Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common type of cancer worldwide and the third most common cause of
cancer-related death (1). Since the
majority of HCC cases occur in patients with chronic liver disease,
especially cirrhosis, treatment modalities selection is determined
not by tumor morphology alone, but also taking into consideration
the liver function and health performance status (2). Therefore, for the HCC treatment in
clinical settings, the Barcelona Clinic Liver Cancer (BCLC) staging
system is used all over the world, instead of the TNM staging
system used for other cancers (3).
Transarterial chemoembolization (TACE) is a widely
used locoregional procedure that is recommended by several
guidelines as a first-line treatment for patients with unresectable
multifocal HCC (4-6).
Recently, calibrated drug-eluting embolics have been developed as
novel embolic devices that can overcome the drawbacks of
conventional TACE (C-TACE) and enable permanent embolization
effects. Several types of microspheres have been introduced for
this purpose. The most commonly used embolics are DC Bead (BTG) and
superabsorbent polymer embolics (SAP) (HepaSphere; Merit Medical
Systems). Retrospective and prospective randomized control trials
have performed a direct comparison between C-TACE and TACE with DC
Bead (7-9).
A meta-analysis including four randomized controlled trials and
eight observational studies showed no significant differences
between TACE with DC Bead and C-TACE in terms of objective response
rates, survival rates, and adverse events (7).
SAP is different from other drug-eluting embolics:
It is provided in a ‘dry state’ and, when exposed to aqueous-based
media, it absorbs fluid and swells to a predictable size (10). Grosso et al (11) and Seki et al (12) reported a promising effect and a high
safety profile for the treatment of HCC in their early experiences.
Until recently, only a few studies have evaluated the efficacy of
TACE with SAP (SAP-TACE) (13-16)
compared with C-TACE; however, no randomized controlled trials have
performed a direct comparison (17).
During the last decade, some chemotherapeutic agents
have been approved for use in patients with advanced-stage HCC,
especially in patients refractory to TACE therapy (18-20).
These oral chemotherapeutic agents demonstrated clinical benefits
in terms of survival, but they can only be administered in patients
with good liver function, i.e. Child-Pugh class A. In a real
clinical setting, preventing the deterioration of liver function
after repeated TACE should be just as important as treatment
efficacy, in the selection of the TACE procedure, because clinical
trials have demonstrated the deterioration of liver function after
repeated TACE (21,22). However, no clinical data with
repeated SAP-TACE have been reported so far.
We compared the early response and safety profile
between the two TACE cohorts in the previous study (16). In the current study, we aimed to
evaluate the long-term survival benefit and liver function
deterioration after repeated SAP-TACE or C-TACE in larger
cohorts.
Materials and methods
Study design
This retrospective, single-center study was
conducted in a TACE-naïve consecutive cohort treated between
January 2011 and August 2016. The study protocol was approved by
our institutional review board and was conducted in accordance with
local laws and the Declaration of Helsinki. All patients provided
written informed consent for the treatment procedures.
Patients
Criteria to perform TACE were as follows: i) HCC
diagnosis with histopathological confirmation and/or radiological
examination based on the European Association for the Study of the
Liver imaging criteria (3); ii)
tumor location and extent not amenable to elective curative
approach (resection and ablation); iii) no previous TACE or
systemic chemotherapy; iv) absence of macrovascular invasion and of
any suspicion of extrahepatic tumor spread; v) Child-Pugh classes A
or B; vi) Eastern Cooperative Oncology Group (ECOG) performance
status scores of 0 to 1; vii) adequate renal function (serum
creatinine <1.5 times the upper limit of the normal range).
We administered C-TACE to consecutive patients
between January 2011 and April 2014; after SAP was approved, we
administered SAP-TACE to consecutive patients between May 2014 and
August 2016, and we did not change the embolics choice, for the
repeated use, in any case.
TACE procedure
Angiography was performed using the Seldinger
technique via the femoral artery. The femoral artery was
catheterized under local anesthesia, and a 4-F catheter (Selecon;
Terumo) was inserted into the hepatic artery. Then, a 1.9-F
microcatheter (Progreat Σ; Terumo) was advanced into the feeder
arteries of each tumor.
In the C-TACE group, a mixture of 5 ml of iodized
oil (Lipiodol; Guerbet Japan) and 50 mg of epirubicin hydrochloride
(Farmorubicin; Pfizer Japan Inc.) were injected to a maximum
administered dose of 50 mg, followed by embolizing with absorbable
gelatin sponge particles (Gelpart; Nippon Kayaku).
In the SAP-TACE group, 50-100 µm HepaSphere
microspheres were prepared as previously reported (12); 25-mg vials of embolics were
preloaded with 25-30 mg of epirubicin dissolved in 5 ml of
non-ionic contrast medium and were left unperturbed for 20 min to
allow the embolics to expand and absorb the epirubicin after
injecting the solution into the vacuum-sealed vials containing the
embolics. The SAP injection was administered until near stasis (the
contrast column cleared within 2-5 heartbeats) (23).
Statistical analysis
Liver function was evaluated at just before first
TACE, after 3 cycles of TACE, and after 5 cycles of TACE by
Child-Pugh class and albumin-bilirubin (ALBI) scores (24). Quantitative differences between the
groups were analyzed using the Mann-Whitney U test, and categorical
differences were analyzed using Fisher's exact or
χ2 test. Survival time was calculated from the
moment of the initial TACE. Survival curves were created using the
Kaplan-Meier method and were compared using the log-rank test.
Prognostic factors related to overall survival were identified by
univariate and multivariate analyses. Multivariate analyses were
performed using the Cox proportional hazards model to identify the
independent prognostic factors. Bonferroni adjustment was used to
correct for multiple comparisons. The level of significance was set
up to P<0.05. Statistical analyses were performed using SPSS
version 25 software for Windows (IBM; SPSS, Inc.).
Results
Clinical characteristics
Baseline characteristics of the patients are shown
in Table I. During 2011-2016, a
total of 155 consecutive patients underwent TACE, including 71
patients treated with C-TACE and 84 patients treated with SAP-TACE.
The follow-up period, patient age, gender, background liver
disease, number of tumors, maximum tumor size, BCLC stage,
Child-Pugh scores, and ALBI scores were not statistically different
between the two groups. The proportion of patients that received
any prior treatment before TACE was significantly different between
the groups (P<0.05). There were no significant differences
between the two groups in terms of laboratory data, the levels of
aspartate aminotransferase, albumin, total bilirubin, prothrombin
time, alpha-fetoprotein (AFP), and des-gamma-carboxy prothrombin
(DCP).
 | Table IBaseline characteristics. |
Table I
Baseline characteristics.
| Variable | C-TACE (n=71) | SAP-TACE (n=84) | P-value |
|---|
| Follow-up period,
months (median; range) | 21 (1-94) | 26 (2-64) | 0.636 |
| Age, years (mean ±
SD) | 73.0±9.4 | 73.4±8.7 | 0.513 |
| Sex, n
(male/female) | 49/22 | 61/23 | 0.723 |
| Etiology, n
(hepatitis C/hepatitis B/other) | 30/11/30 | 33/15/36 | 0.898 |
| Previous treatment, n
(yes/no) | 6/65 | 37/47 | <0.001 |
| Number of tumors | 4.7±4.9 | 6.3±10.2 | 0.906 |
| Maximum tumor size,
mm (mean ± SD) | 49.2±35.3 | 43.7±38.6 | 0.173 |
| Tumor distribution, n
(unilobar/bilobar) | 39/22 | 40/29 | 0.736 |
| BCLC stage, n
(A/B) | 20/51 | 32/52 | 0.233 |
| Child-Pugh class, n
(A/B) | 58/13 | 59/24 | 0.135 |
| ALBI score, n
(1/2/3) | 23/45/3 | 22/58/3 | 0.693 |
| AST, IU/l (mean ±
SD) | 71.0±90.9 | 60.9±53.7 | 0.245 |
| Albumin, g/dl (mean ±
SD) | 3.6±0.5 | 3.5±0.5 | 0.567 |
| Total Bilirubin,
mg/dl (mean ± SD) | 0.9±0.4 | 0.9±0.4 | 0.921 |
| Prothrombin time, %
(mean ± SD) | 81.0±12.3 | 78.4±12.9 | 0.428 |
| AFP, ng/ml (median;
range) | 45.5
(2.0-360100.0) | 22
(1.8-111160.0) | 0.332 |
| DCP, mAU/ml
(median; range) | 151 (8-400000) | 184 (11-54400) | 0.803 |
Overall survival
We evaluated overall survival rates during follow-up
for a median of 25 months. At the time of the analysis, 110
patients had died (59 in the C-TACE cohort, and 51 in the SAP-TACE
cohort). Treatment-related death was not experienced. Median
survival of the C-TACE cohort was 26 months, compared with 28
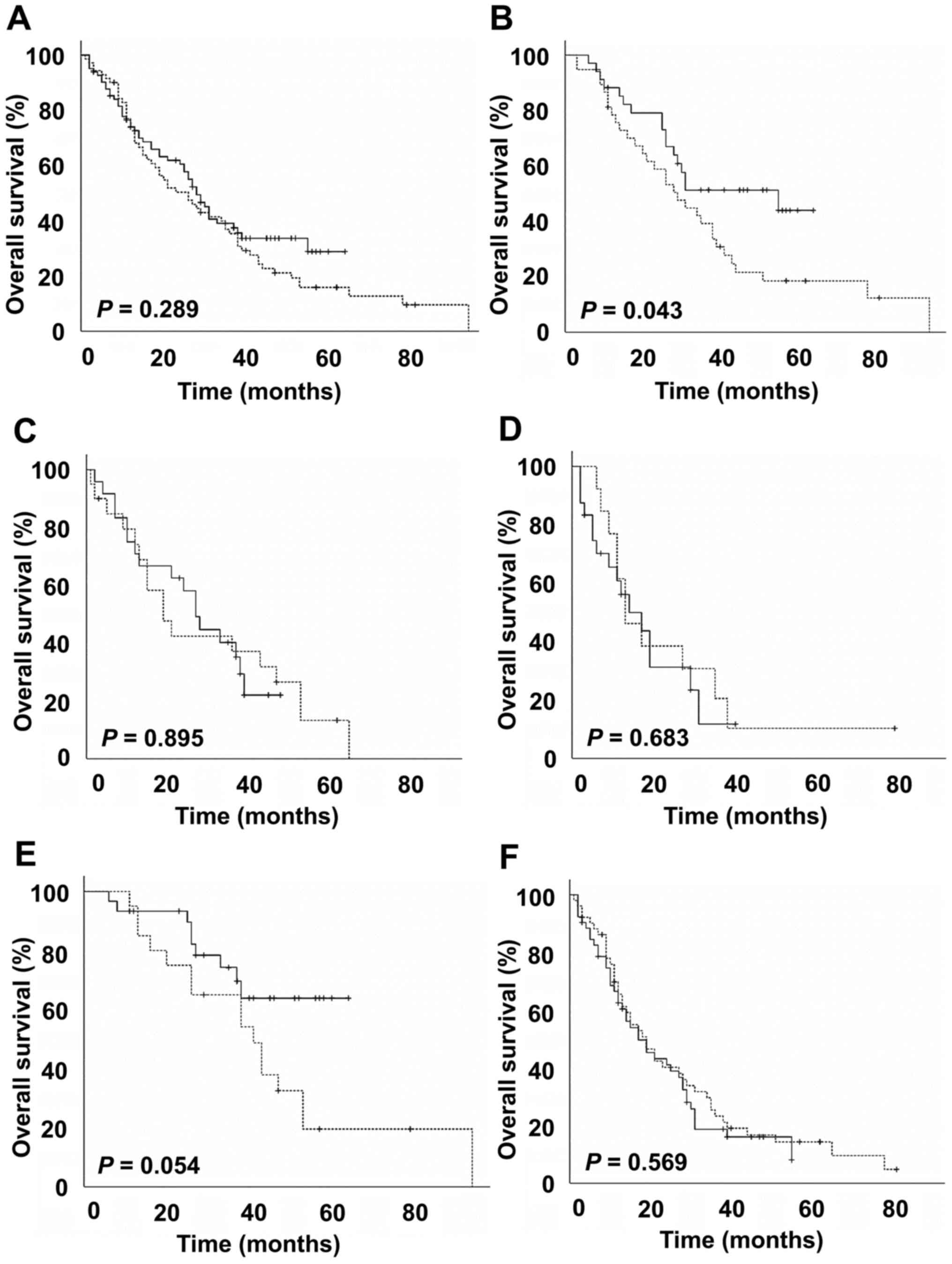
months for the SAP-TACE cohort (Fig.
1A). The overall survival rates were not significantly
different between the two groups (P=0.289). The 1-, 2-, and
3-year overall survival rates were 74, 50 and 35% in the C-TACE
cohort and 75, 60 and 39% in the SAP-TACE cohort, respectively.
Unilobar tumor-distribution, BCLC stage A, Child-Pugh class A, AFP
<400 ng/ml and DCP <1,000 mAU/ml were significant favorable
prognostic factors in the univariate analysis. Age <70,
Child-Pugh class A, AFP <400 ng/ml and DCP <1,000 mAU/ml were
significant favorable prognostic factors in the multivariate Cox
proportional hazard model (Table
II). Treatment modality, i.e., C-TACE or SAP-TACE, did not
affect long-term survival. In a subgroup analysis of patients with
Child-Pugh score of 5, the SAP-TACE cohort showed longer survival
compared to the C-TACE cohort (P=0.043) (Fig. 1B). Subgroup analysis did not show
significant survival differences in patients with Child-Pugh scores
of 6, 7 and 8, and with BCLC stages A and B (Fig. 1C-F).
 | Table IIFactors associated with overall
survival in all patients. |
Table II
Factors associated with overall
survival in all patients.
| | Univariate | Multivariate | |
|---|
| Factor | P-value | P-value | Hazard ratio (95%
CI) |
|---|
| Age (<70 vs. ≥70
years) | 0.696 | 0.037 | 0.625
(0.402-0.972) |
| Sex (male vs.
female) | 0.566 | 0.485 | 0.831
(0.494-1.397) |
| HCV RNA (positive
vs. negative) | 0.299 | 0.434 | 0.842
(0.546-1.297) |
| Tumor distribution
(unilobar vs. bilobar) | <0.001 | 0.161 | 0.701
(0.426-1.153) |
| BCLC stage (A vs.
B) | <0.001 | 0.075 | 0.556
(0.291-1.061) |
| Child-Pugh grade (A
vs. B) | 0.002 | 0.001 | 0.427
(0.256-0.714) |
| AFP (<400 vs.
≥400 ng/ml) | <0.001 | 0.003 | 0.468
(0.284-0.772) |
| DCP (<1,000 vs.
≥1,000 mAU/ml) | <0.001 | 0.007 | 0.512
(0.314-0.835) |
| TACE procedure
(C-TACE vs. SAP-TACE) | 0.289 | 0.319 | 0.804
(0.524-1.234) |
Deterioration of liver function in
patients receiving C-TACE
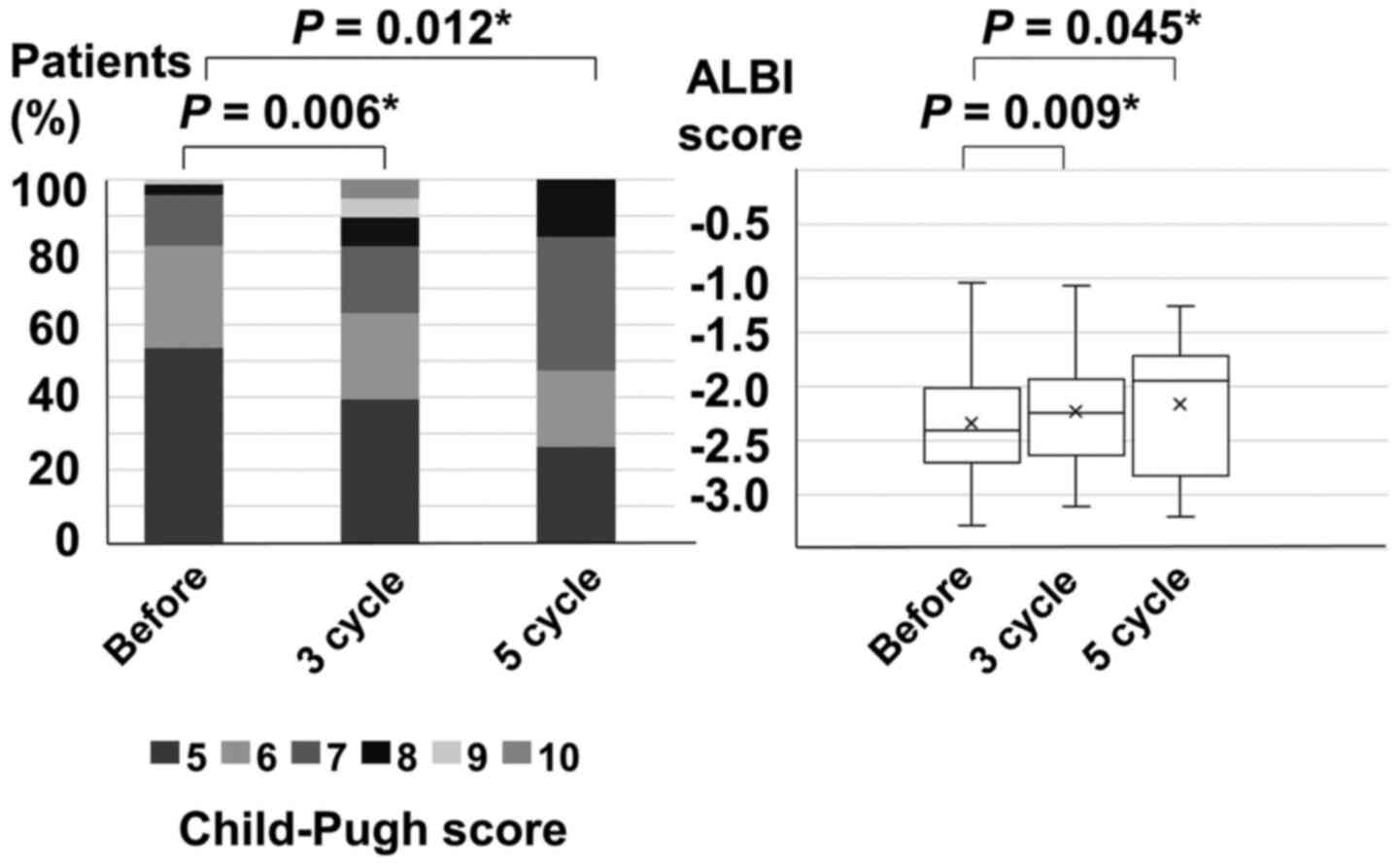
The repeated TACE cycles were three (median) in the
C-TACE cohort. In patients receiving C-TACE, median Child-Pugh
scores were 5 before C-TACE, 6 after 3 cycles of C-TACE, and 7
after 5 cycles of C-TACE. The Child-Pugh scores after 3 and 5 cycle
of C-TACE significantly worsened compared to those before C-TACE
(before vs. 3-cycle, P=0.006; before vs. 5-cycle, P=0.012) (left
side of Fig. 2). The median ALBI
scores were-2.40 before C-TACE, -2.25 after 3 cycles of C-TACE, and
-1.94 after 5 cycles of C-TACE. The ALBI scores after 3 and 5 cycle
C-TACE significantly worsened compared to those before C-TACE
(before vs. 3-cycle, P=0.009; before vs. 5-cycle, P=0.045) (right
side of Fig. 2).
Deterioration of liver function in
patients receiving SAP-TACE
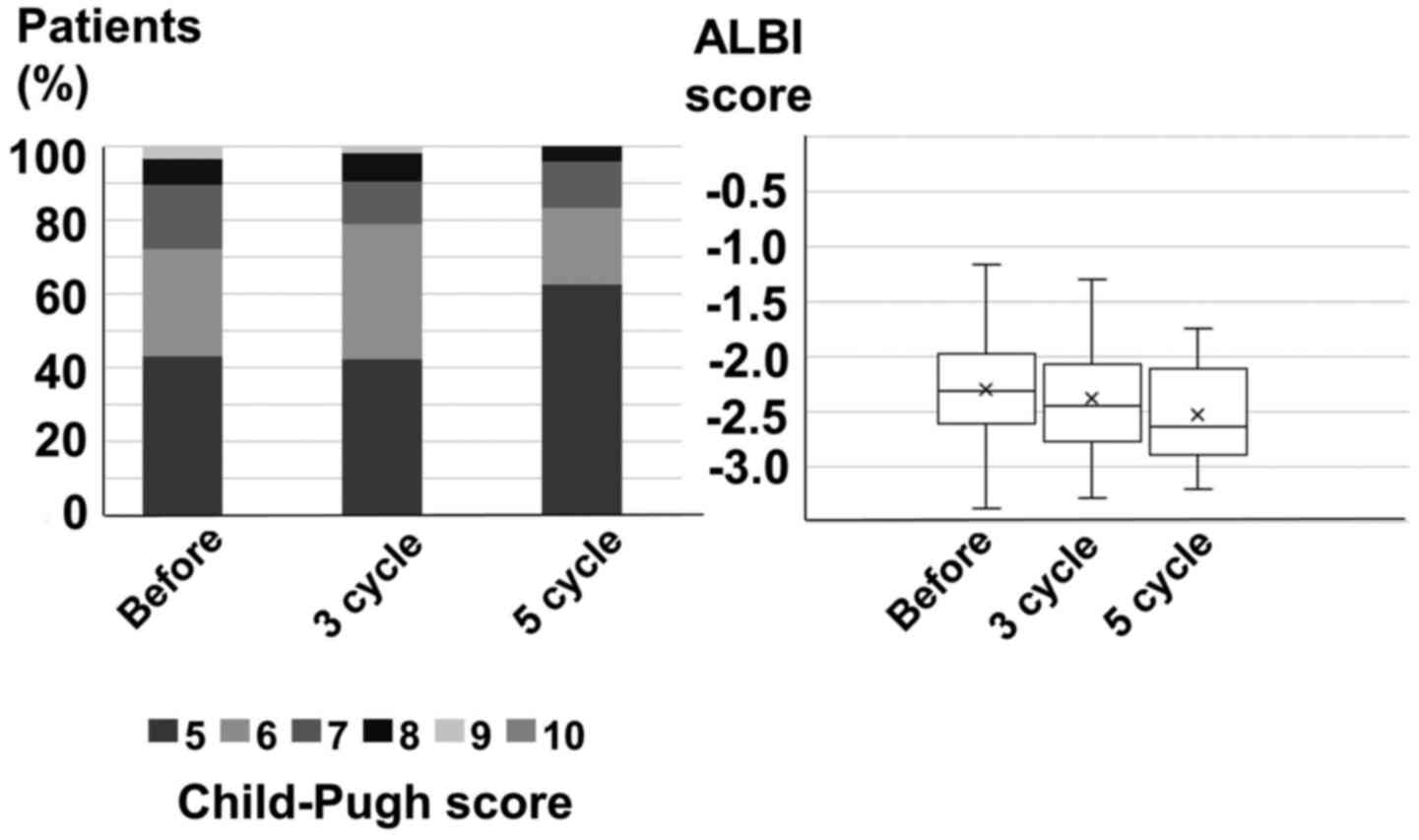
The repeated TACE cycles were three (median) in the
SAP-TACE cohort. In patients receiving SAP-TACE, Child-Pugh scores
were 6 before SAP-TACE, after 3 cycles of SAP-TACE, and after 5
cycles of SAP-TACE. The Child-Pugh scores after 3 and 5 cycles of
SAP-TACE did not change compared to those before SAP-TACE (left
side of Fig. 3). The median ALBI
scores were-2.31 before SAP-TACE, -2.45 after 3 cycles of SAP-TACE,
and -2.64 after 5 cycles of SAP-TACE. The ALBI scores after 3 and 5
cycle of SAP-TACE were not statistically different compared to
those before SAP-TACE (right side of Fig. 3).
Subsequent treatments
Subsequent treatments are showed in Table III. After cessation of C-TACE, 41
patients (57.7%) received secondary treatment with radiofrequency
ablation (RFA) (n=14; 19.7%), surgical resection (n=1; 1.4%),
radiotherapy (n=2; 2.8%), hepatic arterial infusion chemotherapy
(n=15; 21.1%), systemic chemotherapies (n=14; 19.7%). Thirty
patients (42.3%) received no secondary treatment. After cessation
of SAP-TACE, 49 patients (58.3%) received secondary treatment with
RFA (n=22; 26.2%), surgical resection (n=2; 2.4%), radiotherapy
(n=5; 6.0%), hepatic arterial infusion chemotherapy (n=5; 6.0%),
systemic chemotherapies (n=29; 34.5%). Thirty-five patients (41.7%)
received no secondary treatment. The proportions of patients who
received hepatic arterial infusion chemotherapy and systemic
chemotherapies after repeated TACE were different between the
C-TACE cohort and the SAP-TACE cohort (P=0.005 and 0.004,
respectively), although those who received RFA, surgical resection,
and radiotherapy were not different between the C-TACE cohort and
the SAP-cohort (P=0.341, 0.661 and 0.349, respectively).
 | Table IIISubsequent treatment after TACE. |
Table III
Subsequent treatment after TACE.
| Subsequent
treatment | C-TACE (n=71) | SAP-TACE
(n=84) | P-value |
|---|
| RFA, n (%) | 14 (19.7) | 22 (26.2) | 0.342 |
| Surgery, n (%) | 1 (1.4) | 2 (2.4) | 0.661 |
| Radiation, n
(%) | 2 (2.8) | 5 (6.0) | 0.349 |
| Hepatic arterial
infusion, n (%) | 15 (21.1) | 5 (6.0) | 0.005 |
| Systemic
chemotherapy, n (%) | 14 (19.7) | 29 (34.5) | 0.040 |
| None, n (%) | 30 (42.3) | 35 (41.7) | 0.941 |
Discussion
The current study is the first to report a
comparison between the repeated use of C-TACE and SAP-TACE with
respect to long-term overall survival and liver function
deterioration. The current study did not reveal any benefit of
SAP-TACE over C-TACE in long-term survival. The median survival
periods of the C-TACE cohort and the SAP-TACE cohort were 26 and 28
months respectively (P=0.289). However, regarding the deterioration
of liver function after repeated procedures of TACE, unlike C-TACE,
SAP-TACE did not worsen the Child-Pugh and ALBI scores after 3 and
5 cycles of TACE compared with the scores before TACE.
The explanation for the difference in the
deterioration of liver function after the repeated administration
of TACE between SAP-TACE and C-TACE may be related to the incidence
of post-embolization syndrome. Preliminary results from an Italian
multicenter study showed that SAP-TACE was feasible and well
tolerated, with a low complication rate (11). SAP-TACE leads to low plasma levels
of the cytotoxic drug and therefore minimizes toxicity compared to
C-TACE (13). In a randomized
controlled trial, moderate and severe periprocedural pain was less
frequent after TACE with doxorubicin-eluting beads than after
C-TACE (9). In a histological
study, HepaSphere particles penetrated intratumoral vessels
depending on their size but did not reach the hepatic sinusoids or
the peribiliary plexus (10). In
contrast, lipiodol spreads more distally than HepaSphere into the
sinusoids and to the distal portal vein branches, allowing for a
transient dual (arterial and portal) embolization (25). These results could explain why
SAP-TACE using HepaSphere is less invasive than C-TACE using
lipiodol in terms of liver function deterioration after the
procedures. Another study also showed that among 99 evaluated
patients, 90 (90.9%) were not found to have direct damage to the
hepatic arteries after initial SAP-TACE (12).
Recent advances allow chemotherapy with some agents
in cases refractory to TACE in patients with good liver function,
i.e., Child-Pugh class A (18-20).
Johnson et al (24)
introduced a new assessment of liver function, the ALBI grade, that
can evaluate cases with excellent liver function. In patients with
an excellent liver function undergoing chemotherapy, overall
survival outcomes are better than in those with good or poor liver
functions (21,26,27).
In order to ‘pass the baton’ to chemotherapy in TACE-refractory
patients, TACE should be performed repeatedly, with no
deterioration of the liver function caused by the procedures.
Although there were differences between SAP-TACE and
C-TACE with respect to the deterioration of liver function after
repeated TACE, they did not affect long-term outcomes. We
hypothesize that the reason might lie in the treatment selection
differences after refractory TACE. In this study, the choice of
subsequent treatments, including hepatic arterial infusion and
systemic chemotherapy, differed significantly between the two
groups (P=0.005 and 0.04, respectively).
There are some limitations in our study. First, the
study was not randomized and includes the retrospective experience
of a single center. Second, patients in the two cohorts had
different therapeutic backgrounds, i.e., a significantly greater
number of SAP-TACE patients had prior treatment as compared with
C-TACE patients. Differences in biology between primary and
recurrent disease can affect the efficacy of TACE. However, despite
these limitations, the current study is the first to report an
assessment of the choice of TACE. Taking into consideration the
development of new molecular target agents and immune checkpoint
inhibitors (28-30),
we conclude that SAP-TACE might be an appropriate choice for
preserving liver function.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TF, MM, SK, MU and SM made substantial contributions
to conception and design. TF, YS, KK, HA and SN made substantial
contributions to acquisition of data and/or analysis of data. TF
and MM were involved in drafting the manuscript or revising it
critically for important intellectual content. Each author agreed
to be accountable for all aspects of the work in ensuring that
questions related to the accuracy or integrity of any part of the
work are appropriately investigated and resolved. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Institutional
Review Board of Kanagawa Cancer Center and was conducted in
accordance with local laws and the Declaration of Helsinki. All
patients provided written informed consent for the treatment
procedures.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Forner A, Reig M and Bruix J:
Hepatocellular carcinoma. Lancet. 391:1301–1314. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Vauthey JN, Dixon E, Abdalla EK, Helton
WS, Pawlik TM, Taouli B, Brouquet A and Adams RB: American
Hepato-Pancreato-Biliary Association; Society of Surgical Oncology;
Society for Surgery of the Alimentary Tract. Pretreatment
assessment of hepatocellular carcinoma: Expert consensus statement.
HPB (Oxford). 12:289–299. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
European Association for the Study of the
Liver. Electronic address: simpleeasloffice@easloffice.eu;
European Association for the Study of the Liver. EASL clinical
practice guidelines: Management of hepatocellular carcinoma. J
Hepatol. 69:182–236. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Heimbach JK, Kulik LM, Finn RS, Sirlin CB,
Abecassis MM, Roberts LR, Zhu AX, Murad MH and Marrero JA: AASLD
guidelines for the treatment of hepatocellular carcinoma.
Hepatology. 67:358–380. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Omata M, Cheng AL, Kokudo N, Kudo M, Lee
JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, et al:
Asia-Pacific clinical practice guidelines on the management of
hepatocellular carcinoma: A 2017 update. Hepatol Int. 11:317–370.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Facciorusso A, Serviddio G and Muscatiello
N: Transarterial radioembolization vs. chemoembolization for
hepatocarcinoma patients: A systematic review and meta-analysis.
World J Hepatol. 8:770–778. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Facciorusso A, Di Maso M and Muscatiello
N: Drug-eluting beads versus conventional chemoembolization for the
treatment of unresectable hepatocellular carcinoma: A
meta-analysis. Dig Liver Dis. 48:571–577. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Facciorusso A, Mariani L, Sposito C,
Spreafico C, Bongini M, Morosi C, Cascella T, Marchianò A, Camerini
T, Bhoori S, et al: Drug-eluting beads versus conventional
chemoembolization for the treatment of unresectable hepatocellular
carcinoma. J Gastroenterol Hepatol. 31:645–653. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Golfieri R, Giampalma E, Renzulli M, Cioni
R, Bargellini I, Bartolozzi C, Breatta AD, Gandini G, Nani R,
Gasparini D, et al: Randomised controlled trial of
doxorubicin-eluting beads vs. conventional chemoembolisation for
hepatocellular carcinoma. Br J Cancer. 111:255–264. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Osuga K, Khankan AA, Hori S, Okada A,
Sugiura T, Maeda M, Nagano H, Yamada A, Murakami T and Nakamura H:
Transarterial embolization for large hepatocellular carcinoma with
use of superabsorbent polymer microspheres: Initial experience. J
Vasc Interv Radiol. 13:929–934. 2002.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Grosso M, Vignali C, Quaretti P, Nicolini
A, Melchiorre F, Gallarato G, Bargellini I, Petruzzi P, Massa
Saluzzo C, Crespi S and Sarti I: Transarterial chemoembolization
for hepatocellular carcinoma with drug-eluting microspheres:
Preliminary results from an Italian multicentre study. Cardiovasc
Intervent Radiol. 31:1141–1149. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Seki A, Hori S, Kobayashi K and Narumiya
S: Transcatheter arterial chemoembolization with epirubicin-loaded
superabsorbent polymer microspheres for 135 hepatocellular
carcinoma patients: Single-center experience. Cardiovasc Intervent
Radiol. 34:557–565. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
van Malenstein H, Maleux G, Vandecaveye V,
Heye S, Laleman W, van Pelt J, Vaninbroukx J, Nevens F and Verslype
C: A randomized phase II study of drug-eluting beads versus
transarterial chemoembolization for unresectable hepatocellular
carcinoma. Onkologie. 34:368–376. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Duan F, Wang EQ, Lam MG, Abdelmaksoud MH,
Louie JD, Hwang GL, Kothary N, Kuo WT, Hofmann LV and Sze DY:
Superselective chemoembolization of HCC: Comparison of short-term
safety and efficacy between drug-eluting LC beads, quadraspheres,
and conventional ethiodized oil emulsion. Radiology. 278:612–621.
2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kucukay F, Badem S, Karan A, Ozdemir M,
Okten RS, Ozbulbul NI, Kucukay MB, Unlu I, Bostanci EB and Akdogan
M: A single-center retrospective comparison of doxorubicin-loaded
hepasphere transarterial chemoembolization with conventional
transarterial chemoembolization for patients with unresectable
hepatocellular carcinoma. J Vasc Interv Radiol. 26:1622–1629.
2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Morimoto M, Kobayashi S, Moriya S, Ueno M,
Tezuka S, Irie K, Goda Y and Ohkawa S: Short-term efficacy of
transarterial chemoembolization with epirubicin-loaded
superabsorbent polymer microspheres for hepatocellular carcinoma:
Comparison with conventional transarterial chemoembolization. Abdom
Radiol (NY). 42:612–619. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Facciorusso A: Drug-eluting beads
transarterial chemoembolization for hepatocellular carcinoma:
Current state of the art. World J Gastroenterol. 24:161–169.
2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bruix J, Qin S, Merle P, Granito A, Huang
YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, et al:
Regorafenib for patients with hepatocellular carcinoma who
progressed on sorafenib treatment (RESORCE): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet. 389:56–66.
2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kudo M, Finn RS, Qin S, Han KH, Ikeda K,
Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al: Lenvatinib
versus sorafenib in first-line treatment of patients with
unresectable hepatocellular carcinoma: A randomised phase 3
non-inferiority trial. Lancet. 391:1163–1173. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hiraoka A, Kumada T, Kudo M, Hirooka M,
Koizumi Y, Hiasa Y, Tajiri K, Toyoda H, Tada T, Ochi H, et al:
Hepatic function during repeated TACE procedures and prognosis
after introducing sorafenib in patients with unresectable
hepatocellular carcinoma: Multicenter analysis. Dig Dis.
35:602–610. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Miksad RA, Ogasawara S, Xia F, Fellous M
and Piscaglia F: Liver function changes after transarterial
chemoembolization in US hepatocellular carcinoma patients: The
LiverT study. BMC Cancer. 19(795)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lencioni R, de Baere T, Burrel M, Caridi
JG, Lammer J, Malagari K, Martin RC, O'Grady E, Real MI, Vogl TJ,
et al: Transcatheter treatment of hepatocellular carcinoma with
doxorubicin-loaded DC Bead (DEBDOX): Technical recommendations.
Cardiovasc Intervent Radiol. 35:980–985. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Johnson PJ, Berhane S, Kagebayashi C,
Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A,
Palmer D, et al: Assessment of liver function in patients with
hepatocellular carcinoma: A new evidence-based approach-the ALBI
grade. J Clin Oncol. 33:550–558. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Idee JM and Guiu B: Use of Lipiodol as a
drug-delivery system for transcatheter arterial chemoembolization
of hepatocellular carcinoma: A review. Crit Rev Oncol Hematol.
88:530–549. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Pinato DJ, Yen C, Bettinger D, Ramaswami
R, Arizumi T, Ward C, Pirisi M, Burlone ME, Thimme R, Kudo M and
Sharma R: The albumin-bilirubin grade improves hepatic reserve
estimation post-sorafenib failure: Implications for drug
development. Aliment Pharmacol Ther. 45:714–722. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ueshima K, Nishida N, Hagiwara S, Aoki T,
Minami T, Chishina H, Takita M, Minami Y, Ida H, Takenaka M, et al:
Impact of baseline ALBI grade on the outcomes of hepatocellular
carcinoma patients treated with lenvatinib: A multicenter study.
Cancers (Basel). 11(952)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
El-Khoueiry AB, Sangro B, Yau T, Crocenzi
TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, et al:
Nivolumab in patients with advanced hepatocellular carcinoma
(CheckMate 040): An open-label, non-comparative, phase 1/2 dose
escalation and expansion trial. Lancet. 389:2492–2502.
2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Abou-Alfa GK, Meyer T, Cheng AL,
El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park
JW, et al: Cabozantinib in patients with advanced and progressing
hepatocellular carcinoma. N Engl J Med. 379:54–63. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhu AX, Finn RS, Edeline J, Cattan S,
Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A,
et al: Pembrolizumab in patients with advanced hepatocellular
carcinoma previously treated with sorafenib (KEYNOTE-224): A
non-randomised, open-label phase 2 trial. Lancet Oncol. 19:940–952.
2018.PubMed/NCBI View Article : Google Scholar
|