, n | mEHT/w, n | mEHT period,
weeks | mEHT dose, kJ |
|---|
| 1 | 36 | 3 | 12 | 13,464 |
| 2 | 90 | 3 | 30 | 33,660 |
| 3 | 47 | 2 | 23.5 | 17,578 |
| 4 | 87 | 2 | 43.5 | 32,538 |
| 5 | 73 | 3 | 24.3 | 27,302 |
| 6 | 46 | 2 | 23 | 17,204 |
| 7 | 8 | 2 | 4 | 2,992 |
| 8 | 40 | 2 | 20 | 14,960 |
| 9 | 15 | 2 | 7.5 | 5,610 |
| 10 | 44 | 2 | 22 | 16,456 |
 | Table IIIComplementary therapies and local
responses. |
Table III
Complementary therapies and local
responses.
| Case | Combination | Response | CEA1, ng/ml | CEA2, ng/ml |
|---|
| 1 | TDM-1 | PR | 8.1 | 5.1 |
| 2 | mTOR, PTX+BV | PD | 292.8 | 209.8 |
| 3 | (-) | SD | 3.6 | 4.5 |
| 4 | mTOR, Erib.,
PTX+BV | PD | 115.1 | 262.7 |
| 5 | (-) | PD | 12.9 | 45.1 |
| 6 | mTOR | PD | 145.5 | 624.8 |
| 7 | irradiation | PR | 3.7 | 3.8 |
| 8 | (-) | SD | 3.2 | 3.5 |
| 9 | AI,
Fulvestrant | SD | 3.9 | 4.0 |
| 10 | (-) | PR | 10.6 | 2.1 |
 | Table IVResults of statistical analysis. |
Table IV
Results of statistical analysis.
| Factor | PR+SD | PD | P-value |
|---|
| Age, years | 63.6 | 63.3 | 0.953 |
| Stage | | | 0.737 |
| 1 | 0 | 1 | |
| 2 | 3 | 1 | |
| 3 | 1 | 1 | |
| 4 | 2 | 1 | |
| Total mEHT, n | 31.6 | 74.0 | 0.006 |
| mEHT/w, n | 2.0 | 2.5 | 0.312 |
| mEHT period,
weeks | 14.8 | 30.2 | 0.002 |
| mEHT dose, kJ | 11,843 | 27,676 | 0.002 |
| Pre-treat,
total | 3.5 | 6.0 | 0.199 |
| Pre-CT, n | 1.8 | 2.5 | 0.689 |
| Pre-CEA, ng/ml | 5.51 | 141.5 | 0.017 |
| Post-CEA,
ng/ml | 3.85 | 285.6 | 0.009 |
Statistical analysis
The comparison of the distribution between the two
groups of partial response (PR)+stable disease (SD) cases and
progressive disease (PD) cases was conducted using unpaired t-test
for continuous variables [age, total mEHT, mEHT/w, mEHT period,
mEHT dose, pre-treatment, pre-CT, pre-carcinoembryonic antigen
(CEA) and post-CEA] and the Mann-Whitney test for categorical
variables (Stage). P<0.05 was considered to indicate a
statistically significant difference. All analyses were performed
using the JMP15.0 software.
Results
Statistics of mEHT
Out of the 10 cases registered, 5 were stage 3 or 4
preoperatively (Table I). The ER
status was positive in all cases, and HER2 was positive in 1 case.
In 9/10 cases, some treatments were performed before mEHT; however,
due to the lack of a satisfactory antitumor effect, mEHT was
performed or combined with other treatments (Table I). Case 2 received the most
treatments prior to mEHT, including two types of postoperative
adjuvant chemotherapy, 5 types of chemotherapy for tumor
recurrence, two types of hormone therapy, and irradiation. In
addition, Case 2 received two types of chemotherapy in combination
with mEHT (Table III). On the
other hand, Case 10 received no treatment prior to mEHT, following
the patient's request. The statistics of the mEHT are shown in
Table II. As a result, 8-90 mEHTs
were performed. The decision to discontinue was entirely based on
the request of the patient; the most common reason identified was
difficulty in continuing the treatment. Case 7 underwent mEHT only
8 times. The reason for this was that the combined use of
irradiation and mEHT reduced the metastatic skin cancer to PR; the
patient hoped that mEHT would be terminated at the same time as the
termination of irradiation. There were no apparent complications
during mEHT.
Clinical estimation of the PD
case
A summary of the local responses is presented in
Table III. Patients felt
comfortable with warming around the targeted area during treatment.
The elevated body temperature observed was mild, and some patients
presented with sweating without discomfort. In addition, there were
no adverse effects, such as skin blisters, erythema, or dermatitis.
PR was achieved in 3/10 (30%) patients, and so was SD. A total of
4/10 patients (40%) showed PD. All 3 patients (cases 2, 4 and 6)
that were treated with a combination of mEHT and mTOR achieved PD.
They had multiple-organ metastases from the breast cancer and had
undergone multiple sessions of mEHT (46-90). Only case 2 received
anthracycline and taxane for the treatment of breast cancer. Cases
4 and 6 refused chemotherapy and only approved the use of mTOR,
which has relatively few side effects, such as hair loss and
malaise. Therefore, these cases might have deviated from the usual
treatment for advanced breast cancer and do not indicate a low
therapeutic effect of the combination of mEHT and mTOR. However,
2/3 PR patients exhibited a re-increase in tumor size after the
follow-up period. By contrast, another patient recovered and
underwent curative surgery. At the time of writing, she was still
alive with no signs of recurrence (9 months after initial mEHT
therapy). A total of 4 patients judged as PD exhibited worsening of
the local tumor and metastases. Three patients died of cancer
during (2 patients) or after the completion of mEHT (1 patient).
Case 2 was a 66-year-old woman. Bt+Ax was performed in the right
breast. After administering two types of postoperative adjuvant
chemotherapy, hormone therapy was performed. Five years after the
operation, lung, liver and bone metastases occurred. Following
recurrence, seven types of treatment were performed (five types of
chemotherapy, one type of hormone therapy, and radiation therapy).
In addition, case 2 received two types of chemotherapy in
combination with 90 sessions of mEHT for 30 weeks. The tumor did
not grow until 24 weeks after the start of treatment, but
thereafter, lung metastasis gradually worsened, with the eventual
occurrence of pleural effusion. Due to dyspnea, the patient could
not visit the hospital; therefore, mEHT was discontinued. Three
months later, the patient died of cancerous pleurisy. Case 5 was a
74-year-old woman. Bt+SLN was performed for left breast cancer. She
continued hormone therapy following surgery. Three years after the
operation, liver, bone and lymph node metastases occurred. Two
types of chemotherapy, activated autologous lymphocyte therapy and
dendritic cell vaccine therapy were then performed; however, tumor
growth was observed. At her request, mEHT alone was performed for
24 weeks and 73 times without chemotherapy. The symptoms of cough
and dyspnea gradually worsened, and mEHT was discontinued due to
difficulty in visiting the hospital. One month later, the patient
died of cancerous pleurisy. Case 7 was a 75-year-old man with skin
metastasis, lung. Preoperative chemotherapy was performed for stage
IV breast cancer. Although lung metastasis was reduced, skin
metastasis did not change. Eight sessions of mEHT+radiation therapy
were performed, and a reduction in skin metastasis was observed
(PR). Following treatment, he was recommended to undergo surgery
but refused. Two months after the follow-up, chest CT revealed an
exacerbation of lung metastases. Although anticancer drug treatment
was restarted, progressively worsening lung metastases and dyspnea
were observed. The patient eventually died of cancerous pleurisy 6
months after the completion of mEHT.
mEHT monotherapy
A total of 4 patients were treated with mEHT alone,
following their request. As a result, one patient showed PR, two
showed SD, and one showed PD. Case 3 had undergone breast cancer
surgery and postoperative chemotherapy 22 years ago, and a
recurrence of lung metastases was observed 19 years later. Hormone
therapy was continued; however, an exacerbation of lung metastases
was observed. Nevertheless, this time, the patient refused to
receive anticancer drug treatment and only mEHT was performed 47
times. During that time, chest CT revealed no exacerbation of lung
metastases; therefore, the patient was judged to be SD. Case 5 had
undergone breast cancer surgery 5 years ago; 2 years later, she was
diagnosed with liver, bone and lymph node metastases and received
chemotherapy, hormone therapy and activated dendritic cell therapy.
This time, the patient refused to receive anticancer drug
treatment; therefore, only mEHT was performed 73 times. During that
time, the level of the tumor marker CEA was elevated and an
abdominal CT revealed aggravation of liver metastases; therefore,
the patient was judged as PD. Case 8 had multiple lung metastases
on preoperative chest CT; however, the patient refused any
treatment other than surgery; therefore, only mEHT was performed 40
times after mastectomy. During the treatment period, no obvious
subjective symptoms were observed and chest CT revealed no
exacerbation of lung metastases. Therefore, the patient was
considered to be SD. Details regarding the status of case 10 are
provided later.
Statistical evaluation of mEHT
Univariate analysis of the number of various
treatments performed before mEHT and their therapeutic effects are
shown in Table IV. PD patients
received more types of treatments before mEHT than PR+SD patients.
CEA levels before and after mEHT were significantly higher in PD
patients than in PR+SD patients (P=0.017, 0.009), and mEHT was
performed in patients with more advanced cancer. Statistical
analysis of the various parameters of mEHT and their therapeutic
effects are shown in Table IV. The
average number of treatments for PR+SD patients (6 cases) was 31.6
times, and the treatment period 14.8 weeks, which was significantly
less than that for PD cases (number of treatments, 74.0; treatment
period, 30.2 weeks; P=0.002). There were many advanced cancer
patients with PD, and mEHT was often performed in combination with
chemotherapy (75%); however, no clear mEHT-related side effects
were observed, and treatment for long periods was possible.
Clinical estimation of the PR
cases
Showing the details, 2 PR cases are described. The
PR cases 1, 7 and 10 had progression-free survival rates of 2, 7
and 9 months, respectively.
Case 1
Seven years ago, a 58-year-old woman visited our
hospital due to left breast cancer recurrence. The TNM
classification was T1N1M0 stage IIA at that time. Breast-conserving
operation and additional dissection of left axillary lymph nodes
were performed. However, the patient (then aged 65 years old)
developed lung, skin and lymph node metastases. She was positive
for the expression of HER2, ER and PgR. Postoperative radiation
therapy (55 Gy) was performed on the left residual breast tumor
area, and 50 Gy radiotherapy on the left clavicular region. Hormone
therapy (aromatase inhibitor) was continued after the completion of
radiation therapy. A fluorodeoxyglucose-positron emission
tomography scan revealed left chest wall skin invasion (or
metastasis). Left cervical, subclavian and right axillary lymph
node metastases were also observed. Although intravenous
chemotherapy of trastuzumab was administered, metastatic skin
lesions did not respond to these treatments. Combination
chemotherapy with trastuzumab, pertuzumab and docetaxel was
administered; however, intolerable diarrhea occurred. Since an
exacerbation of skin metastasis was observed after this treatment,
the drug was changed to TDM-1. However, there was no improvement in
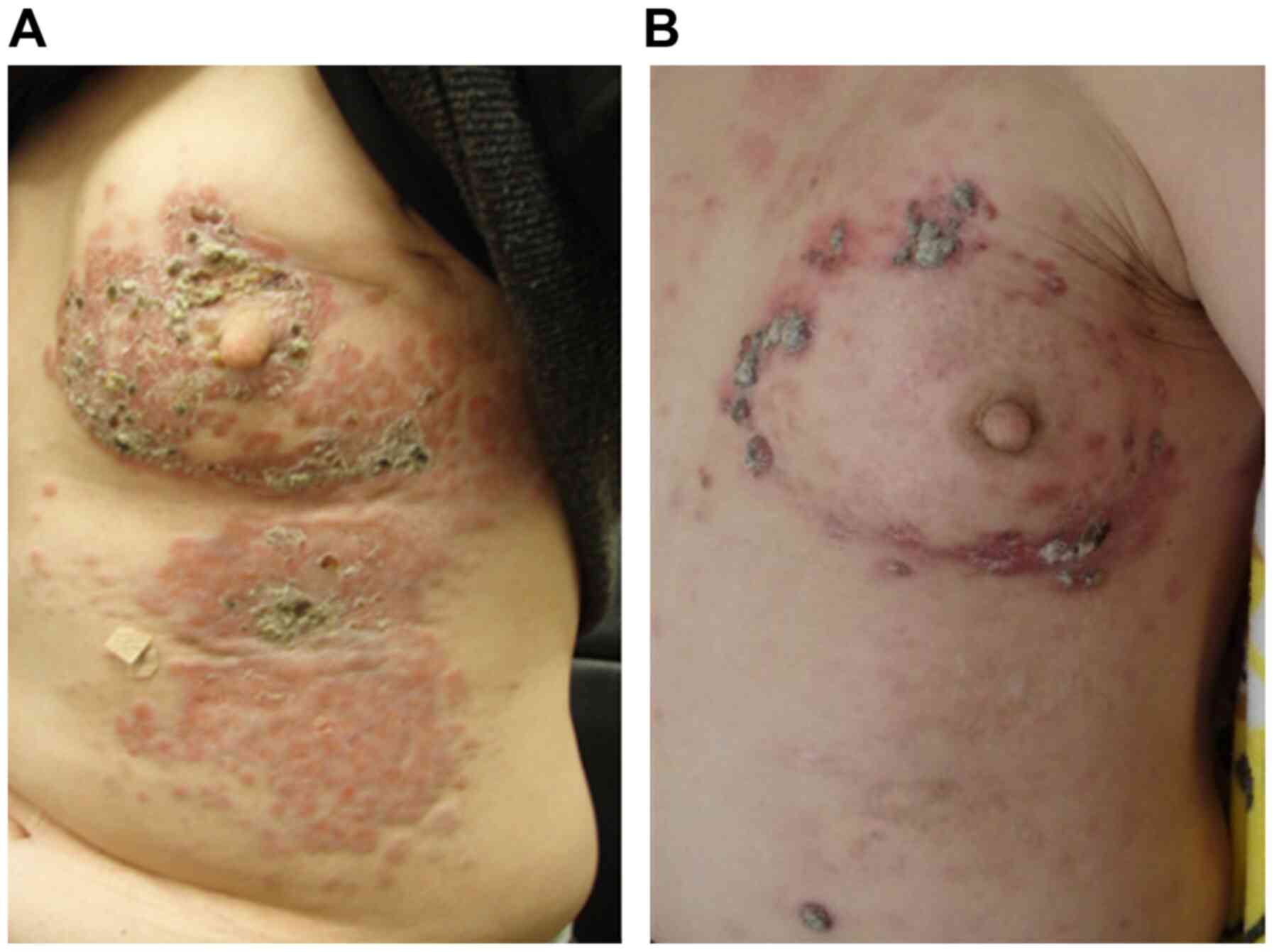
the skin lesions (Fig. 1A).
Finally, mEHT was used for adjuvant therapy using TDM-1. As a
result of the combination of anticancer drug treatment (TDM-1) once
every 3 weeks and mEHT thrice a week, a marked improvement in skin
invasion and metastases was observed (Fig. 1B). During mEHT, right axillary lymph
node metastasis was also reduced without direct intervention.
However, the tumor re-increased after 2 months of post-treatment
evaluation. The tumor metastasized to the brachial plexus. The
patient was alive with disease 1.2 years after the final mEHT
treatment.
Case 10
A 71-year-old woman had observed the presence of a
mass in her right breast for >15 years but decided to ignore it.
Two years ago, she was referred to our university hospital for the
assessment of apparent discharge and bleeding from the protruding
right breast mass. The definite diagnosis was breast cancer. The
patient was recommended to undergo chemotherapy, hormone therapy
and radiation therapy, but she rejected these treatment plans, out
of fear of developing adverse effects. Therefore, she was followed
up without any treatment. However, after the tumor increased in
size with exudation and a foul-smelling odor, she accepted mEHT
monotherapy. At the start of mEHT, an initial blood test showed a
CEA level of 10.4 ng/ml and cancer antigen 15-3 (CA 15-3) of 132
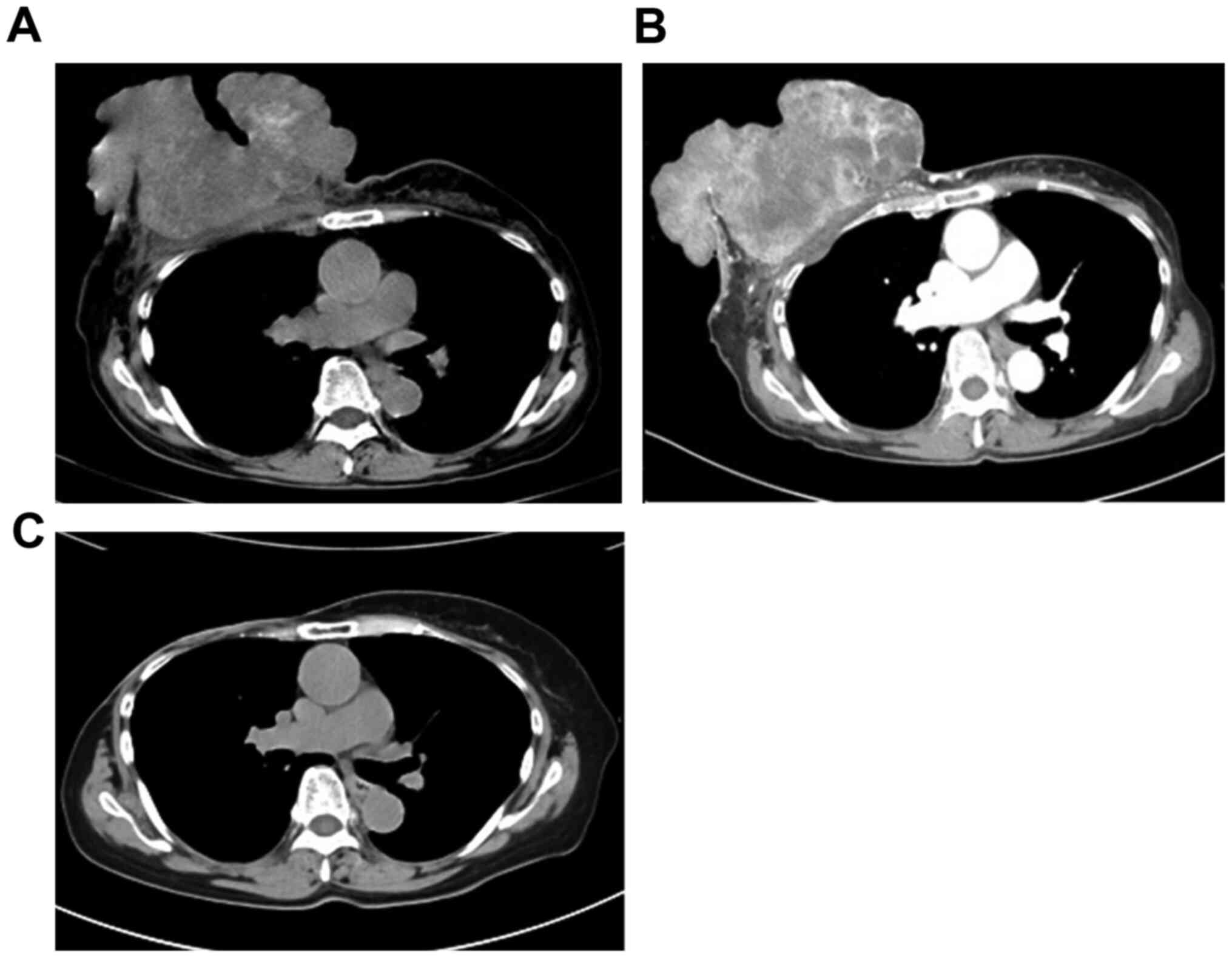
U/ml. CT and magnetic resonance imaging revealed the presence of a
massive tumor measuring 15 cm in diameter in the right breast
(Fig. 2A). Swelling of the axillary
lymph nodes was also observed; however, distant metastasis to other
organs was not detected. mEHT therapy was continued twice a week
for 6 months, resulting in tumor shrinkage, as observed by CT;
therefore, the patient was judged to have achieved PR (Fig. 2B). The preoperative diagnosis was
T4cN3bM0 stage IIIB, which was an indication for right mastectomy
(combined resection of the chest skin and partial large pectoral
muscle), right axillary dissection, and second-stage skin
transplantation. Intraoperative findings revealed that infiltration
into the large pectoral muscle was mild and that it was possible to
avoid total resection of the chest muscle. The skin with changed
color was excised, and the tumor resection margin was
histologically negative. The axillary lymph nodes were dissected to
level II, and it was evaluated that only level I lymph node was
positive for metastasis. The postoperative course was unremarkable,
and she was discharged on postoperative day 14. The pathological
diagnosis of the resected specimen was pT3N1 (level I, 2/24; level
II, 0/14; level III, 0/2) M0 stage IIIA. The tumor was removed at
the curative margin, due to the effectiveness of mEHT. After 3
weeks, the artificial dermis was affixed to the mastectomy part and
grafting was performed from the thigh part of the patient.
Postoperatively, the tumor did not reccur. The CEA level normalized
to 2.1 ng/ml 1 month after the surgery. CA15-3 also normalized to
18.6 U/ml 3 months after the surgery. Nine months after the
surgery, she showed no evidence of the disease (Fig. 2C).
Discussion
As the general lifestyle of people changes, the type
and structure of malignant diseases also changes. The clinical
course of cancer and its treatments have diversified. Furthermore,
the growing of available open-access information has allowed
patients to select their preferred therapies. The widely published
adverse effects deter some individuals from receiving conventional
therapies and favor conservative treatments with the hope of
maintaining a normal life despite the occurrence of cancer.
Hyperthermia is considered a less aggressive antitumor treatment
strategy and sometimes could be applied even in patients who are
unresponsive to conventional treatments (surgery, radiation or
chemotherapy), as well as to new cancer immunotherapies, such as
checkpoint inhibitors, cancer-specific cytotoxic T lymphocytes or
chimeric antigen receptor-T-cell therapy.
In general, cancer cells proliferate autonomously
and randomly. The cytoskeleton and genomic structure of malignant
cells have an inherent instability; therefore, they are more
sensitive to heat than normal cells (16). Utilizing this feature, the concept
of hyperthermia has been established and various therapeutic
approaches have been developed (17), including heating the lesion
isothermally. Most hyperthermia methods use bio-electromagnetic
energy-absorption heating of the cancer tissue of up to 43˚C or
higher temperatures to kill them, mainly by inducing local
necrosis, such as the hyperthermia dose (CEM43˚CTx) calibrated
in vitro. Moreover, many experimental studies have shown
that the obviously heterogenic solid tumors and their blood flow
derail the developed temperature distribution, despite the use of
iso-dose focusing. The usual vasodilatation that occurs in the
vivid part of the tumor and its healthy neighborhood increases
blood flow, possibly facilitating the delivery of chemotherapeutic
drugs and increasing the reaction rate, as well improving the
efficacy of ionization radiation therapies by delivering oxygen
(18). Despite the advantages of
high blood flow, it has several disadvantages, including delivering
nutrients that support tumor growth and helping the dissemination
of the malignant cells by the blood stream, thereby increasing the
incidence of distant metastases (19). On the other hand, the heavily
developed tumors have neo-angiogenetic vessels that form
vasocontraction, increasing the severity of hypoxia and assisting
rapid temperature growth in that part of the tissue (20,21).
This is the reason why local control is significantly good
following the use of this method; however, overall survival is
decreased due to metastases (22-26).
Due to the complex physiological feedback and the
attempt to re-establish thermal homeostasis by increasing blood
flow, as well as by other methods, the effects of conventional
hyperthermia are not stable and mostly insufficient for a lifetime
increase in blood flow. At the end of the 20th day of treatment,
the clinical results for local control following radiation therapy
alone vs. treatment with radiation+hyperthermia for advanced,
recurrent breast cancer were reported to be 41 vs. 59%,
respectively (26). Therefore, many
patients and medical doctors who continue to treat various types of
cancer, including advanced pancreatic cancer and other advanced
cases, without further conventional treatment options are looking
for a more effective therapeutic method, including the safe and
secure hyperthermia treatment. Based on these backgrounds, mEHT is
conducted in accordance with basic and clinical research data,
which is based on the cellular selection of tumor cells, inducing
programmed cell death (apoptosis), in various cancer cells by
causing a temperature gradient and prompting extrinsic pathways to
produce damage-associated molecular patterns (27) and immunogenic cell death (28,29),
thereby producing tumor-specific immune reactions (30) and an abscopal effect (31). The inhibition of protective
autophagy via sublethal hyperthermia in hepatocellular carcinoma
has been shown to enhance hyperthermia-induced apoptosis via the
ATP/AMPK/mTOR signaling pathway (32). Furthermore, it has been reported
that the inhibition of protective autophagy could be a therapeutic
strategy for RAS-induced pancreatic cancer (33). Unfortunately, the combination
therapy with mTOR inhibitor and mEHT used in the present study
resulted in PD in all cases; however, it is possible to continue
long-term treatment for advanced breast cancer cases with multiple
organ metastases. More studies with more cases are needed to
explore the combined treatment of mTOR inhibitor and mEHT. The
following clinical advantages have been reported from this
therapeutic principle: i) Very high heating efficiency for cancer
with a low power (150 W) (34); ii)
modulated electromagnetic waves do not result in burns on the skin
(35), and (3) these waves adequately reach tumors deep
within the body, such as those in the pancreas (36), lung (37), liver (32) and cervix (38).
Due to the lack of awareness and delay in discovery,
elderly patients with breast cancer are sometimes at a very
progressive stage, with skin invasion or other metastases upon
first diagnosis. Therefore, it is essential to consider the risks
and benefits of surgery and anticancer drug treatment for these
patients. When conventional therapies with standard protocols fail,
only palliative care is selected after informed consent. However,
mEHT is recommended as a valid option with few adverse effects for
patients with advanced cancer.
In conclusion, it was reported in the present study
that the use of mEHT is feasible for advanced or recurrent
metastatic breast cancer where pretreatment is ineffective. The
results suggested that mEHT has no side effects and could be
combined with various treatments for a long time.
Acknowledgements
The authors would like to thank Professor Takashi
Kondo (Division of Radiation Oncology, Department of Radiology,
Faculty of Medicine, University of Toyama, Toyama, Japan) for
providing medical advice for this research. The authors would also
like to thank Mrs. Nagisa Isobe (Division of Oncothermia,
Department of Human Science, Faculty of Medicine, University of
Toyama, Toyama, Japan) for technical support in operating the mEHT
system.
Funding
The current study was funded by Teteyamma Machine Co. (Toyama,
Japan).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MK conceived and designed the study. SS, MA and MM
acquired the data. TN and TF analyzed and interpreted the data. TN
drafted the manuscript. MK, SS and TF performed critical revision
of the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All experiments were approved by the Ethics
Committee of the University of Toyama (Toyama, Japan; approval no.
26-13), and written informed consent was obtained from all
participants.
Patient consent for publication
All patients provided written consent for the
publication of their data and images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tremont A, Lu J and Cole JT: Endocrine
therapy for early breast cancer: Updated review. Ochsner J.
17:405–411. 2017.PubMed/NCBI
|
|
2
|
Balaji K, Subramanian B, Yadav P, Radha CA
and Ramasubramanian V: Radiation therapy for breast cancer:
Literature review. Med Dosim. 41:253–257. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cody HS III: Current surgical management
of breast cancer. Curr Opin Obstet Gynecol. 14:45–52.
2002.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ito Y: Chemotherapy and hormone therapy
for breast cancer: Current status and perspective. JMAJ.
45:424–433. 2002.
|
|
5
|
Munagala R, Aqil F and Gupta RC: Promising
molecular targeted therapies in breast cancer. Indian J Pharmacol.
43:236–245. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Vincze G, Szasz O and Szasz A:
Generalization of the thermal dose of hyperthermia in oncology.
Open J Biophys. 5:97–114. 2015.
|
|
7
|
Roussakow S: The History of Ηyperthermia
Rise and Decline. Hindawi Publishing Corporation Conference Papers
in Medicine. Vol 2013, ID428027, 2013. http://www.hindawi.com/archive/2013/428027/.
|
|
8
|
Szasz A, Iluri N and Szasz O: Local
hyperthermia in oncology-to choose or not to choose? In:
Hyperthermia. Huilgol N (ed). InTech, Croatia, pp1-82, 2013.
|
|
9
|
Szasz O, Szasz AM, Minnaar C and Szasz A:
Heating-trends in modern oncological hyperthermia. Open J Biophys.
7:116–144. 2017.
|
|
10
|
Szasz A: Electromagnetic effects in
nanoscale range. In: Cellular Response to Physical Stress and
Therapeutic Applications, cheptor 4. Shimizu T and Kondo T (eds).
Nova Science Publishers, New York, NY, pp55-81, 2013.
|
|
11
|
Papp E, Vancsik T, Kiss E and Szasz O:
Energy absorption by the membrane rafts in the modulated
electro-hyperthermia (mEHT). Open J Biophys. 7:216–229. 2017.
|
|
12
|
Szasz A, Szasz N and Szasz O:
Oncothermia-principles and practices. Springer Science, Heidelberg,
2010.
|
|
13
|
Lee SY and Lee NR: Positive response of a
primary leiomyosarcoma of the breast following salvage hyperthermia
and pazopanib. Korean J Intern Med. 33:442–445. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Schirrmacher V, Stücker W, Lulei M, Bihari
AS and Sprenger T: Long-term survival of a breast cancer patient
with extensive liver metastases upon immune and virotherapy: A case
report. Immunotherapy. 7:855–860. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Anjum F, Razvi N and Massod MA: Breast
cancer therapy: A mini review. MOJ Drug Des Develop Ther. 1:35–38.
2017.
|
|
16
|
Dewey WC, Hopwood LE, Sapareto SA and
Gerweck LE: Cellular responses to combinations of hyperthermia and
radiation. Radiology. 123:463–474. 1977.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Smythe WR and Mansfield PF: Hyperthermia:
Has its time come? Ann Surg Oncol. 10:210–212. 2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Oleson JR: Eugene robertson special
lecture, hyperthermia from the clinic to the laboratory: A
hypothesis. Int J Hyperthermia. 11:315–322. 1995.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Mitsumori M, Zeng ZF, Oliynychenko P, Park
JH, Choi IB, Tatsuzaki H, Tanaka Y and Hiraoka M: Regional
hyperthermia combined with radiotherapy for locally advanced
non-small cell lung cancers: A multi-institutional prospective
randomized trial of the international atomic energy agency. Int J
Clin Oncol. 12:192–198. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Reinhold HS and Endrich B: Tumor
microcirculation as a target for hyperthermia. Int J Hyperthermia.
2:111–137. 1986.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Song CW: Effect of local hyperthermia on
blood-flow and microenvironment: A review. Cancer Res. 44 (Suppl
10):4721s–4730s. 1984.PubMed/NCBI
|
|
22
|
Kay CS, Choi IB, Jang JY, Choi BO, Kim A
and Shinn KS: Thermoradiotherapy in the treatment of locally
advanced nonsmall cell lung cancer. J Korean Soc Ther Radiol Oncol.
14:115–122. 1996.
|
|
23
|
Zolciak-Siwinska A, Piotrkowicz N,
Jonska-Gmyrek J, Nicke-Psikuta M, Michalsky W, Kawczyńska M, Bijok
M and Bujiko K: HDR brachytherapy combined with interstitial
hyperthermia in locally advanced cervical cancer patients initially
treated with concomitant radiochemotherapy-a phase III study.
Radiother Oncol. 109:194–199. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Jones EL, Oleson JR, Prosnitz LR, Samulski
TV, Vujaskovic Z, Yu D, Sanders LL and Dewhirst MW: Randomized
trial of hyperthermia and radiation for superficial tumors. J Clin
Oncol. 23:3079–3085. 2005.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sherar M, Liu FF, Pintilie M, Levin W,
Hunt J, Hill R, Hand J, Vernon C, van Rhoon G, van der Zee J, et
al: Relationship between thermal dose and outcome in
thermoradiotherapy treatments for superficial recurrences of breast
cancer: Data from a phase III trial. Int J Radiat Oncol Biol Phys.
39:371–380. 1997.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Vernon CC, Hand JW, Field SB, Machin D,
Whaley JB, van der Zee J, van Putten WL, van Rhoon GC, van Dijk JD,
González González D, et al: Radiotherapy with or without
hyperthermia in the treatment of superficial localized breast
cancer: Results from five randomized controlled trials.
International collaborative hyperhtermia group. Int J Radiat Oncol
Biol Phys. 35:731–744. 1996.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Forika G, Balogh A, Vancsik T, Zalatnai A,
Petovari G, Benyo Z and Krenacs T: Modulated electro-hyperthermia
resolves radioresistance of Panc1 pancreas adenocarcinoma and
promotes DNA damage and apoptosis in vitro. Int J Mol Sci.
21(5100)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hurwitz MD: Hyperthermia and
immunotherapy: Clinical opportunities. Int J Hyperthermia. 36
(Suppl 1):S4–S9. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Huang L, Li Y, Du Y, Zhang Y, Wang X, Ding
Y, Yang Y, Meng F, Tu J, Luo L and Sun C: Mild photothermal therapy
potentiates anti-PD-L1 treatment for immunologically cold tumors
via an all-in-one and all-in-control strategy. Nat Commun.
10(4871)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
You SH and Kim S: Feasibility of modulated
electro-hyperthermia in preoperative treatment for locally advanced
rectal cancer: Early phase 2 clinical results. Neoplasma.
67:677–683. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Minnaar CA, Kotzen JA, Ayeni OA, VAngu MD
and Bawyens A: Potentiation of the abscopal effect by modulated
electro-hyperthermia in locally advanced cervical cancer patients.
Front Oncol. 10(376)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Jiang J, Chen S, Li K, Zhang C, Tan Y,
Deng Q, Chai Y, Wang X, Chen G, Feng K, et al: Targeting autophagy
enhances heat stress-induced apoptosis via the ATP-AMPK-mTOR axis
for hepatocellular carcinoma. Int J Hyperthermia. 36:499–510.
2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kinsey CG, Camolotto SA, Boespflug AM,
Gillen KP, Foth M, Truong A, Schuman SS, Shea JE, Seipp MT, Yap JT,
et al: Protective autophagy elicited by RAF→MEK→ERK inhibition
suggests a treatment strategy for RAS-driven cancers. Nat Med.
25:620–627. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Van Gool SW, Makalowski J, Feyen O, Prix
L, Schirrmacher V and Stuecker W: The induction of immunogenic cell
death (ICD) during maintenance chemotherapy and subsequent
multimodal immunotherapy for glioblastoma (GBM). Austin Oncol Case
Rep. 3:1–8. 2018.
|
|
35
|
Szasz AM, Minnaar CA, Szentmártoni G,
Szigeti GP and Dank M: Review of the clinical evidences of
modulated electro-hyperthermia (mEHT) method: An update for the
practicing oncologist. Front Oncol. 9(1012)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Fiorentini G, Sarti D, Casadei V, Milandri
C, Dentico P, Mambrini A, Nani R, Fiorentini C and Guadagni S:
Modulated electro-hyperthermia as palliative treatment for
pancreatic cancer: A retrospective observational study on 106
patients. Integr Cancer Ther. 18(1534735419878505)2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ou J, Zhu X, Chen P, Du Y, Lu Y, Peng X,
Bao S, Wang J, Zhang X, Zhang T and Pang CLK: A randomized phase II
trial of best supportive care with or without hyperthermia and
vitamin C for heavily pretreated, advanced, refractory
non-small-cell lung cancer. J Adv Res. 24:175–182. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Minnaar CA, Kotzen JA, Ayeni OA, Naidoo T,
Tunmer M, Sharma V, Vangu MD and Baeyens A: The effect of modulated
electro-hyperthermia on local disease control in HIV-positive and
-negative cervical cancer women in South Africa: Early results from
a phase III randomised controlled trial. PLoS One.
14(e0217894)2019.PubMed/NCBI View Article : Google Scholar
|