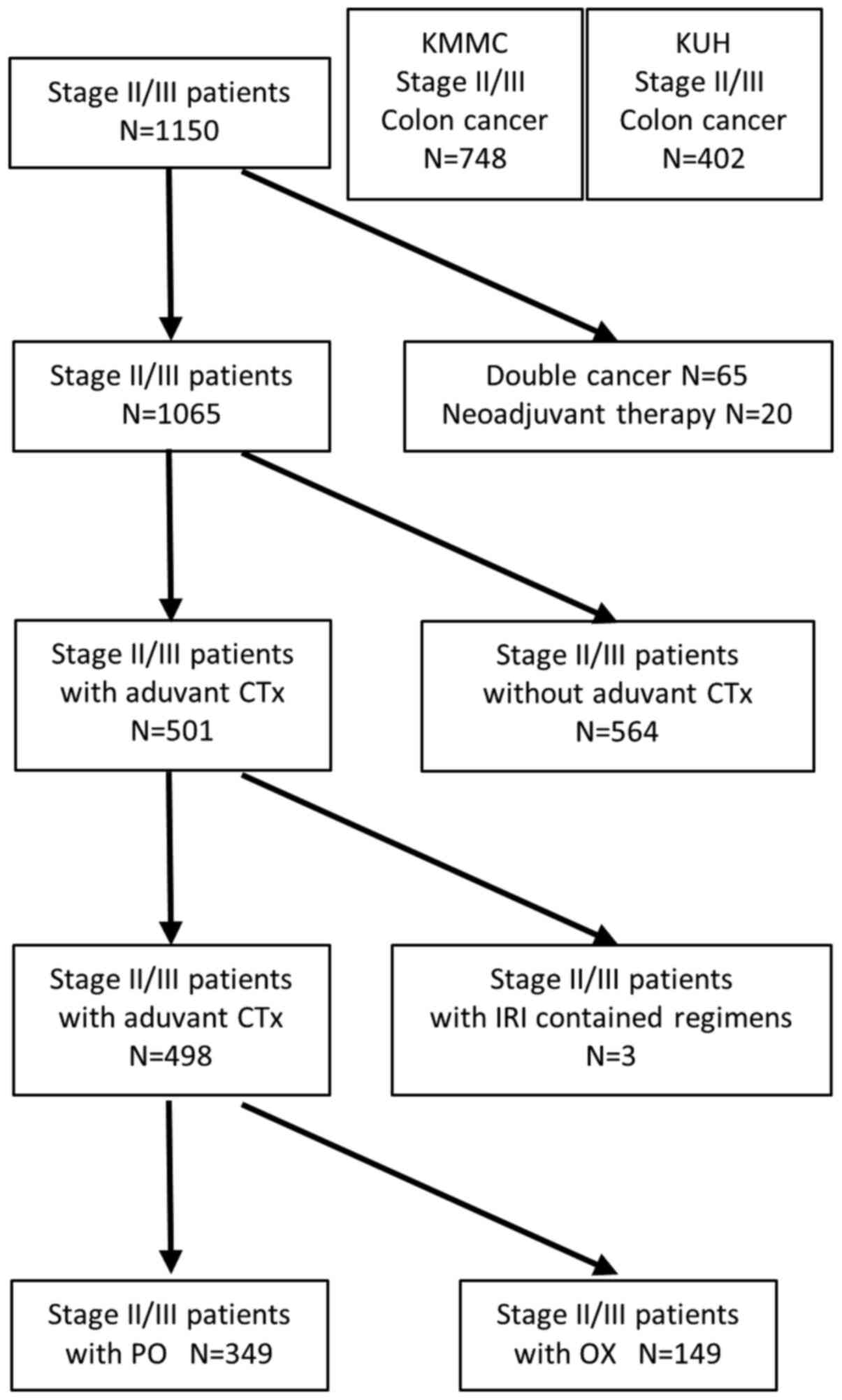
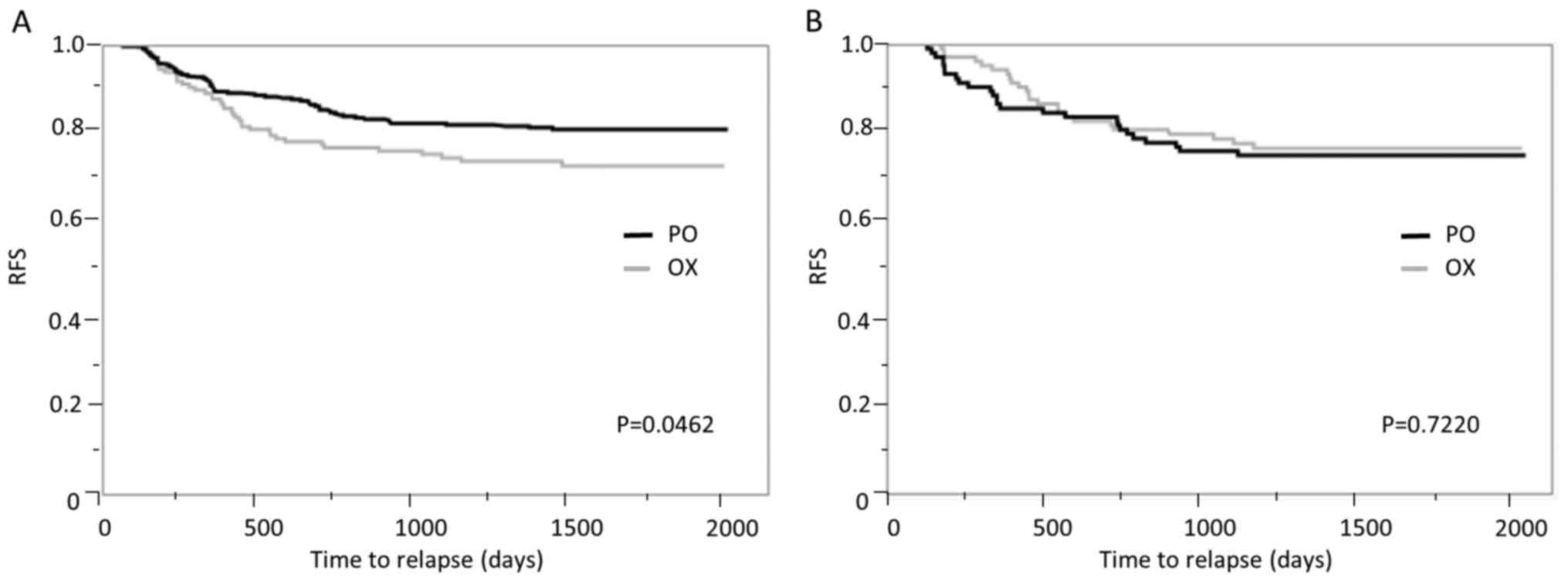
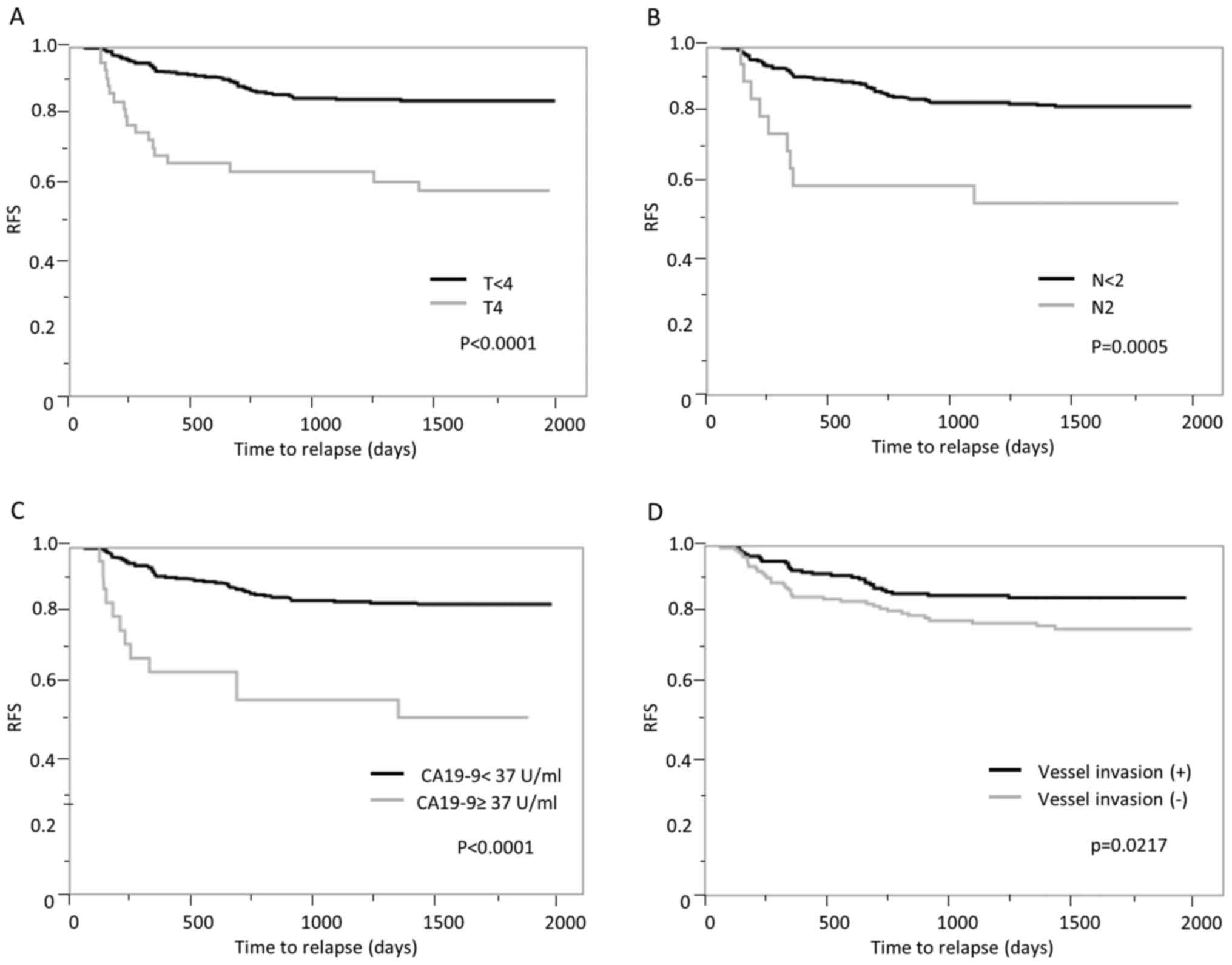
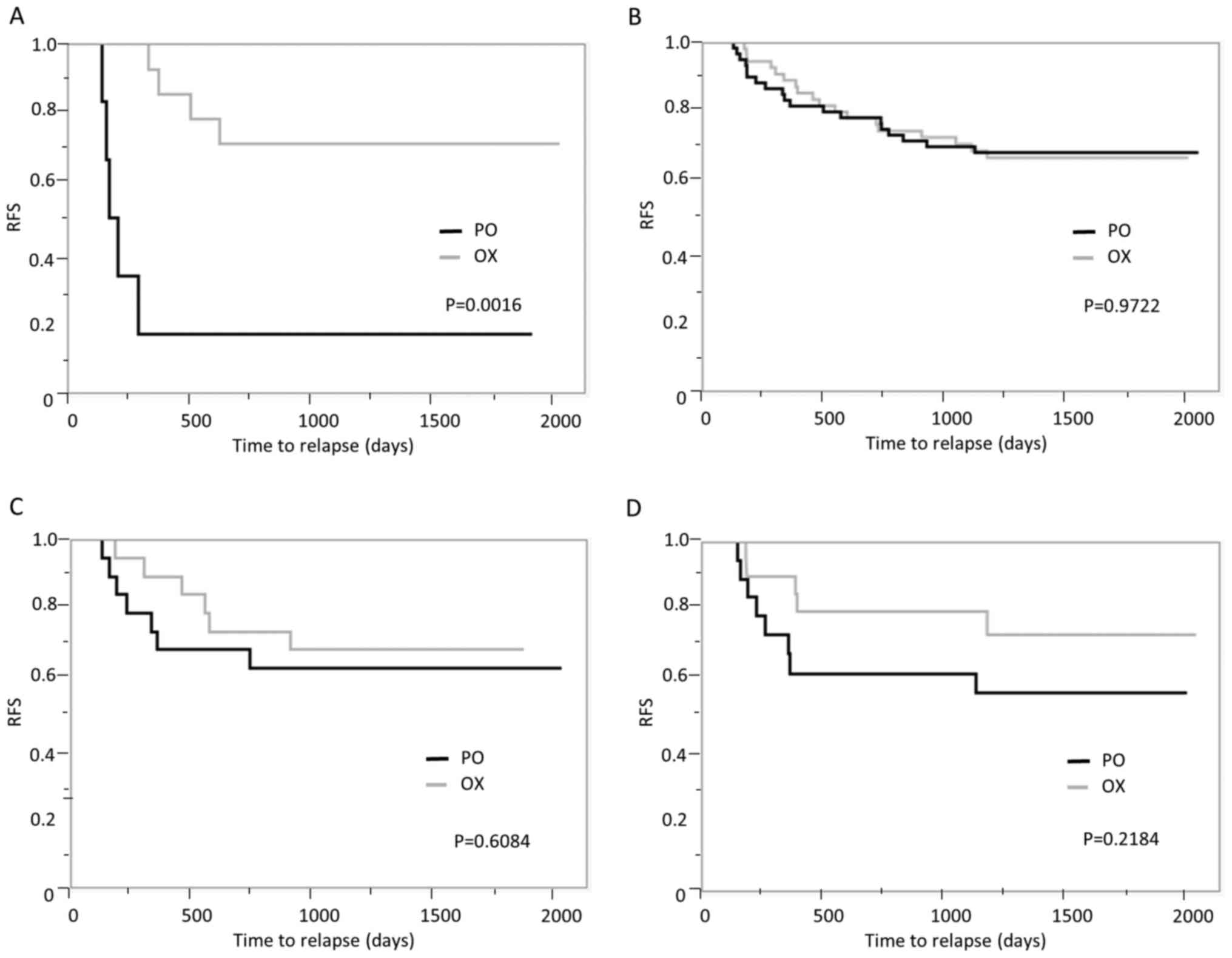
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Japanese National Cancer Center: Cancer
Registry and Statistics: Cancer Information Service. http://ganjoho.jp/reg_stat/statistics/dl/index.html#incidence.
Accessed December 2020.
|
|
3
|
Inomata M, Shiroshita H, Uchida H, Bandoh
T, Akira S, Yamaguchi S, Kurokawa Y, Seki Y, Eguchi S, Wada N, et
al: Current status of endoscopic surgery in Japan: The 14th
national survey of endoscopic surgery by the Japan society for
endoscopic surgery. Asian J Endosc Surg. 13:7–18. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Survival statistics of Japanese
association of Clinical Cancer Centers: Cancer survival rates at
Japanese Association of Clinical Cancer Centers. https://kapweb.chiba-cancer-registry.org/usage?lang=en.
Accessed December 2020.
|
|
5
|
André T, Boni C, Mounedji-Boudiaf L,
Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan
P, Bridgewater J, et al: Oxaliplatin, fluorouracil, and leucovorin
as adjuvant treatment for colon cancer. N Engl J Med.
350:2343–2351. 2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
André T, Boni C, Navarro M, Tabernero J,
Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F
and de Gramont A: Improved overall survival with oxaliplatin,
fluorouracil, and leucovorin as adjuvant treatment in stage II or
III colon cancer in the MOSAIC trial. J Clin Oncol. 27:3109–3116.
2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kuebler JP, Wieand HS, O'Connell MJ, Smith
RE, Colangelo LH, Yothers G, Petrelli NJ, Findlay MP, Seay TE,
Atkins JN, et al: Oxaliplatin combined with weekly bolus
fluorouracil and leucovorin as surgical adjuvant chemotherapy for
stage II and III colon cancer: Results from NSABP C-07. J Clin
Oncol. 25:2198–2204. 2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Haller DG, Tabernero J, Maroun J, de Braud
F, Price T, Van Cutsem E, Hill M, Gilberg F, Rittweger K and
Schmoll HJ: Capecitabine plus oxaliplatin compared with
fluorouracil and folinic acid as adjuvant therapy for stage III
colon cancer. J Clin Oncol. 29:1465–1471. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hoff PM, Saad ED, Costa F, Coutinho AK,
Caponero R, Prolla G and Gansl RC: Literature review and practical
aspects on the management of oxaliplatin-associated toxicity. Clin
Colorectal Cancer. 11:93–100. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lembersky BC, Wieand HS, Petrelli NJ,
O'Connell MJ, Colangelo LH, Smith RE, Seay TE, Giguere JK, Marshall
ME, Jacobs AD, et al: Oral uracil and tegafur plus leucovorin
compared with intravenous fluorouracil and leucovorin in stage II
and III carcinoma of the colon: Results from National Surgical
Adjuvant Breast and Bowel Project Protocol C-06. J Clin Oncol.
24:2059–2064. 2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Shimada Y, Hamaguchi T, Mizusawa J, Saito
N, Kanemitsu Y, Takiguchi N, Ohue M, Kato T, Takii Y, Sato T, et
al: Randomised phase III trial of adjuvant chemotherapy with oral
uracil and tegafur plus leucovorin versus intravenous fluorouracil
and levofolinate in patients with stage III colorectal cancer who
have undergone Japanese D2/D3 lymph node dissection: Final results
of JCOG0205. Eur J Cancer. 50:2231–2240. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Twelves C, Scheithauer W, McKendrick J,
Seitz JF, Van Hazel G, Wong A, Díaz-Rubio E, Gilberg F and Cassidy
J: Capecitabine versus 5-fluorouracil/folinic acid as adjuvant
therapy for stage III colon cancer: Final results from the X-ACT
trial with analysis by age and preliminary evidence of a
pharmacodynamic marker of efficacy. Ann Oncol. 23:1190–1197.
2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yoshida M, Ishiguro M, Ikejiri K,
Mochizuki I, Nakamoto Y, Kinugasa Y, Takagane A, Endo T, Shinozaki
H, Takii Y, et al: ACTS-CC study group. S-1 as adjuvant
chemotherapy for stage III colon cancer: A randomized phase III
study (ACTS-CC trial). Ann Oncol. 25:1743–1749. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Oki E, Murata A, Yoshida K, Maeda K,
Ikejiri K, Munemoto Y, Sasaki K, Matsuda C, Kotake M, Suenaga T, et
al: A randomized phase III trial comparing S-1 versus UFT as
adjuvant chemotherapy for stage II/III rectal cancer (JFMC35-C1:
ACTS-RC). Ann Oncol. 27:1266–1272. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Benson AB III, Schrag D, Somerfield MR,
Cohen AM, Figueredo AT, Flynn PJ, Krzyzanowska MK, Maroun J,
McAllister P, Van Cutsem E, et al: American society of clinical
oncology recommendations on adjuvant chemotherapy for stage II
colon cancer. J Clin Oncol. 22:3408–3419. 2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yu Z, Chen Z, Wu J, Li Z and Wu Y:
Prognostic value of pretreatment serum carbohydrate antigen 19-9
level in patients with colorectal cancer: A meta-analysis. PLoS
One. 12(e0188139)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Betge J, Pollheimer MJ, Lindtner RA,
Kornprat P, Schlemmer A, Rehak P, Vieth M, Hoefler G and Langner C:
Intramural and extramural vascular invasion in colorectal cancer:
Prognostic significance and quality of pathology reporting. Cancer.
118:628–638. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Akagi Y, Adachi Y, Ohchi T, Kinugasa T and
Shirouzu K: Prognostic impact of lymphatic invasion of colorectal
cancer: A single-center analysis of 1,616 patients over 24 years.
Anticancer Res. 33:2965–2970. 2013.PubMed/NCBI
|
|
19
|
Brierley JD, Gospodarowicz MK and
Wittekind C (eds): TNM Classification of Malignant Tumours. 8th
edition. John Wiley and Sons, Inc., New York, NY, 2017.
|
|
20
|
Japanese Society for Cancer of the Colon
and Rectum: Japanese Classification of Colorectal, Appendiceal and
Anal Carcinoma, Third English edition. Kanehara & Co., Ltd.,
Tokyo, 2019.
|
|
21
|
Dindo D, Demartines N and Clavien PA:
Classification of surgical complications: A new proposal with
evaluation in a cohort of 6336 patients and results of a survey.
Ann Surg. 240:205–213. 2004.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Watanabe Y, Watanabe M, Suehara N, Saimura
M, Mizuuchi Y, Nishihara K, Iwashita T and Nakano T: Billroth-I
reconstruction using an overlap method in totally laparoscopic
distal gastrectomy: Propensity score matched cohort study of short-
and long-term outcomes compared with Roux-en-Y reconstruction. Surg
Endosc. 33:3990–4002. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hashiguchi Y, Muro K, Saito Y, Ito Y,
Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M,
et al: Japanese society for cancer of the colon and rectum (JSCCR)
guidelines 2019 for the treatment of colorectal cancer. Int J Clin
Oncol. 25:1–42. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Burton S, Norman AR, Brown G, Abulafi AM
and Swift RI: Predictive poor prognostic factors in colonic
carcinoma. Surg Oncol. 15:71–78. 2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhou W, Yang F, Peng J, Wang F, Lin Y,
Jiang W, Yang X, Li L, Lu Z, Wan D, et al: High pretreatment serum
CA19-9 level predicts a poor prognosis for patients with stage III
colon cancer after curative resection and adjuvant chemotherapy. J
Cancer. 10:3810–3818. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chapman MA, Buckley D, Henson DB and
Armitage NC: Preoperative carcinoembryonic antigen is related to
tumour stage and long-term survival in colorectal cancer. Br J
Cancer. 78:1346–1349. 1998.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Benson AB, Venook AP, Al-Hawary MM,
Cederquist L, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D,
Engstrom PF, et al: NCCN guidelines insights: Colon cancer, version
2.2018. J Natl Compr Canc Netw. 16:359–369. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hamaguchi T, Shimada Y, Mizusawa J,
Kinugasa Y, Kanemitsu Y, Ohue M, Fujii S, Takiguchi N, Yatsuoka T,
Takii Y, et al: Capecitabine versus S-1 as adjuvant chemotherapy
for patients with stage III colorectal cancer (JCOG0910): An
open-label, non-inferiority, randomised, phase 3, multicentre
trial. Lancet Gastroenterol Hepatol. 3:47–56. 2018.PubMed/NCBI View Article : Google Scholar
|