Introduction
Primary cutaneous CD30+
lymphoproliferative disorders (LPDs) are rare diseases but
represent the second most frequent form of cutaneous T-cell
lymphoma after mycosis fungoides (1,2).
According to the 2018 update of the WHO-EORTC classification,
CD30+ T-cell LPDs range from lymphomatoid papulosis
(LYP) to primary cutaneous anaplastic large-cell lymphoma (PCALCL)
and constitute a spectrum of disorders expressing CD30 antigen in
tumor cells (1,3,4). The
two conditions feature different presentation and clinical course:
LYP is characterized by papulonodular lesions undergoing
spontaneous regression over a chronic course of years to decades,
while PCALCL manifests with solitary or grouped, rapidly growing
and ulcerating tumors or plaques. PCALCL typically affects older
males and in 20% of cases presents multifocal skin involvement. In
30% of cases, PCALCL tends to spontaneous regression, yet to a
lesser extent and duration than LYP (1). Diagnosis is based on histopathology
and immunohistochemistry revealing CD30+ large
pleomorphic or anaplastic T-cells; in PCALCL at least 75% of tumor
cells are CD30+ (1,3).
Extra-cutaneous disease spreading should be ruled out with staging
procedures including whole body PET-CT scan and, in the case of
multifocal tumors, extracutaneous disease and abnormal laboratory
exams, bone marrow examination (1).
Management of PCALCL should be tailored to each case taking into
account the severity of cutaneous involvement and the indolent
nature of the disease, with 5-year survival rates between 76-96%
(1). The disease has an intrinsic
tendency to skin relapse in ~39% of patients, associated to a
limited risk of systemic spreading in only 13% of cases (2). In cases of localized disease, a
wait-and-see strategy can be appropriate as spontaneous complete
remission occurs in up to 22% of cases after a median period of 2
months (range 1-6 months) (1,2).
Surgical excision or local radiation can be additionally considered
based on tumor burden (2). The
approach to multifocal or disseminate disease should consider that
aggressiveness of therapy does not influence the frequency of
relapse and that consequently the aim of treatment cannot be
sustained remission. Alternatives in disseminate or progressive
forms include single cytotoxic drugs, such as methotrexate, or
polychemotherapy regimens based on anthracyclines, such as CHOP
(3). Brentuximab vedotin (BV) has
recently shown promising results in the treatment of PCALCL even if
the optimal dosing is currently not defined.
Case presentation
A 62-year-old Caucasian man was referred to our
Dermatology Department complaining the abrupt appearance and quick
advancement of cutaneous nodules, associated with a sensation of
skin pain and tenderness. The patient reported the onset of a
single lesion in the right frontal region, progressively increasing
in size since June 2019, followed by the appearance of other
nodules in the scalp and finally by the rapid development of
another nodular left frontal lesion in August 2019. The patient had
a personal history of arterial hypertension, gastroduodenal ulcer
and bilateral ischemic optic neuropathy in therapy with atenolol,
perindopril, indapamide, amlodipine, acetylsalicylic acid and
pantoprazole. Clinical examination revealed diffuse nodules on the
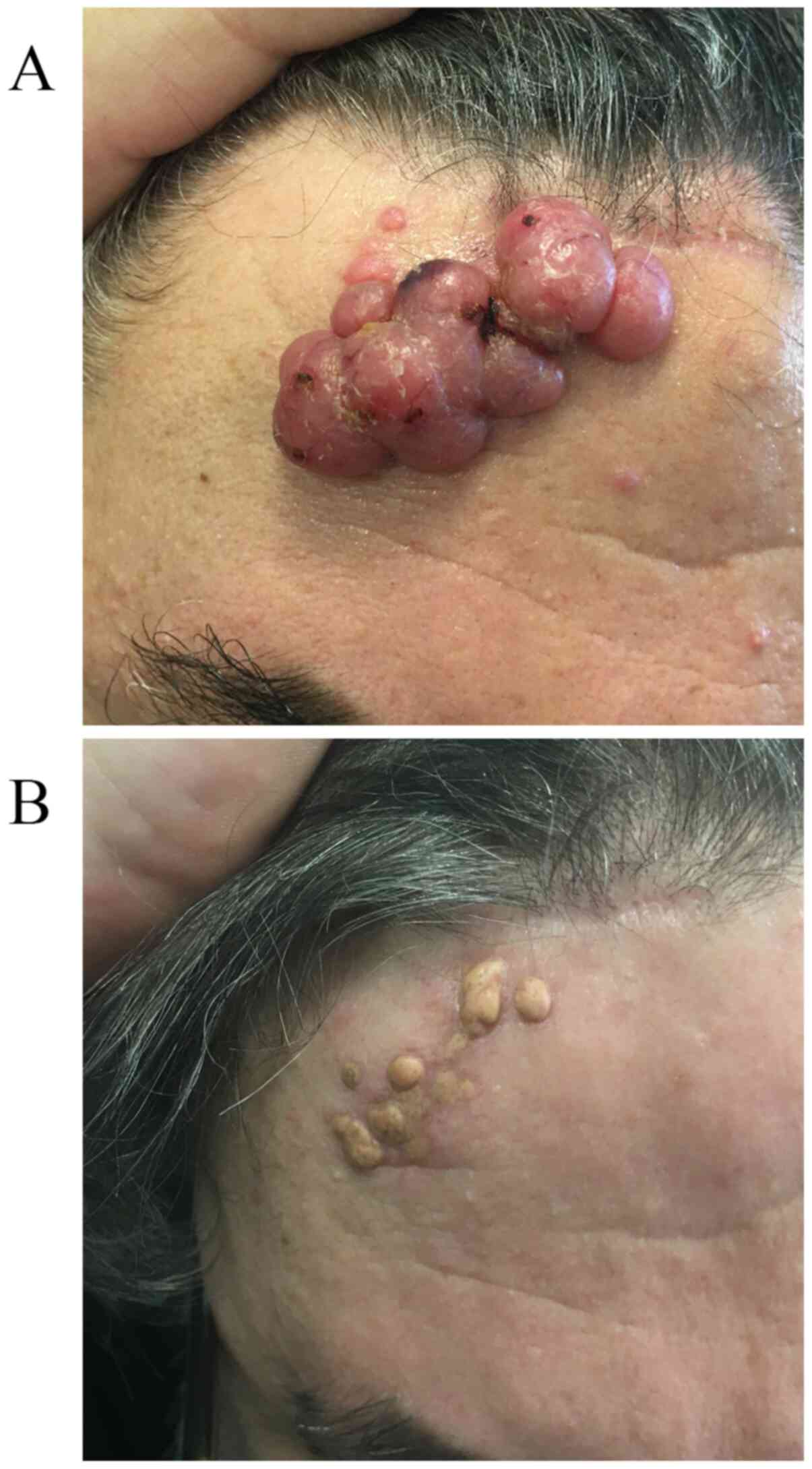
scalp and on the frontal area, where the two major lesions, 4.5 cm
diameter in the right frontal region (Fig. 1A) and 8 cm in the left, were
observed. The nodular lesions were elevated ~3 cm from the
surrounding skin, firm to touch and presented an ulcerated
erythematous surface. The characteristics of lesions and the
history of rapid growth were highly suggestive for a cutaneous
localization of lymphoproliferative disease.
Multiple biopsy specimens were obtained in September
and October, setting a differential diagnosis between LYP and
PCALCL. A CT-scan ruled out extra-cutaneous localizations of
disease and peripheral blood cytometry showed no abnormal
immunophenotypes. Finally, a bone marrow specimen was obtained, and
the results proved normal bone cellularity with no signs of
cellular atypia and a maintained leuco-erithroblastic ratio.
As soon as the diagnosis was confirmed by histology,
we started therapy with methotrexate 7.5 mg sc weekly and
prednisone 25 mg po daily. At the follow-up visit, scheduled after
one month, we found a size increase in the lesions and we raised
the dose of methotrexate up to 15 mg weekly and considered
alternative treatments. Because of the clinical extent and
aggressive behavior of the disease we decided to start therapy with
BV at the dosing of 180 mg (1.8 mg/kg) every three weeks. Clinical
assessment at three weeks from the first infusion of BV showed an
important decrease in volume of lesions, with the prominent left
frontal reduced to 3.5 cm and the right one to less than 1 cm in
diameter. Two weeks after the second infusion, we appreciated a
complete remission of all skin lesions with only mild
hyperpigmented and fibrotic results (Fig. 1B).
The patient is scheduled for 16 infusions of
brentuximab 180 mg every three weeks, four of which have been
already administered. The disease is in complete remission and the
patient complained no adverse events following treatment. Monthly
monitoring of laboratory tests before and during treatment showed
no abnormal values.
Discussion
Currently, there is no standard therapeutic approach
for cases of multifocal PCALCL. Combination chemotherapy has been
frequently administered for multifocal primary cutaneous disease,
even though no specific regimen has been reported as superior and
doxorubicin-based combinations, such as CHOP (cyclophosphamide,
doxorubicin, vincristine and prednisone), are more frequently used.
On the basis of a review by Shehan et al relapses seem to
occur more frequently in patients receiving traditional
chemotherapy and this result doesn't correlate with a more
aggressive treatment of extensive disease (3). Moreover, the Dutch Cutaneous Lymphoma
Group found that all patients treated with CHOP regimen developed
one or more relapses in the skin and authors suggested not to use
traditional chemotherapy for multifocal ALCL involving the skin
(5). Radiotherapy or low-dose
methotrexate, that was initially administered to our patient, have
also been proposed as first-line treatment for multifocal or
relapsing PCALCL limited to skin (5,6).
Moreover, systemic retinoids including bexarotene, interferon
alpha-2a and thalidomide have been effective treatments for
multifocal PCALCL (1). Etoposide
monotherapy and autologous bone-marrow transplantation have also
been proposed for relapsed multifocal disease (3).
Finally, BV is an antibody-drug conjugate (ADC) that
combines a chimeric anti-CD30 antibody to the anti-microtubule
agent monomethyl auristatin E (MMAE) (7,8). Upon
binding to CD30 receptor, the ADC is internalized by endocytosis
and undergoes consequent lysosomal degradation allowing MMAE to
bind microtubules and cause cell cycle arrest and apoptosis
(7). CD30 is a cell surface
leukocyte antigen constituted by a type I transmembrane
glycoprotein and an extracellular domain homologous to tumor
necrosis factor and nerve growth factor receptor family members.
CD30 is normally expressed in activated T, B, and natural killer
lymphocytes. Certain LPDs (including Hodgkin lymphoma, ALCL, CTCL,
a fraction of diffuse large B-cell lymphomas and of follicular
lymphomas) and Kaposi sarcoma express the CD30 antigen as well
(2,9). The function of CD30 has not been
defined yet, but it seems implicated in both cell death and
proliferation (9). Targeted
delivery of MMAE to CD30 expressing tumor cells makes BV a
well-suited target for immunotherapy. Based on our observation of
significant disease response after the first BV infusion and
complete remission after the second one, we suggest that high-dose
short course therapy with BV could be an appropriate approach for
PCALCL. While therapeutic management of Hodgkin lymphoma (HL) with
BV is better established and more clinical data are available,
PCALCL treatment protocol is not yet well defined. BV is approved
by the FDA for both HL and ALCL at an intravenous dose of 1.8 mg/kg
every 3 weeks (7). While treatment
is usually administered for a maximum of 16 cycles or until either
disease progression or unacceptable toxicity occur, multiple
studies have been conducted in recent years with different dosing
regimens of BV (7). Fanale et
al in a phase I study with CD30+ LPDs administered a
0.4-1.4 mg/Kg/dose weekly for 3 weeks every 28 days obtaining an
overall response rate (ORR) of 59% and a complete response (CR) of
34% (10). A phase II study of BV
in PCALCL enrolled 11 patients receiving six doses each of 0.4-1.2
mg/kg, achieving an ORR of 82% and a CR in 55% of cases (9). Though limited, these results support
the hypothesis that clinical response to BV in PCALCL could be
dose-related and suggest use of maximal dosing equivalent to 1.8
mg/kg. Moreover, the fast response observed in our case report
could be explained by the high percentage of CD30+ cells
present in PCALCL on which the drug exerts a targeted mechanism of
action. PCALCL expresses CD30 antigen in at least 75% of tumor
mass, thus implicating that after each cycle of BV ~75% of cells
die as a result of direct killing. In addition, such a rapid
dimensional decrease could be the result of an indirect action of
BV. In fact, MMAE produce a well-known toxic effect by diffusing
into surrounding stroma, destroying not only cells internalizing
the ADC but also proximal tumor cells (7). Furthermore, our patient experienced a
quick reduction in lesion size after the first dose, which is
consistent with the pharmacokinetics of MMAE gaining maximum
concentration approximately 1-3 days post infusion (7).
High dose protocols over a short course may be
associated with a beneficial safety profile. Therapy with BV has
been related to toxicities mostly of grade 1 or 2. The most common
adverse reactions, observed in >20% of cases, have been
peripheral sensory neuropathy, fatigue, diarrhea, neutropenia,
vomiting, pyrexia, anemia, upper respiratory tract infections,
fever, thrombocytopenia (7,11). However, serious adverse reactions
such as peripheral motor neuropathy, septic shock, supraventricular
arrhythmia, progressive multifocal leukoencephalopathy and one case
of Steven-Johnson syndrome were reported in phase II trials
(7). Peripheral neuropathy is the
most common side effect, experienced by 55% of patients,
responsible for treatment discontinuation in 12% of cases and for
dose-reduction in 10% (7).
Moreover, this side effect seems to be more common when the drug is
administered weekly rather than every three weeks. Younes et
al reported 22% of patients experiencing peripheral neuropathy
with 1.8 mg/kg every 3 weeks of BV compared to 66% of cases
described by Fanale et al with a weekly dose of 0.4-1.4
mg/kg (10,12). Although usually reversible,
peripheral neuropathy is typically an effect of cumulative
toxicity, thus encouraging reduction in number of cycles and
frequency of administration (7).
In the end, considering an estimate cost of ~200.000
euros for 16 cycles of BV in a patient of 80 kg, a short cycle may
considerably improve cost-effectiveness (13).
In conclusion, PCALCL has a generally indolent
behavior even in disseminated presentations and shows frequent
cutaneous relapses that maintain responsiveness to treatments.
Well-designed clinical trials are needed to determine the efficacy
of high dose short course therapy with BV and to assess whether
there are other potential advantages over standard 16 cycle
protocol.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
EM, PM and AS prepared the manuscript. EM and PM
performed the literature analysis search. AS, MA, SF and DM
conceived and designed the current study. AS and MA drafted and
critically revised the manuscript for important intellectual
content. AS prepared the figures. MA gave final approval of the
version to be published. All authors read and approved the final
manuscript. MA and AS confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent for publication of their
clinical details and clinical images was obtained from the
patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kempf W, Pfaltz K, Vermeer MH, Cozzio A,
Ortiz-Romero PL, Bagot M, Olsen E, Kim YH, Dummer R, Pimpinelli N,
et al: EORTC, ISCL, and USCLC consensus recommendations for the
treatment of primary cutaneous CD30-positive lymphoproliferative
disorders: Lymphomatoid papulosis and primary cutaneous anaplastic
large-cell lymphoma. Blood. 118:4024–4035. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Liu HL, Hoppe RT, Kohler S, Harvell JD,
Reddy S and Kim YH: Cd30+ cutaneous lymphoproliferative
disorders: The stanford experience in lymphomatoid papulosis and
primary cutaneous anaplastic large cell lymphoma. J Am Acad
Dermatol. 49:1049–1058. 2003.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Shehan JM, Kalaaji AN, Markovic SN and
Ahmed I: Management of multifocal primary cutaneous
CD30+ anaplastic large cell lymphoma. J Am Acad
Dermatol. 51:103–110. 2004.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Willemze R, Cerroni L, Kempf W, Berti E,
Facchetti F, Swerdlow SH and Jaffe ES: The 2018 update of the
WHO-EORTC classification for primary cutaneous lymphomas. Blood.
133:1703–1714. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bekkenk MW, Geelen FA, van Voorst Vader
PC, Heule F, Geerts ML, van Vloten WA, Meijer CJ and Willemze R:
Primary and secondary cutaneous CD30(+) lymphoproliterative
disorders: A report from the Dutch Cutaneous Lymphoma Group on the
long-term follow-up data of 219 patients and guidelines for
diagnosis and treatment. Blood. 95:3653–3661. 2000.PubMed/NCBI
|
|
6
|
Artemi P, Wong D, Mann S and Regan W: CD30
(Ki-1)-positive primary cutaneous T-cell lymphoma: Report of
spontaneous resolution. Australas J Dermatol. 38:206–208.
1997.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Minich SS: Brentuximab vedotin: A new age
in the treatment of hodgkin lymphoma and anaplastic large cell
lymphoma. Ann Pharmacother. 46:377–383. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Donato EM, Fernández-Zarzoso M, Hueso JA
and de la Rubia J: Brentuximab vedotin in hodgkin lymphoma and
anaplastic large-cell lymphoma: An evidence-based review. Onco
Targets Ther. 11:4583–4590. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Duvic M, Reddy SA, Pinter-Brown L, Korman
NJ, Zic J, Kennedy DA, Lorenz J, Sievers EL and Kim YH: A phase II
study of SGN-30 in cutaneous anaplastic large cell lymphoma and
related lymphoproliferative disorders. Clin Cancer Res.
15:6217–6224. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Fanale MA, Forero-Torres A, Rosenblatt JD,
Advani RH, Franklin AR, Kennedy DA, Han TH, Sievers EL and Bartlett
NL: A phase I weekly dosing study of brentuximab vedotin in
patients with relapsed/refractory CD30-positive hematologic
malignancies. Clin Cancer Res. 18:248–255. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gravanis I, Tzogani K, van Hennik P, de
Graeff P, Schmitt P, Mueller-Berghaus J, Salmonson T, Gisselbrecht
C, Laane E, Bergmann L, et al: The European medicines agency review
of brentuximab vedotin (Adcetris) for the treatment of adult
patients with relapsed or refractory CD30+ hodgkin
lymphoma or systemic anaplastic large cell lymphoma: Summary of the
scientific assessment of the committee. Oncologist. 21:102–109.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Younes A, Bartlett NL, Leonard JP, Kennedy
DA, Lynch CM, Sievers EL and Forero-Torres A: Brentuximab vedotin
(SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med.
363:1812–1821. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Prince HM, Kim YH, Horwitz S, Dummer R,
Scarisbrick J, Quaglino P, Zinzani PL, Wolter P, Sanches JA,
Ortiz-Romero PL, et al: Brentuximab vedotin or physician's choice
in CD30-positive cutaneous T-cell lymphoma (ALCANZA): An
international, open-label, randomised, phase 3, multicentre trial.
Lancet. 390:555–566. 2017.PubMed/NCBI View Article : Google Scholar
|