Introduction
Colon cancer (CC) is the third highest cause of
cancer-associated mortality in the United States (1). In Japan, CC is the leading cause of
mortality in women and the third highest cause in men. A total of
101,952 patients were newly diagnosed with CC in 2017 and 35,414
patients died due to CC in 2018(2).
Surgery is the most suitable treatment for patients with resectable
CC and the number of laparoscopic surgeries performed in Japan is
increasing (3). In advanced CC with
distant metastases, prolonged survival be achieved by
multidisciplinary therapy such as surgery, chemotherapy,
immunotherapy and radiation, but patients still have a poor
prognosis; the 5-year overall survival rate is 16.7% in Japan
(4). Because recurrent CC also has
a poor prognosis, recurrence prevention contributes to extending
survival. Postoperative adjuvant chemotherapy aims to decrease
recurrence in patients with stage II/III disease following R0
surgery.
Oxaliplatin (OX), a therapeutic in the
platinum-based antineoplastic family, acts by blocking DNA
replication and is a key drug together with fluorinated pyrimidine.
Based on the results of Multicenter International Study of
OX/5-FU-LV in the Adjuvant Treatment of CC (MOSAIC), National
Surgical Adjuvant Breast and Bowel Project (NSABP) and capecitabine
+ OX adjuvant (XELOXA) trials (5-8),
OX-containing regimens such as folinic acid + fluorouracil (FU) +
OX (FOLFOX), infused 5-FU + leucovorin + OX (FLOX) and peroral
capecitabine + infused OX (CapOX) are standard therapies for
postoperative adjuvant chemotherapy for patients with CC. However,
in OX induction, persistent OX-induced peripheral neuropathy is
often an adverse effect and a number of patients are concerned
about this refractory symptom (9).
While OX-containing regimens are a worldwide
standard in the adjuvant setting for postoperative patients with
CC, PO fluorinated pyrimidine-based therapies, such as
uracil-tegafur/leucovorin (UFT/LV), tegafur/gimeracil/oteracil
(S-1) and capecitabine are commonly used, especially in Japan for
patients with CC with a relatively low risk of recurrence (5-8).
Treatment with such PO fluorinated pyrimidine agents is also
standard, based on the results of NSABP, Japan Clinical Oncology
Group, Xeloda in Adjuvant CC Therapy (ACT), ACT Stage III CC and
Stage II/III Rectal Cancer trials (10-14).
T4, N2, high levels of tumor markers, including
carcinoembryonic antigen (CEA) and cancer antigen (CA)19-9, and
lymphatic or vessel permeation have been reported as high-risk
factors for CC recurrence (15-18);
however, a subgroup analysis of previous randomized trials did not
show superiority of OX-containing regimens in patients with these
high-risk factors (5,6,8). Thus,
it remains controversial as to which patients should be treated
with OX-containing regimens. The present study aimed to identify
patients with high relapse rates by analyzing clinicopathological
factors among those treated with PO fluorinated pyrimidine agents
without OX and used propensity score matching analysis to determine
whether patients with certain characteristics should receive more
effective chemotherapeutic agent-containing regimens, such as those
that incorporate OX.
Materials and methods
Patients and data collection
The inclusion criteria were consecutive patients
with CC with curative resection who underwent adjuvant chemotherapy
and were histologically diagnosed as stage II/III colon
adenocarcinoma at two tertiary medical institutions (Kitakyushu
Municipal Medical Center, Kitakyushu, and the Department of Surgery
and Oncology at Kyushu University, Higaki, Japan) between September
2009 and March 2016. Patients with two or more cancer lesions or
who had been treated with preoperative adjuvant therapy were
excluded. Medical records of 1,065 patients with stage II/III CC
were retrospectively reviewed and 501 patients with postoperative
adjuvant chemotherapy were identified. Three patients treated with
irinotecan-containing regimens were excluded. Finally, a total of
349 and 149 patients treated with PO and OX-containing regimens,
respectively, were enrolled for subsequent analysis. The median
observation period was 1,689 days. The informed consent requirement
was waived because of the retrospective nature of the study, in
which patient data was kept confidential. All procedures conformed
to the ethical guidelines of the Japanese Government and the
Declaration of Helsinki. This retrospective study was approved by
the ethics committee of Kitakyushu Municipal Medical Center
(approval no. 201801055).
Clinicopathological factors
Preoperative variables were institution, sex, age,
tumor location, preoperative bowel obstruction and preoperative
serum levels of CEA and CA19-9. The intra- and postoperative
variables were surgical procedure (open or laparoscopic), blood
loss volume, operating time, number of harvested lymph nodes,
hospital stay length and morbidity. Histological variables were
tumor differentiation, T and N stage and lymphatic or vessel
invasion. Tumor location was defined as follows: Right colon,
including cecum, ascending and transverse colon; and left colon,
including descending and sigmoid colon and rectosigmoid junction.
The pathological tumor stage was defined according to the Union for
International Cancer Control (UICC) TNM classification (19). Tumor differentiation was defined as
well-, moderately and poorly differentiated (20). Postoperative complications were
evaluated using the Clavien-Dindo classification (21) as follows: Grade I, deviation from
the normal course without need for treatment; grade II,
complication requiring pharmacological treatment; grade III,
complication requiring surgical, endoscopic or radiological
intervention; grade IV, life-threatening complication requiring
intensive care unit management; and grade V, patient mortality. In
the present study, complications greater than grade II were defined
as postoperative complications. Postoperative mortality indicated
30-day or in-hospital deaths.
Adjuvant chemotherapy regimens
Postoperative adjuvant chemotherapy, regimens and
dosages were determined by individual attending physicians,
referring to the multi-disciplinary cancer board. Patients who
received adjuvant chemotherapy for stage II cancer without high
risk factors for recurrence (T4, lymphatic or vascular invasion,
preoperative bowel obstruction and high histological grade) were
excluded from the present study. All high-risk stage II and stage
III patients were considered suitable for adjuvant chemotherapy,
except for those intolerant to chemotherapy and those who did not
consent to chemotherapy. In most cases, postoperative adjuvant
chemotherapy was administered to patients with stage III colorectal
cancer (CRC), but it was not recommended for all patients with
stage II CRC. OX-containing regimens were recommended primarily for
patients with ≥4 lymph node metastases (N2), tumor-invaded adjacent
organs or perforated visceral peritoneum (T4), but OX could be
initiated at other stages following a decision by either the
patient or the treating physician referring to the
multi-disciplinary cancer board.
Statistical analysis
The association between two variables was analyzed
using chi-squared or Fisher's exact test as appropriate. For the
survival analysis, recurrence-free survival (RFS) was adopted as
the primary endpoint. Recurrence data included the presence and
date of recurrence and the last follow-up date in patients without
recurrence. The last follow-up date was December 2020. The survival
analysis was performed using Kaplan-Meier analysis and the curves
were compared using the log-rank test. Continuous variables were
expressed as the median and the range was assessed using
Mann-Whitney U test. The cutoff of the tumor marker was determined
according to the upper normal limit at Kitakyushu Municipal Medical
Center and Kyushu University Hospital. Multivariate survival
analysis was evaluated by Cox's proportional hazard model. In order
to minimize the impact of selection bias and other confounding
factors, propensity score-matched analysis was performed as
previously described (22).
Propensity scores were generated using clinicopathological
characteristics including institution, age, sex, tumor location and
differentiation, pathological T and N stage and lymphatic or vessel
invasion. Propensity scores were matched using a caliper width of
0.05 multiplied by the standard deviation of values calculated by a
logistic regression model. Each patient with an OX-containing
regimen was matched to a patient with a PO regimen using a
one-to-one nearest neighbor-matching algorithm without replacement.
Following matching, there were 105 patients in each group. All
statistical analyses were performed using JMP 15 software (SAS
Institute, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
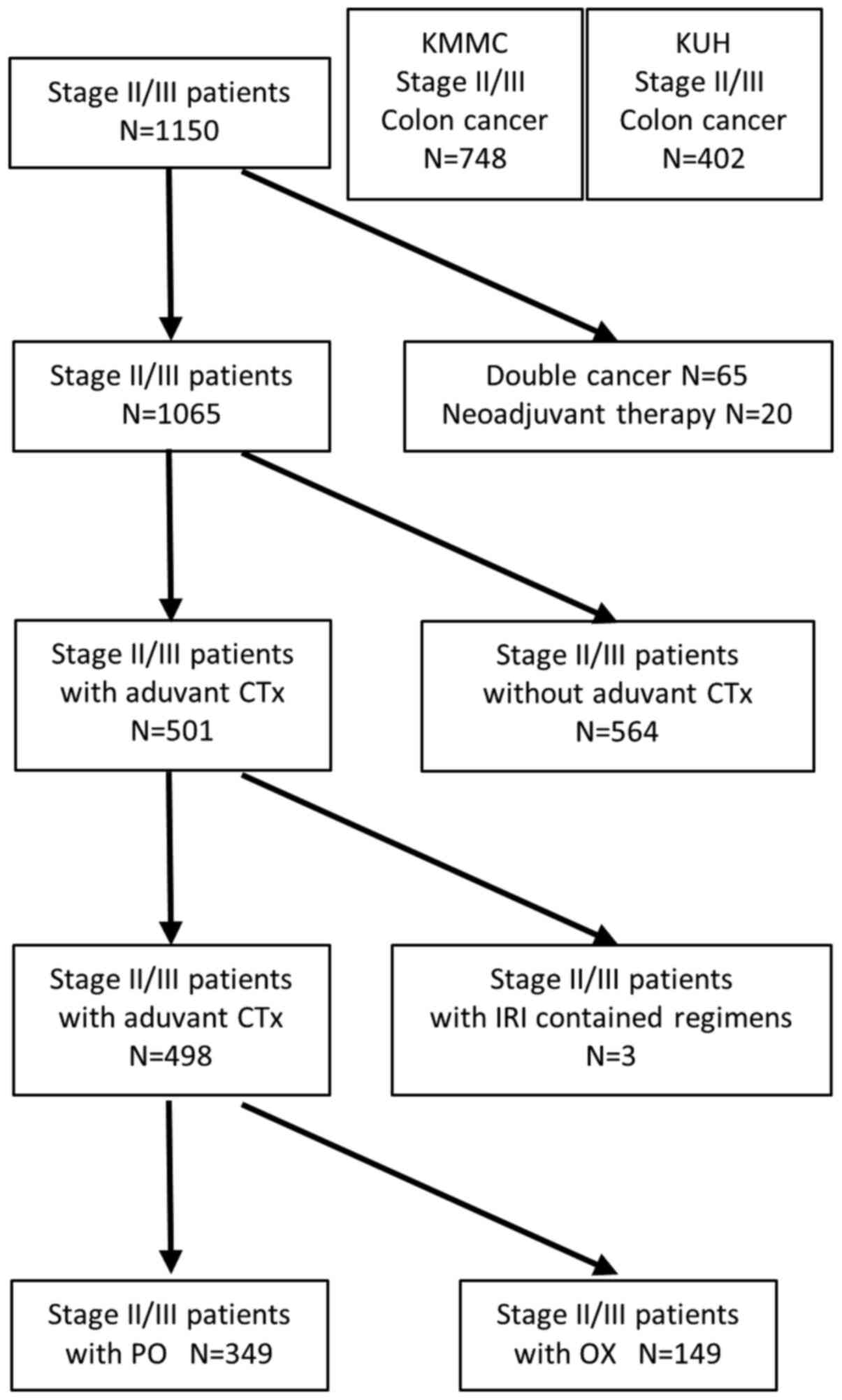
Fig. 1 shows the
distribution of patients. Among all patients who underwent curative
resection and were diagnosed with colon adenocarcinoma, 85 patients
who either had ≥2 cancer occurrences or underwent preoperative
therapy were excluded. A total of 564 patients received no
postoperative adjuvant chemotherapy, 149 patients were treated with
OX-containing regimens and 349 patients were treated with PO
fluorinated pyrimidine agent. In addition, we also excluded three
patients who received irinotecan-containing regimens (folinic acid
+ FU + irinotecan and irinotecan + S-1).
Table I details the
chemotherapy regimens of the PO and OX groups. Patients in the PO
group were administered sole UFT and 5'-deoxy-5-fluorouridine
regimens at a relatively early stage.
 | Table IChemotherapy regimens administered to
patients with colorectal cancer. |
Table I
Chemotherapy regimens administered to
patients with colorectal cancer.
| A, Peroral group |
|---|
| Regimen | n |
|---|
| Cap | 202 |
| UFT/LV | 85 |
| S-1 | 29 |
| UFT | 31 |
| 5'-DFUR | 2 |
| Total | 349 |
| B, OX group |
| Regimen | n |
| CapOX | 93 |
| mFOLFOX6 | 53 |
| SOX | 3 |
| Total | 149 |
A number of patients without adjuvant chemotherapy
were T3N0 patients without other high-risk factors for recurrence.
According to UICC TNM stage, among the stage II patients, almost
all patients who underwent adjuvant chemotherapy were high-risk
stage II (high histological grade, T4 and lymphatic or vessel
invasion). There were 20 patients in the OX group and 103 patients
in the PO group, which indicates that a large number of regimens
for patients with stage II CRC in the adjuvant setting were PO
regimens without OX. Among the stage III patients, there were 129
patients in the OX group and 246 patients in the PO group. Two
patients without adjuvant chemotherapy died postoperatively; one
patient with perforating peritonitis and sepsis due to CC died of
multiple organ failure 2 days after emergency surgery; another
patient with arteriosclerosis obliterans and poorly controlled
diabetes died of multiple organ failure due to anastomotic leakage
4 months after elective surgery. No chemotherapy-associated
mortality was identified in the PO and OX groups.
Table II details
the characteristics of patients in the OX and PO groups. Compared
with the PO group, the age of the OX group patients was younger
(P>0.001), poorly differentiated adenocarcinoma was more
frequently observed (P=0.023) and T (P=0.033), N (P>0.001), and
UICC TNM stage (P>0.0001) were advanced. Vessel or lymphatic
invasion was also observed more frequently (P=0.019). The
proportion of patients in the OX group differed between the two
institutions (P=0.007), reflecting the policy of each institution
regarding the use of OX-containing regimens. Tumor location, tumor
markers, preoperative bowel obstruction, surgical procedure, blood
loss volume, operating time, number of harvested lymph nodes,
postoperative hospital stay and morbidity were not different
between the two groups. These results suggested physician selection
bias as a large proportion of N1 patients were treated with PO
chemotherapy, while patients with more aggressive N2 almost
exclusively received OX-containing regimens.
 | Table IICharacteristics of patients with colon
cancer treated with adjuvant chemotherapy. |
Table II
Characteristics of patients with colon
cancer treated with adjuvant chemotherapy.
| Characteristic | OX group | PO group | P-value |
|---|
| N | 149 | 349 | |
| Male/female | 77/72 | 142/207 | 0.024a |
| Mean age, years | 61.8 (30.0-84.0) | 66.7 (31.0-89.0) |
>0.001a |
| Institution
(KMMC/KU) | 78/71 | 239/110 | 0.007a |
| Tumor location | | | 0.465 |
|
Right | 55 (36.9%) | 141 (40.4%) | |
|
Left | 92 (63.1%) | 208 (59.6%) | |
| Tumor marker | | | |
|
Median
CEA | 3.6 (0.6-168.0) | 3.0 (0.4-262.1) | 0.529 |
|
Median
CA19-9 | 9.0 (1.5-2351.8) | 8.0 (1.8-592.2) | 0.342 |
| Bowel
obstruction | 22 (14.8%) | 37 (10.6%) | 0.196 |
| Surgical
procedure | | | 0.388 |
|
Laparoscopic | 132 (88.6%) | 318 (91.1%) | |
|
Open | 17 (11.4%) | 31 (8.9%) | |
| Median blood loss
volume, ml | 30 (2-2965) | 30 (3-11010) | 0.516 |
| Median operating
time, min | 251 (88-752) | 241 (90-594) | 0.114 |
| Number of lymph
nodes | 25 (3-97) | 21 (2-112) | |
| Hospital stay,
days | 13 (5-137) | 13 (7-125) | 0.738 |
| Postoperative
complications | 25 (16.8%) | 50 (14.3%) | 0.487 |
| Tumor
differentiation | | | 0.023a |
|
Well | 70 (47.0%) | 200 (57.3%) | |
|
Moderate | 56 (37.6%) | 121 (34.7%) | |
|
Poor | 23 (15.4%) | 28 (8.0%) | |
| T stage | | | 0.033a |
|
T1 | 3 (2.0%) | 16 (4.6%) | |
|
T2 | 17 (11.4%) | 30 (8.6%) | |
|
T3 | 97 (65.1%) | 258 (73.9%) | |
|
T4 | 32 (21.5%) | 45 (12.9%) | |
| N stage | | |
>0.001a |
|
N0 | 20 (13.4%) | 103 (29.5%) | |
|
N1 | 72 (48.3%) | 226 (64.8%) | |
|
N2 | 57 (38.3%) | 20 (5.7%) | |
| Vessel or lymphatic
invasion | 81 (54.4%) | 150 (43.0%) | 0.019a |
| TNM stage | | |
>0.001a |
|
II | 20 (13.4%) | 103 (29.5%) | |
|
III | 129 (86.6%) | 180 (70.5%) | |
Comparison of RFS between patients
treated with PO and OX-containing regimens
In the PO group, 66 of 349 (18.9%) patients had a
recurrence of CC, with initial relapse in the liver (n=28) or lung
(n=19), tumor dissemination (n=12) and lymph node (n=15), local
(n=3) or bone recurrence (n=1) including duplication (data not
shown). However, in the OX group, 40 of 149 (26.8%) patients had CC
recurrence, with initial relapse in the liver (n=12) or lung
(n=13), tumor dissemination (n=15) and lymph node (n=10), local
(n=5) or bone recurrence (n=1; data not shown). Patients in the OX
group experienced tumor dissemination and local recurrence more
frequently than those in the PO group. The 5-year RFS rate was
80.8% in the PO group and 71.9% in the OX group (stage IIA, 85.1
and 83.3; IIB, 58.3 and 50.0; IIC, 66.7 and 71.4; IIIA, 93.3 and
100.0; IIIB, 80.5 and 71.3 and IIIC, 45.7 and 61.9%, respectively;
data not shown).
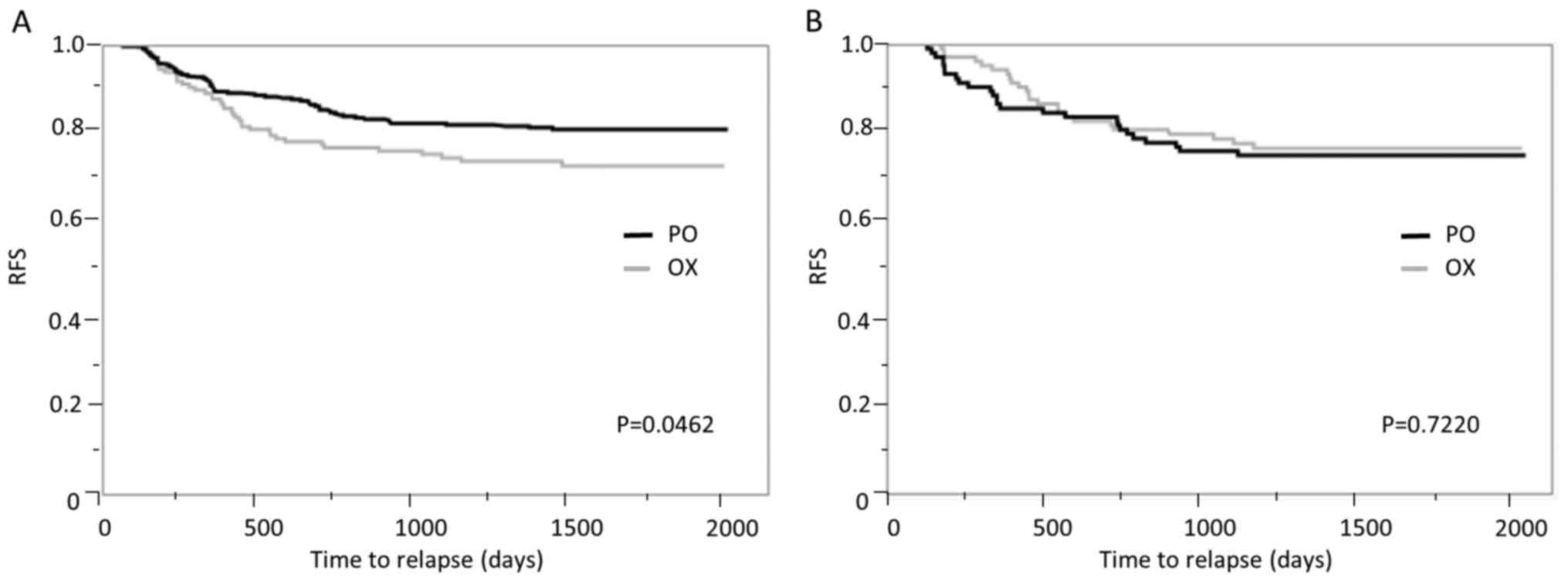
Previous studies have shown that OX administration
tends to improve RFS (6-8).
In order to minimize the impact of any selection bias in comparing
the recurrence risk between the two groups and to elucidate the
effects of OX for patients with CC, propensity score matching was
performed. Patient characteristics following matching are presented
in Table III. Before matching,
the prognosis of OX group patients was significantly poorer than
that of PO group (P=0.0462; Fig.
2A), potentially due to strong therapeutic selection bias.
After matching, the prognosis of the OX group was better than that
of the PO group, although this was not significant (P=0.7220;
Fig. 2B), which suggests an
additional effect of OX for patients with stage II/III CC.
 | Table IIICharacteristics of patients with
colon cancers treated with adjuvant chemotherapy following
propensity score matching. |
Table III
Characteristics of patients with
colon cancers treated with adjuvant chemotherapy following
propensity score matching.
| Characteristic | OX group | PO group | P-value |
|---|
| N | 105 | 105 | |
| Male/female | 50/55 | 49/56 | 0.890 |
| Mean age,
years | 62.6
(30.0-84.0) | 62.9
(31.0-89.0) | 0.667 |
| Institution
(KMMC/KU) | 53/52 | 49/56 | 0.581 |
| Tumor location | | | 0.573 |
|
Right | 40 (38.1%) | 44 (41.9%) | |
|
Left | 65 (61.9%) | 61 (58.1%) | |
| Tumor marker | | | |
|
Median
CEA | 3.8
(0.6-116.0) | 2.8
(0.5-262.1) | 0.088 |
|
Median
CA19-9 | 9.0
(1.5-2351.8) | 8.5
(1.8-592.2) | 0.657 |
| Bowel
obstruction | 13 (12.4%) | 11 (10.5%) | 0.664 |
| Surgical
procedure | | | 0.818 |
|
Laparoscopic | 94 (89.5%) | 95 (90.5%) | |
|
Open | 11 (10.5%) | 10 (9.5%) | |
| Median blood loss
volume, ml | 30 (2-2965) | 30 (3-4300) | 0.900 |
| Median operating
time, min | 251 (88-752) | 241 (90-778) | 0.646 |
| No. of lymph
nodes | 25 (3-97) | 21 (2-112) | |
| Hospital stay,
days | 13 (5-137) | 13 (7-125) | 0.738 |
| Postoperative
complications | 14 (13.3%) | 15 (14.3%) | 0.842 |
| Tumor
differentiation | | | 0.904 |
|
Well | 50 (47.6%) | 50 (47.6%) | |
|
Moderate | 41 (39.1%) | 43 (40.9%) | |
|
Poor | 14 (13.3%) | 12 (11.4%) | |
| T stage | | | 0.468 |
|
T1 | 3 (2.9%) | 7 (6.7%) | |
|
T2 | 11 (10.5%) | 7 (6.7%) | |
|
T3 | 72 (68.6%) | 72 (68.6%) | |
|
T4 | 19 (18.1%) | 19 (18.1%) | |
| N stage | | | 0.238 |
|
N0 | 18 (17.1%) | 28 (26.7%) | |
|
N1 | 64 (61.0%) | 58 (55.2%) | |
|
N2 | 23 (21.9%) | 19 (18.1%) | |
| Vessel or lymphatic
invasion | 55 (52.4%) | 60 (57.1%) | 0.488 |
| TNM stage | | |
>0.001a |
|
II | 14 (13.4%) | 103 (29.5%) | |
|
III | 63 (86.6%) | 180 (70.5%) | |
Risk factors of cancer recurrence for
patients with PO regimens
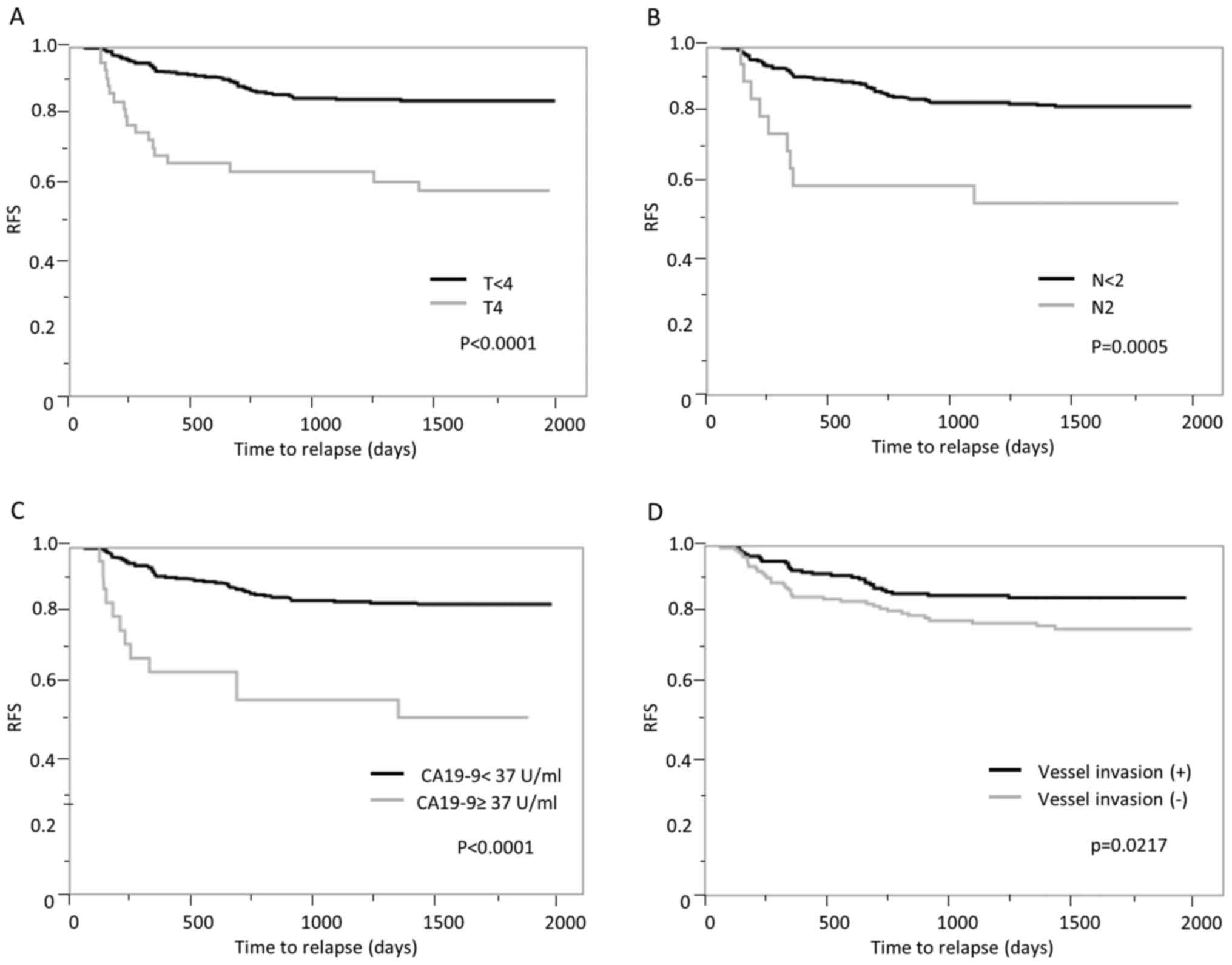
The RFS curve of the PO group is shown in Fig. 3. Univariate analysis showed that T4
(P<0.0001; Fig. 3A), N2
(P=0.0005; Fig. 3B), high CA19-9
(P<0.0001; Fig. 3C) and
lymphatic or vessel invasion (P=0.0217; Fig. 3D) were significantly associated with
worse RFS. Multivariate analysis indicated that high CA19-9 [hazard
ratio (HR), 3.367; 95% CI, 1.773-6.393; P=0.0002], T4 (HR, 2.947;
95% CI, 1.698-5.113; P=0.0001), N2 (HR, 2.704; 95% CI, 1.304-5.609;
P=0.0075) and lymphatic or vessel invasion (HR, 1.675; 95% CI,
1.014-2.765; P=0.0437) were independent predictive factors for
cancer recurrence (Table IV).
 | Table IVUnivariate and multivariate Cox
proportional hazards analysis of predictive factors for cancer
relapse in peroral group. |
Table IV
Univariate and multivariate Cox
proportional hazards analysis of predictive factors for cancer
relapse in peroral group.
| A,
Preoperative |
|---|
| | Univariate
analysis | Multivariate
analysis |
|---|
| Factor | P-value | Risk ratio (95%
CI) | P-value |
|---|
| Institution | 0.0824 | | |
| Bowel
obstruction | 0.8897 | | |
| Tumor location | 0.8396 | | |
| CEA >5
ng/ml | 0.3429 | | |
| CA19-9 >37
U/ml |
<0.0001a | 3.367
(1.773-6.393) | 0.0002a |
| B, Surgical |
| | Univariate
analysis | Multivariate
analysis |
| Factor | P-value | Risk ratio (95%
CI) | P-value |
| Procedure,
laparoscopic vs. open | 0.7307 | | |
| Blood loss volume
≥300 ml | 0.4415 | | |
| Operating time ≥300
min | 0.1515 | | |
| Postoperative
complications | 0.0800 | | |
| C,
Histological |
| | Univariate
analysis | Multivariate
analysis |
| Factor | P-value | Risk ratio (95%
CI) | P-value |
| Grade | 0.7605 | | |
| T4 vs. T≤3 |
<0.0001a | 2.947
(1.698-5.113) | 0.0001a |
| N2 vs. N≤1 | 0.0005a | 2.704
(1.304-5.609) | 0.0075a |
| Lymphatic or vessel
invasion | 0.0217a | 1.675
(1.014-2.765) | 0.0437a |
Subgroup analysis of predictive risk
factors for cancer relapse in the propensity score-matched
cohort
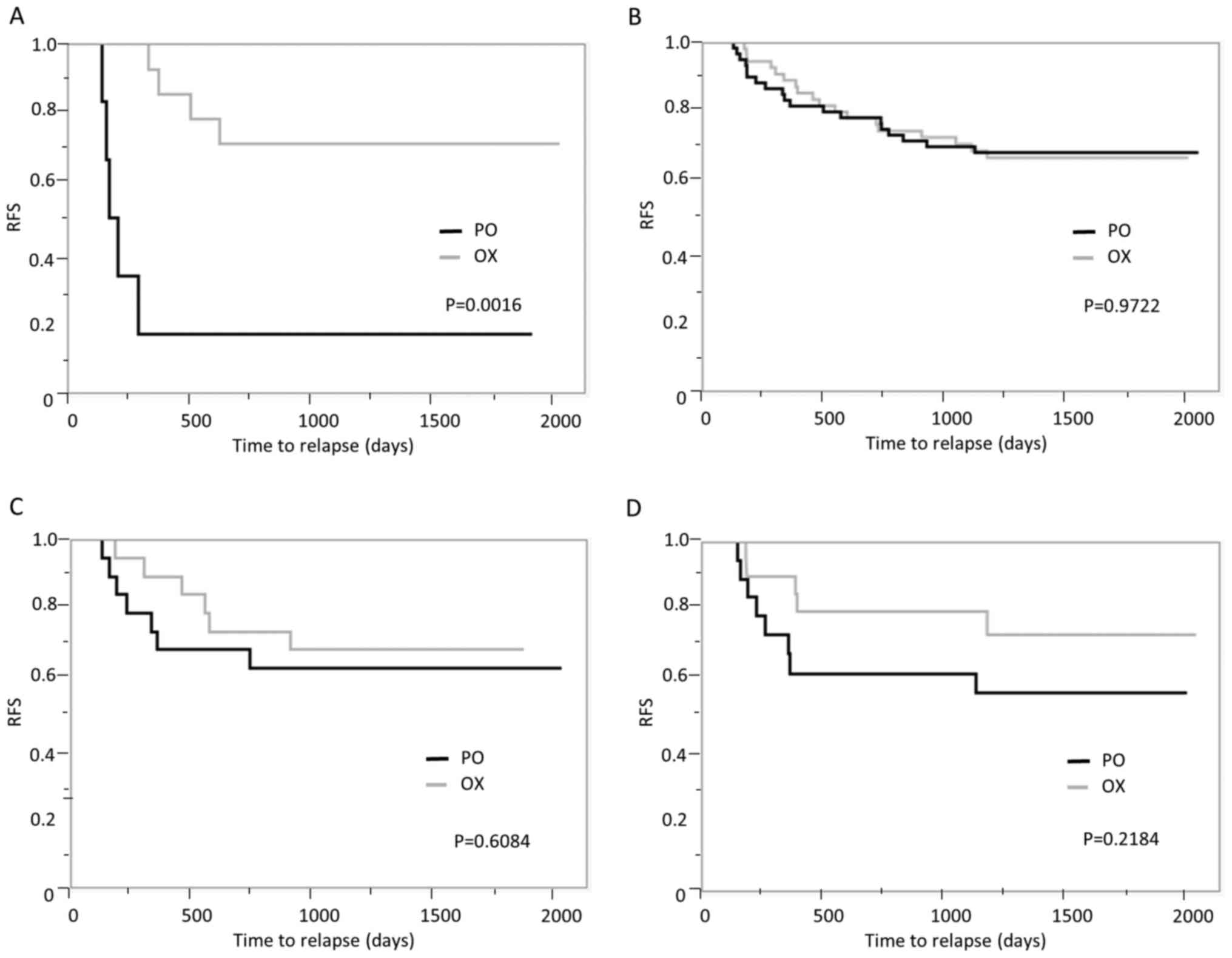
In order to compare the recurrence risk between
patients with PO and OX-containing regimens, subgroup analysis was
performed using the propensity score-matched cohort to minimize
selection bias. Subgroup analysis of patients with T4, N2, high
CA19-9 and lymphatic or vessel invasion revealed an additional
effect of OX in the high CA19-9 subgroup (P=0.0016; Fig. 4A). This was not demonstrated in the
subgroup with lymphatic or vessel invasion (P=0.9722; Fig. 4B) but there was an association with
a prognostic benefit of OX in the T4 and N2 subgroups (P=0.6084 and
P=0.2184, respectively; Fig. 4C and
D), which suggests that patients
with T4, N2 and high CA19-9 may benefit from OX-containing regimens
such as FOLFOX and CapOX.
Discussion
In Japan, postoperative adjuvant chemotherapy
following R0 resection is recommended for patients with high-risk
stage II or III CRC by the Japanese Society for Cancer of the Colon
and Rectum (JSCCR) guidelines 2019. The recommended regimens
include not only OX-containing regimens, such as FOLFOX and CapOX,
but also 5-FU/LV, capecitabine, UFT/LV and S-1(23). At Kitakyushu Municipal Medical
Center and Kyushu University Hospital, administration of PO
fluorinated pyrimidine-based therapy without OX is common,
especially for patients with high-risk stage II without lymph node
metastasis, relatively low-risk stage III without T4 and ≥N2 or
high-risk stage III with advanced age or comorbidity and patients
who prefer to avoid adverse effects, such as persistent peripheral
neuropathy. This may reflect the situation in other hospitals in
Japan. The stage at which patients with CRC should receive adjuvant
chemotherapy is known but it remains controversial as to which
patients should be treated with OX-containing regimens.
The present study investigated the predictive
factors for recurrence following curative resection in patients
with CC treated with PO adjuvant chemotherapy. Our findings
indicated that patients with T4, N2, high CA19-9 and lymphatic or
vessel invasion had a high risk of recurrence when treated with PO
regimens as postoperative adjuvant chemotherapy. It has been
previously reported that T4, N2, high CA19-9 and lymphatic or
vessel invasion are poor prognostic factors that affect the
survival rate of patients with CC (15-18),
but to the best of our knowledge, the present study is the first to
demonstrate that these factors are associated with high recurrence
rate in patients receiving PO chemotherapy regimens. In Japan, PO
regimens are widespread, therefore these results may reflect
real-world use of peroral adjuvant chemotherapy for stage II/III CC
in Japan.
Previous proportional hazards model survival
analyses have identified T4, N2, high tumor marker, postoperative
complications and lymphatic or vessel invasion as poor prognostic
factors (15-18,24-26).
Here, patients in the PO group with high CA19-9, T4, N2, and
lymphatic or vessel invasion had a poor prognosis, but high CEA and
postoperative complications were not predictive factors for cancer
relapse. Previous studies have reported that high expression levels
of tumor markers, such as CEA and CA19-9, affect survival in
patients with CC (16,25,26).
The present study also indicated that high CA19-9 was an
independent predictive marker of recurrence; moreover, high CA19-9
was the only factor to reveal a significant improvement in RFS
following OX administration in the propensity score-matched cohort,
despite the small number of patients. CEA has also been reported to
be a marker of poor prognosis (26), but this was not reflected in the
present study and cancer recurrence in patients with high
preoperative CEA levels has been observed. The cutoff value of the
tumor marker was determined according to the upper limit of normal
at Kitakyushu Municipal Medical Center and Kyushu University
Hospital to eliminate arbitrariness. Because there is no validated
or well-established cutoff for elevated CEA, another appropriate
cutoff value for CEA may exist. Postoperative morbidity, such as
anastomotic failure, ileus and surgical site infection, was not a
recurrence risk factor in this study. Adjuvant chemotherapy could
not be administered to these frail patients in principle; these
patients were assigned to a group without adjuvant chemotherapy and
only patients with relatively mild complications began treatment
with PO regimens.
Randomized control trials, such as MOSAIC, NSABP
C-07 and XELOXA, have shown that the addition of OX to fluorinated
pyrimidine-based therapies improves patient outcome in
postoperative adjuvant settings (5-8)
and OX-containing regimens are recommended as adjuvant chemotherapy
for patients with CC by National Comprehensive Cancer Network and
JSCCR guidelines (22,27). However, a subgroup analysis of these
trials did not demonstrate that OX-containing regimens decrease the
risk of cancer recurrence in patients with T4, N2 and lymphatic or
vessel invasion, which suggests that the prognostic benefit of OX
is limited in these patients compared with other patients in these
previous studies (5-8).
Subgroup analysis before propensity score matching
also failed to demonstrate the additional effect of OX in patients
with high tumor markers, T4, and lymphatic or vessel invasion.
Propensity score matching analysis to adjust clinicopathological
factors also showed that OX-containing regimens improved RFS in the
high CA19-9 subgroup and that there was an association with RFS
improvement in T4 or N2 patients treated with OX-containing
regimens compared with PO regimens. The lack of significance may be
due to the small number of patients. These results suggest that OX
may confer a benefit to prognosis for patients without these
factors.
A limitation of the present study was the
retrospective analysis of patients with CC who were treated at two
tertiary medical institutions. In a previous randomized controlled
trial, S-1 did not show non-inferiority compared with capecitabine
for disease-free survival (28).
S-1 was administered prior to this study and there was a potential
for selection bias if S-1-treated patients were excluded.
Therefore, patients treated with S-1 were included. In addition,
the inclusion of patients and physician preferential regimens
conferred a selection bias. Strong selection biases can exist when
patients without adjuvant chemotherapy are excluded. However, the
present study limited patients to those with adjuvant chemotherapy
because the aim was to determine predictive factors for cancer
recurrence in patients treated with PO regimens as adjuvant
chemotherapy and to investigate the additional effect of OX in
patients with stage II/III CC. Propensity score matching analysis
was performed to minimize the effect of other clinicopathological
factors influencing survival.
The present study demonstrated that high CA19-9, T4
and lymphatic or vessel invasion were predictive markers for cancer
recurrence in patients with PO regimens. CC recurrence predictive
factors for patients treated with PO regimens were determined;
OX-containing regimens may outcome in patients with T4, N2, and
high CA19-9. These data promote OX as a therapeutic regimen for
patients with stage II/III CC.
Acknowledgements
The authors would like to thank Dr Nikki March from
Edanz Group for editing a draft of this manuscript.
Funding
The present study was supported by the Japan Society for the
Promotion of Science Grant-in-Aid for Research Activity Start-up
and Young Scientists (grant nos. 19K24016 and 20K17621) and Kanae
Foundation.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YM, YT and MN designed the study. MS, YK and YW
analyzed the data. SN, KN and KO performed statistical analysis and
data interpretation. ST performed histological diagnoses. YM, YT,
MS, YK, SN and TN performed surgery and patient care. KN and KO
contributed to data acquisition. YM drafted the manuscript. YM and
MS confirm the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
All procedures conformed to the ethical guidelines
of the Japanese Government and the Declaration of Helsinki. The
study was approved by the ethics committee of Kitakyushu Municipal
Medical Center (approval no. 201801055).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Japanese National Cancer Center: Cancer
Registry and Statistics: Cancer Information Service. http://ganjoho.jp/reg_stat/statistics/dl/index.html#incidence.
Accessed December 2020.
|
|
3
|
Inomata M, Shiroshita H, Uchida H, Bandoh
T, Akira S, Yamaguchi S, Kurokawa Y, Seki Y, Eguchi S, Wada N, et
al: Current status of endoscopic surgery in Japan: The 14th
national survey of endoscopic surgery by the Japan society for
endoscopic surgery. Asian J Endosc Surg. 13:7–18. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Survival statistics of Japanese
association of Clinical Cancer Centers: Cancer survival rates at
Japanese Association of Clinical Cancer Centers. https://kapweb.chiba-cancer-registry.org/usage?lang=en.
Accessed December 2020.
|
|
5
|
André T, Boni C, Mounedji-Boudiaf L,
Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan
P, Bridgewater J, et al: Oxaliplatin, fluorouracil, and leucovorin
as adjuvant treatment for colon cancer. N Engl J Med.
350:2343–2351. 2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
André T, Boni C, Navarro M, Tabernero J,
Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F
and de Gramont A: Improved overall survival with oxaliplatin,
fluorouracil, and leucovorin as adjuvant treatment in stage II or
III colon cancer in the MOSAIC trial. J Clin Oncol. 27:3109–3116.
2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kuebler JP, Wieand HS, O'Connell MJ, Smith
RE, Colangelo LH, Yothers G, Petrelli NJ, Findlay MP, Seay TE,
Atkins JN, et al: Oxaliplatin combined with weekly bolus
fluorouracil and leucovorin as surgical adjuvant chemotherapy for
stage II and III colon cancer: Results from NSABP C-07. J Clin
Oncol. 25:2198–2204. 2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Haller DG, Tabernero J, Maroun J, de Braud
F, Price T, Van Cutsem E, Hill M, Gilberg F, Rittweger K and
Schmoll HJ: Capecitabine plus oxaliplatin compared with
fluorouracil and folinic acid as adjuvant therapy for stage III
colon cancer. J Clin Oncol. 29:1465–1471. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hoff PM, Saad ED, Costa F, Coutinho AK,
Caponero R, Prolla G and Gansl RC: Literature review and practical
aspects on the management of oxaliplatin-associated toxicity. Clin
Colorectal Cancer. 11:93–100. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lembersky BC, Wieand HS, Petrelli NJ,
O'Connell MJ, Colangelo LH, Smith RE, Seay TE, Giguere JK, Marshall
ME, Jacobs AD, et al: Oral uracil and tegafur plus leucovorin
compared with intravenous fluorouracil and leucovorin in stage II
and III carcinoma of the colon: Results from National Surgical
Adjuvant Breast and Bowel Project Protocol C-06. J Clin Oncol.
24:2059–2064. 2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Shimada Y, Hamaguchi T, Mizusawa J, Saito
N, Kanemitsu Y, Takiguchi N, Ohue M, Kato T, Takii Y, Sato T, et
al: Randomised phase III trial of adjuvant chemotherapy with oral
uracil and tegafur plus leucovorin versus intravenous fluorouracil
and levofolinate in patients with stage III colorectal cancer who
have undergone Japanese D2/D3 lymph node dissection: Final results
of JCOG0205. Eur J Cancer. 50:2231–2240. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Twelves C, Scheithauer W, McKendrick J,
Seitz JF, Van Hazel G, Wong A, Díaz-Rubio E, Gilberg F and Cassidy
J: Capecitabine versus 5-fluorouracil/folinic acid as adjuvant
therapy for stage III colon cancer: Final results from the X-ACT
trial with analysis by age and preliminary evidence of a
pharmacodynamic marker of efficacy. Ann Oncol. 23:1190–1197.
2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yoshida M, Ishiguro M, Ikejiri K,
Mochizuki I, Nakamoto Y, Kinugasa Y, Takagane A, Endo T, Shinozaki
H, Takii Y, et al: ACTS-CC study group. S-1 as adjuvant
chemotherapy for stage III colon cancer: A randomized phase III
study (ACTS-CC trial). Ann Oncol. 25:1743–1749. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Oki E, Murata A, Yoshida K, Maeda K,
Ikejiri K, Munemoto Y, Sasaki K, Matsuda C, Kotake M, Suenaga T, et
al: A randomized phase III trial comparing S-1 versus UFT as
adjuvant chemotherapy for stage II/III rectal cancer (JFMC35-C1:
ACTS-RC). Ann Oncol. 27:1266–1272. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Benson AB III, Schrag D, Somerfield MR,
Cohen AM, Figueredo AT, Flynn PJ, Krzyzanowska MK, Maroun J,
McAllister P, Van Cutsem E, et al: American society of clinical
oncology recommendations on adjuvant chemotherapy for stage II
colon cancer. J Clin Oncol. 22:3408–3419. 2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yu Z, Chen Z, Wu J, Li Z and Wu Y:
Prognostic value of pretreatment serum carbohydrate antigen 19-9
level in patients with colorectal cancer: A meta-analysis. PLoS
One. 12(e0188139)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Betge J, Pollheimer MJ, Lindtner RA,
Kornprat P, Schlemmer A, Rehak P, Vieth M, Hoefler G and Langner C:
Intramural and extramural vascular invasion in colorectal cancer:
Prognostic significance and quality of pathology reporting. Cancer.
118:628–638. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Akagi Y, Adachi Y, Ohchi T, Kinugasa T and
Shirouzu K: Prognostic impact of lymphatic invasion of colorectal
cancer: A single-center analysis of 1,616 patients over 24 years.
Anticancer Res. 33:2965–2970. 2013.PubMed/NCBI
|
|
19
|
Brierley JD, Gospodarowicz MK and
Wittekind C (eds): TNM Classification of Malignant Tumours. 8th
edition. John Wiley and Sons, Inc., New York, NY, 2017.
|
|
20
|
Japanese Society for Cancer of the Colon
and Rectum: Japanese Classification of Colorectal, Appendiceal and
Anal Carcinoma, Third English edition. Kanehara & Co., Ltd.,
Tokyo, 2019.
|
|
21
|
Dindo D, Demartines N and Clavien PA:
Classification of surgical complications: A new proposal with
evaluation in a cohort of 6336 patients and results of a survey.
Ann Surg. 240:205–213. 2004.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Watanabe Y, Watanabe M, Suehara N, Saimura
M, Mizuuchi Y, Nishihara K, Iwashita T and Nakano T: Billroth-I
reconstruction using an overlap method in totally laparoscopic
distal gastrectomy: Propensity score matched cohort study of short-
and long-term outcomes compared with Roux-en-Y reconstruction. Surg
Endosc. 33:3990–4002. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hashiguchi Y, Muro K, Saito Y, Ito Y,
Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M,
et al: Japanese society for cancer of the colon and rectum (JSCCR)
guidelines 2019 for the treatment of colorectal cancer. Int J Clin
Oncol. 25:1–42. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Burton S, Norman AR, Brown G, Abulafi AM
and Swift RI: Predictive poor prognostic factors in colonic
carcinoma. Surg Oncol. 15:71–78. 2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhou W, Yang F, Peng J, Wang F, Lin Y,
Jiang W, Yang X, Li L, Lu Z, Wan D, et al: High pretreatment serum
CA19-9 level predicts a poor prognosis for patients with stage III
colon cancer after curative resection and adjuvant chemotherapy. J
Cancer. 10:3810–3818. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chapman MA, Buckley D, Henson DB and
Armitage NC: Preoperative carcinoembryonic antigen is related to
tumour stage and long-term survival in colorectal cancer. Br J
Cancer. 78:1346–1349. 1998.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Benson AB, Venook AP, Al-Hawary MM,
Cederquist L, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D,
Engstrom PF, et al: NCCN guidelines insights: Colon cancer, version
2.2018. J Natl Compr Canc Netw. 16:359–369. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hamaguchi T, Shimada Y, Mizusawa J,
Kinugasa Y, Kanemitsu Y, Ohue M, Fujii S, Takiguchi N, Yatsuoka T,
Takii Y, et al: Capecitabine versus S-1 as adjuvant chemotherapy
for patients with stage III colorectal cancer (JCOG0910): An
open-label, non-inferiority, randomised, phase 3, multicentre
trial. Lancet Gastroenterol Hepatol. 3:47–56. 2018.PubMed/NCBI View Article : Google Scholar
|