Introduction
Colorectal cancer (CRC) is the most common neoplasm
in the gastrointestinal tract worldwide (1) and is considered to be have a good
prognosis among the gastrointestinal cancers. However, the
prognosis of unresectable colorectal cancer (uCRC) due to
progression or metastasis is worse, and tools are required for
predicting the disease course and first-line chemotherapy
response.
Recently, there has been an argument supporting the
role of inflammation in malignant tumor growth and progression
(2). Emerging studies have
demonstrated that inflammatory markers, such as the
neutrophil-to-lymphocyte ratio (NLR) play substantial roles in the
prediction of survival in different malignant tumors, including
colorectal, breast, ovarian, gastric and bladder cancers (3-8).
The researchers focused mainly on pretreatment inflammatory
markers, while the dynamic changes in inflammatory markers after
chemotherapy were not considered. Changes in inflammatory markers
during chemotherapy might be a valuable tool to assess prognosis
because chemotherapy may change the inflammatory response. However,
even though changes in systemic inflammatory markers might
dynamically reflect the modification of the balance between the
host inflammatory response and the immune response against cancer
during chemotherapy, their value is not fully understood.
NLR has been reported as an independent predictive
factor for the prognosis of uCRC (9,10).
Relationships between cancer treatment outcomes and
inflammation-based indicators, including NLR,
platelet-to-lymphocyte ratio (PLR), lymphocyte-to-monocyte ratio
(LMR), and modified Glasgow prognostic score, have been widely
studied. Among these, the NLR is a representative index. An
elevated NLR reflects greater systemic inflammation, which can
induce cancer progression via the production of pro-inflammatory
and angiogenic cytokines, and is associated with reduced
tumor-specific immunity, including a reduced number of
tumor-infiltrating lymphocytes in the tumor microenvironment
(11,12). Although NLR is considered a
promising prognostic biomarker based on previous reports, it is
unlikely to be used in clinical practice because there is no
consensus on the cutoff value. A recent meta-analysis evaluating
NLR as a prognostic biomarker in patients with CRC also noted this
heterogeneity on the cutoff level as a critical limitation
(13). Whereas, there are a few
reports about the change in NLR during chemotherapy (6), examining the association between
change in NLR and prognosis would obviate the need to set the
cutoff level. This study aimed to explore the prognostic impact of
the change in NLR during first-line chemotherapy on outcomes in
patients with uCRC.
Patients and methods
Study population
This was a retrospective single-institutional study
and included 71 patients who received first-line chemotherapy for
uCRC between April 2012 and April 2019 at the Department of
Coloproctology, Aizu Medical Center, Fukushima Medical University.
The Fukushima Medical University ethics committee approved this
study. Patients' characteristics data were collected from medical
records, and all patients presented with histologically confirmed
colorectal adenocarcinoma. The exclusion criteria were: i) Clinical
confirmation of acute infection, systemic inflammation or other
autoimmune disorders; ii) patients who received steroid therapy;
iii) patients with hematologic disorders; iv) patients diagnosed
with synchronous second malignancy arising from different regions;
v) patients who were given first-line chemotherapy for less than 3
months; vi) patients who had undergone curative resection after
chemotherapy and vii) patients who had been injected with
granulocyte-colony stimulating factor (G-CSF) within one month.
Chemotherapy regimens
Chemotherapy was administered after the diagnosis of
uCRC. First-line chemotherapy protocols were oxaliplatin-based,
irinotecan-based or oxaliplatin plus irinotecan-based, and
molecular targeted drugs were added to all cases based on
RAS status and by the discretion of the attending
physician.
Tumor response and data
collection
All patients underwent baseline computed tomography
(CT) screening before chemotherapy, and follow-up imaging was
performed every 3 months. The response evaluation criteria for
solid tumors (RECIST) were used to evaluate radiological responses.
Baseline characteristics included age, sex, performance status,
tumor location, metastatic organs, histology, RAS status,
blood cell count (white blood cells, neutrophils, lymphocytes,
monocytes, platelets), albumin, alkaline phosphatase (ALP), lactate
dehydrogenase (LDH), carcinoembryonic antigen (CEA), and
carbohydrate antigen 19-9 (CA 19-9). Inflammation-based indicators,
including NLR, LMR and PLR, and other laboratory data, including
LDH, ALP, albumin, CEA, and CA 19-9, were obtained retrospectively,
both before chemotherapy and 3 months after chemotherapy
initiation. We analyzed the relationship between laboratory data
that showed significant changes during chemotherapy and overall
survival (OS) and progression-free survival (PFS).
Endpoints
The primary endpoint of this study was to determine
whether a change in NLR is a prognostic factor for patients with
uCRC treated with chemotherapy. The secondary endpoints were to
examine the association of prognosis with the change in the index
calculated from inflammatory markers such as LMR and PLR and the
change in the tumor markers of colorectal cancer, such as CEA and
CA 19-9.
Statistical analysis
Quantitative data were reported as median (range).
All statistical analyses were performed using EZR (14). The Wilcoxon signed rank test was
used to compare paired data, the Mann-Whitney U test was used to
continuous variables, and the Chi-square tests (Fisher's exact
tests or Pearson's Chi-square test) were used to compare discrete
variables. To identify prognostic factors, Cox's proportional
hazards model was used for univariate analyses. For univariate
analyses, the laboratory data that changed significantly during
chemotherapy were examined in two groups: Decrease and increase,
and overall survival time was used as the time variable. OS and RFS
analyses conducted using the Kaplan-Meier method and the log-rank
test were used to determine the significance of the survival
curves. P-values <0.05 were considered statistically
significant.
Results
The baseline characteristics of patients are
summarized in Table I. In this
study, the median follow-up period was 21.0 (5.1-73.4) months. The
gender was predominantly male, and most of the patients were in
good general condition with Eastern Cooperative Oncology Group
(ECOG) performance scores of 0 to 1 in 98.6% of cases. In terms of
tumor location, right-sided colon, left-sided colon, and rectum had
almost the same proportions. The proportions of patients who
previously received adjuvant chemotherapy and who had metachronous
metastasis were similar. There were more cases of using an
anti-VEGF antibody as a molecular-targeted drug in addition to
chemotherapy, although there was an equal number of cases of wild
type and mutant type in RAS status.
 | Table IBaseline characteristics. |
Table I
Baseline characteristics.
| Characteristic | N (%) |
|---|
| Follow-up period
(months)a | 21.0 (5.1-73.4) |
| Age
(years)a | 66 (37-84) |
| Sex | |
|
Male | 53 (74.6) |
|
Female | 18 (25.4) |
| Performance score
(ECOG) | |
|
0 | 57 (80.3) |
|
1 | 15 (18.3) |
|
2 | 1 (1.4) |
| Tumor location | |
|
Right | 22 (31.0) |
|
Left | 49 (69.0) |
| Colon/rectum | |
|
Colon | 44 (62.0) |
|
Rectum | 27 (38.0) |
| Primary lesion
removal before chemotherapy | |
|
Yes | 48 (67.6) |
|
No | 23 (32.4) |
| Adjuvant
chemotherapy | |
|
Yes | 27 (33.8) |
|
No | 47 (66.2) |
| Indication for
chemotherapy | |
|
Unresectable
primary lesion | 3 (4.2) |
|
Unresectable
local recurrence | 4 (5.6) |
|
Distant
metastasis | 66 (93.0) |
|
Synchronous/metachronous metastasis | |
|
Synchronous | 40 (56.3) |
|
Metachronous | 29 (40.8) |
| Metastasis sites | |
|
Liver | 38 (53.5) |
|
Lung | 31 (43.7) |
|
Lymph
node | 17 (23.9) |
|
Peritoneum/local | 14 (19.7) |
| Number of metastasis
sites | |
|
0 | 2 (2.8) |
|
1 | 39 (54.9) |
|
≥2 | 30 (42.2) |
| Histology | |
|
Differentiated | 67 (94.4) |
|
Non-differentiated | 4 (5.6) |
| RAS gene
status | |
|
Wild | 31 (43.7) |
|
Mutant | 31 (43.7) |
|
Unknown | 9 (12.7) |
The treatment characteristics of the patients are
summarized in Table II.
Chemotherapy was administered after the diagnosis of uCRC.
First-line chemotherapy was oxaliplatin-based in 30 cases,
irinotecan-based in 34 cases, and oxaliplatin plus irinotecan-based
in 7 cases. Molecular targeted drugs included anti-VEGF antibody
(bevacizumab) in 58 cases and anti-EGFR antibody (cetuximab or
panitumumab) in 13 cases. The total number of chemotherapy regimens
were as follows: One regimen, 19 cases; 2-4 regimens, 42 cases; and
5-7 regimens, 10 cases. The best responses to chemotherapy were
complete response (CR), 2 cases; partial response (PR), 30 cases;
stable disease (SD), 28 cases; and progressive disease (PD), 11
cases. There were 10 patients (14.1%) who received palliative
resection of the primary lesion/metastatic lesions after
chemotherapy induction.
 | Table IITreatment characteristics. |
Table II
Treatment characteristics.
| Treatment | N (%) |
|---|
| First-line
chemotherapy | |
|
Oxaliplatin
based | 30 (42.3) |
|
Irrinotecan
based | 34 (47.9) |
|
Oxaliplatin
+ Irrinotecan | 7 (9.9) |
| Molecular targeted
drugs | |
|
Anti-VEGF | 58 (81.7) |
|
Anti-EGFR | 13 (18.3) |
| Total number of
regimens | |
|
1 | 19 (26.8) |
|
2-4 | 42 (59.2) |
|
5-7 | 10 (14.1) |
| Palliative surgery
after chemotherapy | |
|
Yes | 10 (14.1) |
|
No | 61 (85.9) |
| Chemotherapy
response | |
|
CR | 2 (2.8) |
|
PR | 30 (42.3) |
|
SD | 28 (39.4) |
|
PD | 11 (15.5) |
Changes in inflammation-based indicators, other
laboratory data, and tumor markers are shown in Table III. Significant changes between
pre-chemotherapy and 3 months after initiation of chemotherapy were
observed for NLR and PLR among the inflammation-based indicators,
and LDH and ALP levels among other laboratory data. CEA and CA 19-9
as tumor markers showed significant changes. Among these factors,
we analyzed NLR, PLR, ALP, CEA, and CA 19-9, which showed a
significant difference in terms of change during chemotherapy,
using Cox's univariate analyses (Table
IV). Only the change in NLR was statistically significant with
respect to change and prognosis pre-chemotherapy and 3 months after
the initiation of chemotherapy.
 | Table IIIChanges in inflammation-based
indicators, other laboratory data and tumor markers. |
Table III
Changes in inflammation-based
indicators, other laboratory data and tumor markers.
| Marker | Pre-chemotherapy,
median (range) | 3 months after
chemotherapy, median (range) |
P-valuea |
|---|
| NLR | 2.7 (1.2-9.8) | 1.6 (0.5-14.1) | <0.0001 |
| LMR | 4.0 (0.8-13.3) | 4.0 (0.6-10.2) | 0.9247 |
| PLR | 159.6
(69.6-504.9) | 123.1
(50.3-823.5) | <0.0001 |
| LDH (IU/l) | 216.0
(118.0-4068.0) | 212.0
(112.0-1732.0) | 0.0077 |
| ALP (IU/l) | 282.0
(139.0-5561.0) | 261.0
(37.4-1,297.0) | 0.0003 |
| ALB (g/dl) | 3.7 (2.1-4.8) | 3.7 (2.4-4.6) | 0.9541 |
| CEA (ng/ml) | 21.8
(1.7-22527.0) | 7.7
(1.9-8086.0) | <0.0001 |
| CA19-9 (U/ml) | 40.9
(0-16926.0) | 20.8
(0.6-23868.0) | 0.0001 |
 | Table IVCox's univariate analyses for changes
in NLR, PLR, ALP, CEA and CA 19-9. |
Table IV
Cox's univariate analyses for changes
in NLR, PLR, ALP, CEA and CA 19-9.
| Marker | Hazard
ratioa | 95% CI | P-value |
|---|
| NLR | 3.950 | 1.937-8.054 | 0.0002 |
| PLR | 1.325 | 0.716-2.453 | 0.3703 |
| ALP (IU/l) | 0.755 | 0.428-1.332 | 0.3318 |
| CEA (ng/ml) | 1.186 | 0.659-2.134 | 0.5695 |
| CA19-9 (U/ml) | 1.173 | 0.673-2.043 | 0.5740 |
Based on these results, we considered the NLR
prechemotherapy and 3 months after the initiation of chemotherapy
to be a prognostic factor for patients with uCRC and conducted our
analysis. Table V shows the
relationship between clinicopathologic factors in the two groups:
There were 61 cases with a decrease in NLR and 10 cases with an
increase in NLR during this period. Statistically significant
differences were seen in the indications of chemotherapy. Table VI shows the relationship between
the chemotherapeutic factors with NLR decrease and increase groups,
there was a significant different only in the number of total
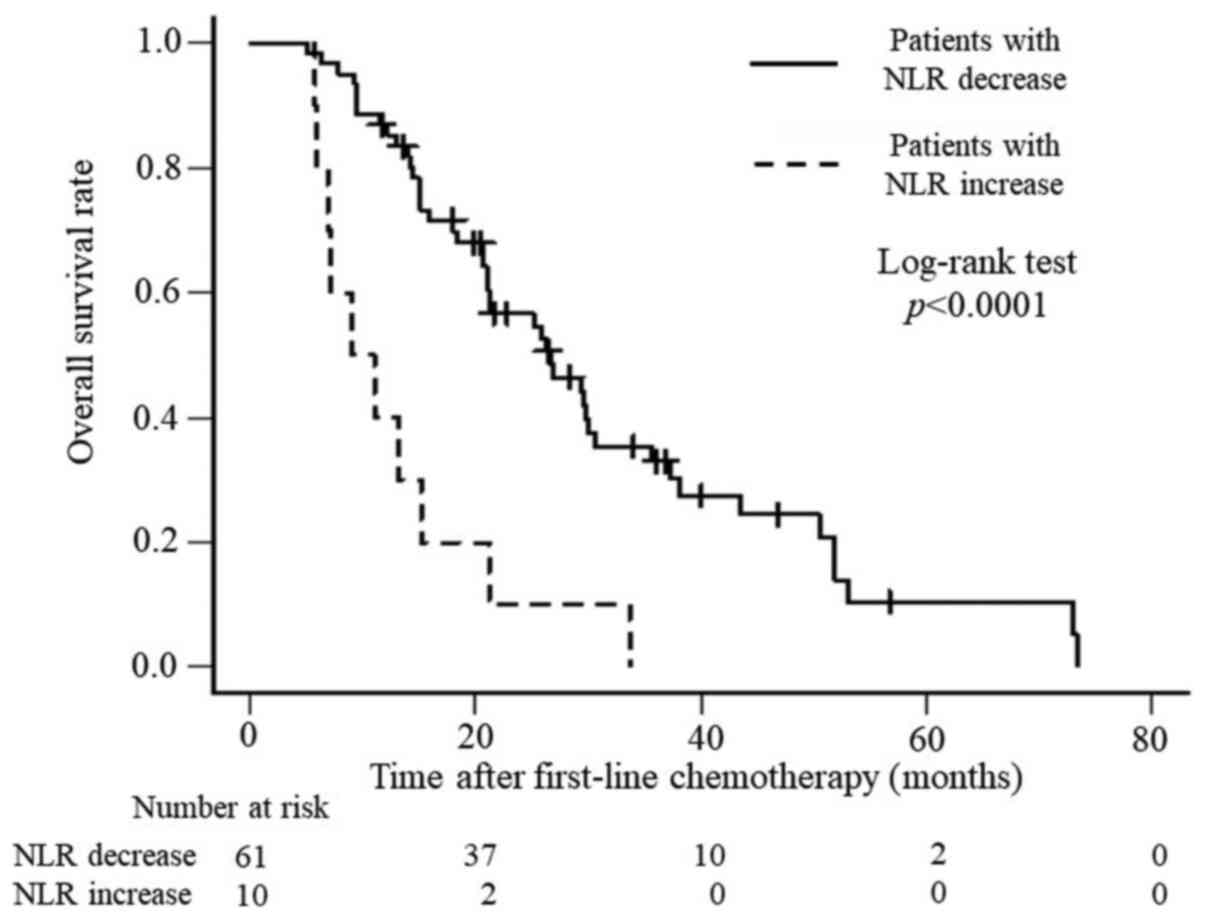
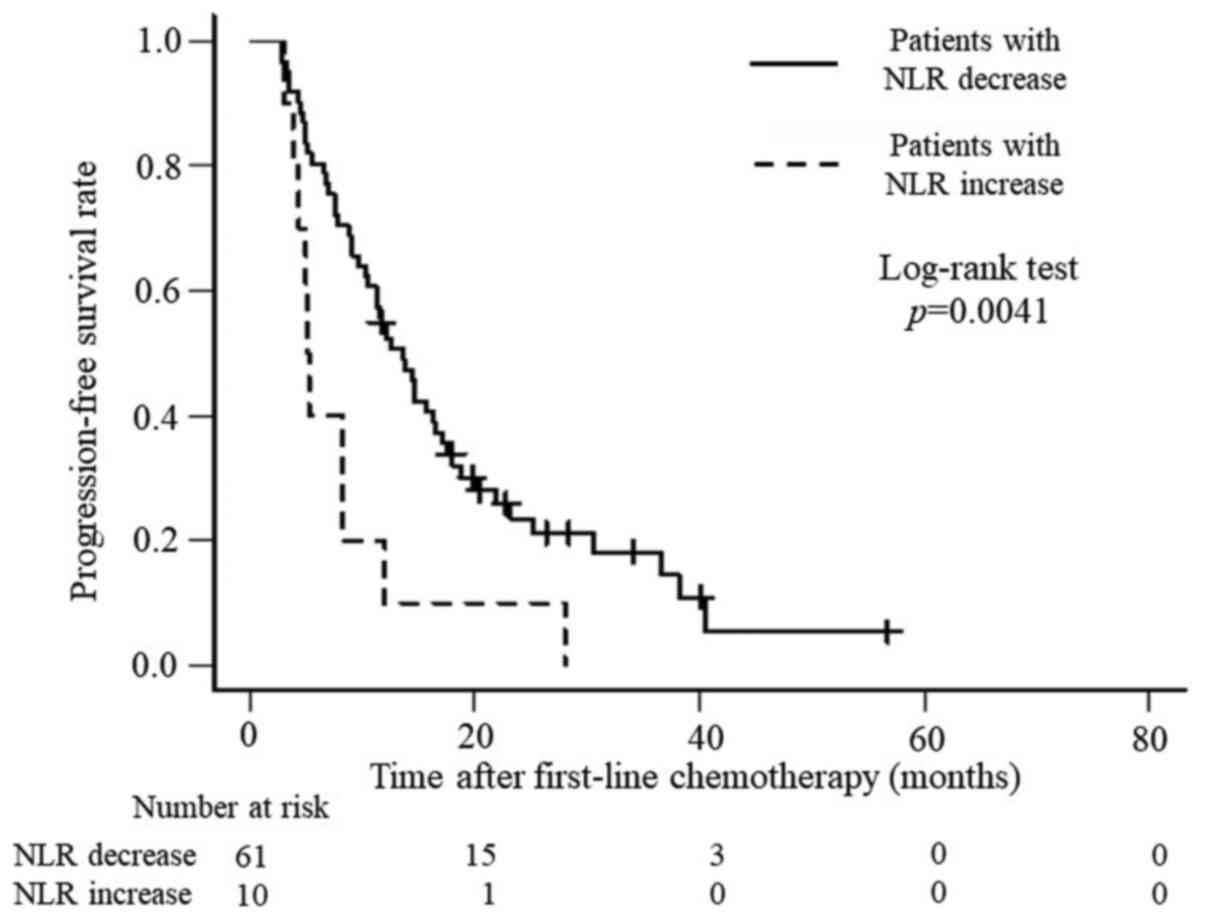
chemotherapy regimens. In addition, OS and PFS were analyzed. OS
was significantly better for patients with a decreased NLR than for
those with an increased NLR (P<0.0001), with median survival
times of 26.6 months and 10.1 months, respectively (Fig. 1), whereas PFS was 13.6 months and
5.2 months (P=0.0041), respectively (Fig. 2).
 | Table VRelationship between NLR and
clinicopathologic factors. |
Table V
Relationship between NLR and
clinicopathologic factors.
| Clinicopathologic
factor | NLR decrease
(n=61) | NLR increase
(n=10) | P-value |
|---|
| Age, years; median
(range) | 65 (32-84) | 68 (58-80) | 0.5905 |
| Sex | | | 0.4493 |
|
Male | 47 | 6 | |
|
Female | 14 | 4 | |
| Performance
status | | | 0.1901 |
|
0 | 51 | 6 | |
|
1-2 | 10 | 4 | |
| Tumor location | | | 0.7671 |
|
Right | 18 | 4 | |
|
Left | 43 | 6 | |
| Colon/rectum | | | >0.9999 |
|
Colon | 38 | 6 | |
|
Rectum | 23 | 4 | |
| Primary lesion
removal before chemotherapy | | | >0.9999 |
|
Yes | 41 | 7 | |
|
No | 20 | 3 | |
| Adjuvant
chemotherapy | | | 0.4193 |
|
Yes | 19 | 5 | |
|
No | 42 | 5 | |
| Indication of
chemotherapy | | | 0.2886 |
|
Unresectable
primary lesion or local recurrence | 3 | 2 | |
|
Unresectable
metastasis | 58 | 8 | |
|
Synchronous/metachronous metastasis | | | 0.8369 |
|
Synchronous | 35 | 5 | |
|
Metachronous | 24 | 5 | |
| Metastasis
site | | | |
|
Liver | 34 | 4 | 0.5600 |
|
Lung | 26 | 5 | 0.9267 |
|
Lymph
node | 16 | 1 | 0.4746 |
|
Peritoneum/local | 11 | 3 | 0.6506 |
| Number of
metastasis sites | | | 0.6164 |
|
0-1 | 34 | 7 | |
|
≥2 | 27 | 3 | |
| Histology | | | 0.1658 |
|
Differenciated | 59 | 8 | |
|
Non-differenciated | 2 | 2 | |
| RAS gene
status | | | 1 |
|
Wild | 27 | 4 | |
|
Mutant | 27 | 4 | |
 | Table VIRelationship between NLR and
chemotherapeutic factors. |
Table VI
Relationship between NLR and
chemotherapeutic factors.
| Chemotherapeutic
factor | NLR decrease
(n=61) | NLR increase
(n=10) | P-value |
|---|
| First-line
chemotherapy | | | 0.8535a |
|
Oxaliplatin | 30 | 4 | |
|
Irinotecan | 25 | 5 | |
|
Oxaliplatin
+ Irinotecan | 6 | 1 | |
| Molecular targeted
drug (first-line chemotherapy) | | | 0.7703a |
|
Anti-VEGF | 49 | 9 | |
|
Anti-EGFR | 12 | 1 | |
| Number of total
regimens, median (range) | 3 (1-7) | 1 (1-4) | 0.0240b |
| Palliative surgery
after chemotherapy | | | 0.3297a |
|
Yes | 10 | 0 | |
|
No | 51 | 10 | |
| Response to
chemotherapy | | | 0.3077a |
|
CR | 2 | 0 | |
|
PR | 28 | 2 | |
|
SD | 23 | 5 | |
|
PD | 8 | 3 | |
| CR + PR + SD vs.
PD | 53 vs. 8 | 7 vs. 3 | 0.3700a |
Discussion
CRC is considered to have a good prognosis among the
gastrointestinal cancers. However, the prognosis of uCRC due to
progression or metastasis is poor. Although uCRC is treated with
multidisciplinary therapy, mainly chemotherapy, its survival rate
has not reached the desired level. CEA and CA 19-9 are considered
useful markers in the treatment of colorectal cancer, but they are
highly variable and not accurate enough to be recognized as
prognostic markers for patients with uCRC treated with
chemotherapy. Therefore, the need for predictive and prognostic
markers during chemotherapy for uCRC is increasing.
In this study, we examined the NLR, LMR, and PLR,
which have been reported to be useful as inflammatory markers, but
only the NLR and PLR showed a significant difference between
prechemotherapy and 3 months after chemotherapy initiation.
Finally, only NLR correlated with prognosis. Patients were divided
into two groups: Decreased NLR and increased NLR, and their
clinicopathological factors were compared. However, there was no
difference in the response to chemotherapy, but there was a
difference in the total number of chemotherapy regimens. There was
also a significant difference in the CEA and CA 19-9 level between
prechemotherapy and 3 months after the initiation of chemotherapy,
but this did not correlate with prognosis.
The NLR has been suggested as a prognostic marker in
various solid tumors (3-8,15,16).
NLR is a factor related to systemic inflammation, which is
associated with cancer growth. Systemic inflammation may lead to
tumor initiation through genetic mutations, genomic instability,
and epigenetic modifications. Inflammation promotes tissue repair
responses that induce the proliferation of premalignant cells and
increase their viability. It is also involved in angiogenesis,
immunosuppression, inhibition of apoptosis, and DNA damage,
ultimately and contributing to metastatic spread (12,17). A
high NLR indicates a relatively elevated neutrophil count and
depressed lymphocyte count. Neutrophils are thought to produce
vascular endothelial growth factor and various matrix proteases
(18).
Another promising application of inflammatory-based
scores as predictive biomarkers is a longitudinal change before and
after treatment. A similar previous study in patients with
metastatic gastric cancer reported that constantly elevated NLR or
an increase in NLR during chemotherapy correlated with poor OS,
PFS, and chemotherapy response (19). There was study compared the
preoperative NLR and the differences in NLR preoperative and
one-month postoperative by determine the cutoff value from receiver
operating characteristic (ROC) curve analysis, respectively, with
prognosis in colorectal cancer resection cases (20). That study reported better OS and
disease-free survival (DFS) when the preoperative NLR was low and
when the NLR was reduced by resection. Another similar study also
reported that when preoperative NLR was low and 7 days
postoperative NLR was low, and when preoperative NLR was high and
postoperative NLR was low in colorectal cancer surgical treatment,
OS and PFS were better (21). Those
studies needed cutoff value determined by ROC curve analysis that
was cumbersome. Contrarily, a study examining changes in NLR with
uCRC patients before and after two cycles of chemotherapy (FOLFIRI
+ bevacizumab) reported that an increase NLR led to significantly
longer OS than a decrease NLR, in patients with SD (22). That result was a different from our
study in the manner that they were shorter period which evaluate
the change in NLR and were limited to SD patients.
Divided of the patients into the NLR decrease and
increase groups resulted in significant differences in OS and PFS.
There was also a significant difference in the number of
chemotherapy regimens. Furthermore, the large differences between
the two groups in terms of both OS and PFS suggest that patients
with an increased NLR may have had rapid cancer progression that
would have made the continuation of chemotherapy difficult. These
results may also indicate that patients with an increased NLR
during chemotherapy have a systemic poor immunologic condition that
does not improve with chemotherapy. An advanced state of so-called
cancer cachexia is assumed. Although these results may be limited
to the early period of chemotherapy, a study of patients undergoing
late-line chemotherapy treated with TAS-102, which is an oral
combination of trifluridine and tipiracil, also reported that the
NLR before initiation of treatment was significantly negatively
associated with OS and PFS (23).
The relationship between changes in NLR and prognosis should also
be confirmed in late-line chemotherapy patients.
In this study, dynamic changes in NLR statistically
correlated with OS and PFS. Therefore, we believe that the change
in NLR during chemotherapy may be more accurate in predicting
prognoses after chemotherapy than the NLR calculated before
chemotherapy. The ability to predict prognosis at an early phase
after the introduction of chemotherapy will provide useful
information for the selection of subsequent treatments and may
contribute towards improving patients' quality of life. It should
also be emphasized that the ROC curve analysis does not need to be
used to determine the cutoff value, which makes it easier to
determine.
The limitations of this study are its retrospective
observational nature, small sample size, and its setting in a
single institute in Japan, non-exclusion of some factors affecting
NLR values. Although many studies have found the NLR to be useful
as a prognostic biomarker, the cutoff level for NLR has been
calculated using the median value of NLR or using receiver
operating characteristic curve analyses, which may be difficult to
use in routine clinical practice. For this reason, we recommend
that the results of this study be validated prospectively without
the need to establish a cutoff value. Further high-quality studies
with larger cohorts are required to confirm this finding.
In conclusions, although OS and PFS correlated with
changes in NLR prechemotherapy and 3 months after the initiation of
chemotherapy, only the number of chemotherapy regimens showed an
association between changes in NLR in clinicopathological factors.
The ability to predict prognosis at an early phase after the
introduction of chemotherapy will provide useful information for
the selection of subsequent treatment protocols and may contribute
toward the improvement of patients' quality of life.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SE designed the study. TN, SE, NI, DT, DN, MA and KU
acquired the data. TN and SE confirm the authenticity of all the
raw data. TN, KT and SE analyzed and interpreted the data and
drafted the manuscript. SE and KT performed critical revision of
the manuscript. SE supervised the study. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of the Fukushima Medical University (approval no. General
2020-198). Written informed consent was not obtained due to the
retrospective nature of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444.
2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Li MX, Liu XM, Zhang XF, Zhang JF, Wang
WL, Zhu Y, Dong J, Cheng JW, Liu ZW, Ma L and Lv Y: Prognostic role
of neutrophil-to-lymphocyte ratio in colorectal cancer: A
systematic review and meta-analysis. Int J Cancer. 134:2403–2413.
2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pistelli M, De Lisa M, Ballatore Z,
Caramanti M, Pagliacci A, Battelli N, Ridolfi F, Santoni M,
Maccaroni E, Bracci R, et al: Pre-treatment neutrophil to
lymphocyte ratio may be a useful tool in predicting survival in
early triple negative breast cancer patients. BMC Cancer.
15(195)2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Nomelini RS, Carrijo Chiovato AF,
Abdulmassih FBF, da Silva RC, Tavares-Murta BM and Murta EFC:
Neutrophil-to-lymphocyte ratio and platelet count as prognostic
factors in ovarian malignancies. J Cancer Res Ther. 15:1226–1230.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Aizawa M, Gotohda N, Takahashi S, Konishi
M and Kinoshita T: Predictive value of baseline
neutrophil/lymphocyte ratio for T4 disease in wall-penetrating
gastric cancer. World J Surg. 35:2717–2722. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dirican A, Kucukzeybek Y, Somali I, Erten
C, Demir L, Can A, Bahriye Payzin K, Vedat Bayoglu I, Akyol M,
Koseoglu M, et al: The association of hematologic parameters on the
prognosis of patients with metastatic renal cell carcinoma. J BUON.
18:413–419. 2013.PubMed/NCBI
|
|
8
|
Kang M, Jeong CW, Kwak C, Kim HH and Ku
JH: Preoperative neutrophil-lymphocyte ratio can significantly
predict mortality outcomes in patients with non-muscle invasive
bladder cancer undergoing transurethral resection of bladder tumor.
Oncotarget. 8:12891–12901. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tsai PL, Su WJ, Leung WH, Lai CT and Liu
CK: Neutrophil-lymphocyte ratio and CEA level as prognostic and
predictive factors in colorectal cancer: A systematic review and
meta-analysis. J Cancer Res Ther. 12:582–589. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kishi Y, Kopetz S, Chun YS, Palavecino M,
Abdalla EK and Vauthey JN: Blood neutrophil-to-lymphocyte ratio
predicts survival in patients with colorectal liver metastases
treated with systemic chemotherapy. Ann Surg Oncol. 16:614–622.
2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chen ZY, Raghav K, Lieu CH, Jiang ZQ, Eng
C, Vauthey JN, Chang GJ, Qiao W, Morris J, Hong D, et al: Cytokine
profile and prognostic significance of high neutrophil-lymphocyte
ratio in colorectal cancer. Br J Cancer. 112:1088–1097.
2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Malietzis G, Giacometti M, Kennedy RH,
Athanasiou T, Aziz O and Jenkins JT: The emerging role of
neutrophil to lymphocyte ratio in determining colorectal cancer
treatment outcomes: A systematic review and meta-analysis. Ann Surg
Oncol. 21:3938–3946. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lee DY, Hong SW, Chang YG, Lee WY and Lee
B: Clinical significance of preoperative inflammatory parameters in
gastric cancer patients. J Gastric Cancer. 13:111–116.
2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nagasaki T, Akiyoshi T, Fujimoto Y,
Konishi T, Nagayama S, Fukunaga Y and Ueno M: Prognostic impact of
neutrophil-to-lymphocyte ratio in patients with advanced low rectal
cancer treated with preoperative chemoradiotherapy. Dig Surg.
32:496–503. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899.
2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to virchow? Lancet. 357:539–545. 2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bozkurt O, Firat ST, Dogan E, Cosar R,
Inanc M and Ozkan M: The prognostic value of the change in
neutrophil-to-lymphocyte ratio during first-line palliative
chemotherapy in patients with metastatic gastric cancer: A
retrospective study. J BUON. 24:1992–1999. 2019.PubMed/NCBI
|
|
20
|
Guo D, Han A, Jing W, Chen D, Jin F, Li M,
Kong L and Yu J: Preoperative to postoperative change in
neutrophil-to-lymphocyte ratio predict survival in colorectal
cancer patients. Future Oncol. 14:1187–1196. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cui M, Xu R and Yan B: A persistent high
neutrophil-to-lymphocyte ratio predicts poor prognosis in patients
with colorectal cancer undergoing resection. Mol Clin Oncol.
13(63)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Formica V, Luccchetti J, Cunningham D,
Smyth EC, Ferroni P, Nardecchia A, Tesauro M, Cereda V, Guadagni F
and Roselli M: Systemic inflammation, as measured by the
neutrophil/lymphocyte ratio, may have differential prognostic
impact before and during treatment with fluorouracil, irinotecan
and bevacizumab in metastatic colorectal cancer patients. Med
Oncol. 31(166)2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Matsuda A, Yamada T, Matsumoto S,
Sakurazawa N, Kawano Y, Shinozuka E, Sekiguchi K, Suzuki H and
Yoshida H: Pretreatment neutrophil-to-lymphocyte ratio predicts
survival after TAS-102 treatment of patients with metastatic
colorectal cancer. Anticancer Res. 39:4343–4350. 2019.PubMed/NCBI View Article : Google Scholar
|