Introduction
Lung cancer has the second highest incidence and
highest mortality rates in both males and females worldwide
(1). It is well-known that surgical
resection plays an important role in the comprehensive treatment of
lung cancer. The preoperative evaluation and postoperative
prediction of pulmonary function (PF) is essential for lung
resection, as PF can predict the risk of perioperative
complications, and long-term disability and mortality following
major lung resection (2-4).
Furthermore, the prediction of the postoperative PF has been
associated with long-term survival following surgery compared with
that in preoperative lung function (5). Several methods have been developed to
predict the postoperative PF (6-8),
such as perfusion scans and segment counting methods; however, it
has been suggested that these methods are inaccurate (9-11).
The postoperative PF could be theoretically determined by the
residual parenchymal volume following lung resection, since the
adult lung generally does not have the ability to regenerate new
alveolar septal tissues (12). In
addition, the compensatory expansion of the remaining lung is not
simply a consequence of hyperexpansion of the pre-existing alveolar
septal tissue, but is accompanied by compensatory growth of the
residual lung in volume and weight (13). Based on this theory, volumetric
computed tomography (CT) has been widely used in several studies to
observe the perioperative changes in lung volume or for analyzing
the correlation between lung volume and PF tests (PFTs) (14-19).
Therefore, volumetric CT has been considered to be more reliable
and accurate in predicting postoperative PF compared with that in
the segment-counting method (18-20).
However, previous studies have only analyzed the changes in lung
volume at two time points, preoperatively and postoperatively.
Therefore, the present study aimed to continuously analyze the
changes in the early postoperative lung volume in patients with
non-small cell lung cancer (NSCLC) following video-assisted
thoracic surgery (VATS) using volumetric CT.
Materials and methods
Clinical data collection
A total of 34 patients (58.56±9.00 years) with
NSCLC, who planned to undergo VATS lobectomy in June 2019, were
enrolled in the present study. The study was approved by the Human
Ethics Committee of The Affiliated Hospital of Guizhou Medical
University (Guizhou, China; approval no. 2020-244). Written
informed consent was provided by all the patients, for the use of
their data in scientific research at the beginning of enrollment.
The clinical and radiological data of the patients, including sex,
age, body mass index (BMI), smoking status, surgical sides and
chest CT scans were prospectively collected within 1 week
preoperatively (T0), and at 1 (T1), 3 (T2) and 6 months (T3)
following surgery.
CT scan
The CT images were acquired using a LightSpeed VCT
64-detector scanner (GE Healthcare), with subjects holding their
breath at the end of inspiration without contrast. The following CT
parameters were used: 64x0.625 mm detector configuration, 0.969
pitch, 120 kVp tube energy, 250 mA tube current and 0.4 sec gantry
rotation (or 100 mAs). The CT scan images were saved as DICOM
format.
Surgical procedure
All surgical procedures were performed by the same
team using uniportal VATS, as previously described (21).
Lung volume measurement
The CT scan data was loaded into the Chest Imaging
Platform and analyzed using the 3D Slicer software (version 4.10.2;
macOS; https://download.slicer.org/). The
Interactive Lobe Segmentation module was used to segment the lung
lobes, and the Label Statistics module under the Quantification
menu was used to compute the left and right sides, and whole lung
volume (Fig. S1).
Statistical analysis
All the data from the recorded measurements was
manually entered into the different analyses by the same
researcher. Descriptive statistics were used to describe the
demographic characteristics. Age and BMI were categorized into two
groups (low and high) according to their mean values. Continuous
variables were presented as the mean ± standard deviation, while
categorical values were presented as numbers. Net compensatory
expansion volume was calculated as the lung volume at the current
observation time minus the prior volume. Mixed two-way ANOVA was
utilized to compare the differences between subgroups, and
χ2 or Fisher's exact test was used for categorical data.
Greenhouse-Geisser was used to adjust the degrees of freedom for
the average number of tests of significance if P<0.05 from the
Mauchly's Test of Sphericity. A two-tailed P<0.05 was considered
to indicate a statistically significant difference. All statistical
analyses were performed using the SPSS v22.0 software (SPSS
Inc.).
Results
Demographic characteristics
A total of 38 patients with NSCLC underwent VATS in
June 2019 at the Department of Thoracic Surgery, The Affiliated
Hospital of Guizhou Medical University, (Guizhou, China). None of
the patients were treated with pneumonectomy or complex lobectomy.
All the procedures were successfully performed using VATS, without
converting to thoracotomy. Among all the patients, two were lost to
follow-up at 1 and 3 months following surgery, respectively, and
two at 6 months. Finally, there were 34 patients at the end of
follow-up and were included in the datasets. The demographic
characteristics of the included patients are listed in Table I. A total of 19 male patients were
included. Among all the patients, 17 underwent left lateral VATS,
while the remaining 17 received right lateral VATS. When the
patients were divided by sex, there was no significant difference
for the clinicopatholoical variables, except for smoking status
where all eight (23.529%) smokers were males.
 | Table IDemographic characteristics of the
patients in the study. |
Table I
Demographic characteristics of the
patients in the study.
| | Sex | |
|---|
| Variable | Female (n=19) | Male (n=15) | P-value |
|---|
| Age, years | | |
>0.9999a |
|
Low | 10 | 8 | |
|
High | 9 | 7 | |
| BMI | | | 0.510a |
|
Low | 9 | 9 | |
|
High | 10 | 6 | |
| Smoking status | | |
<0.001b |
|
No | 19 | 7 | |
|
Yes | 0 | 8 | |
| Smoking in
males | | | - |
|
No | - | 7 | |
|
Yes | - | 8 | |
| Surgical side | | | 0.491a |
|
Left | 11 | 6 | |
|
Right | 8 | 9 | |
Changes on the whole, left and right
lung volume over time
The volume of the whole, left and right lung at four
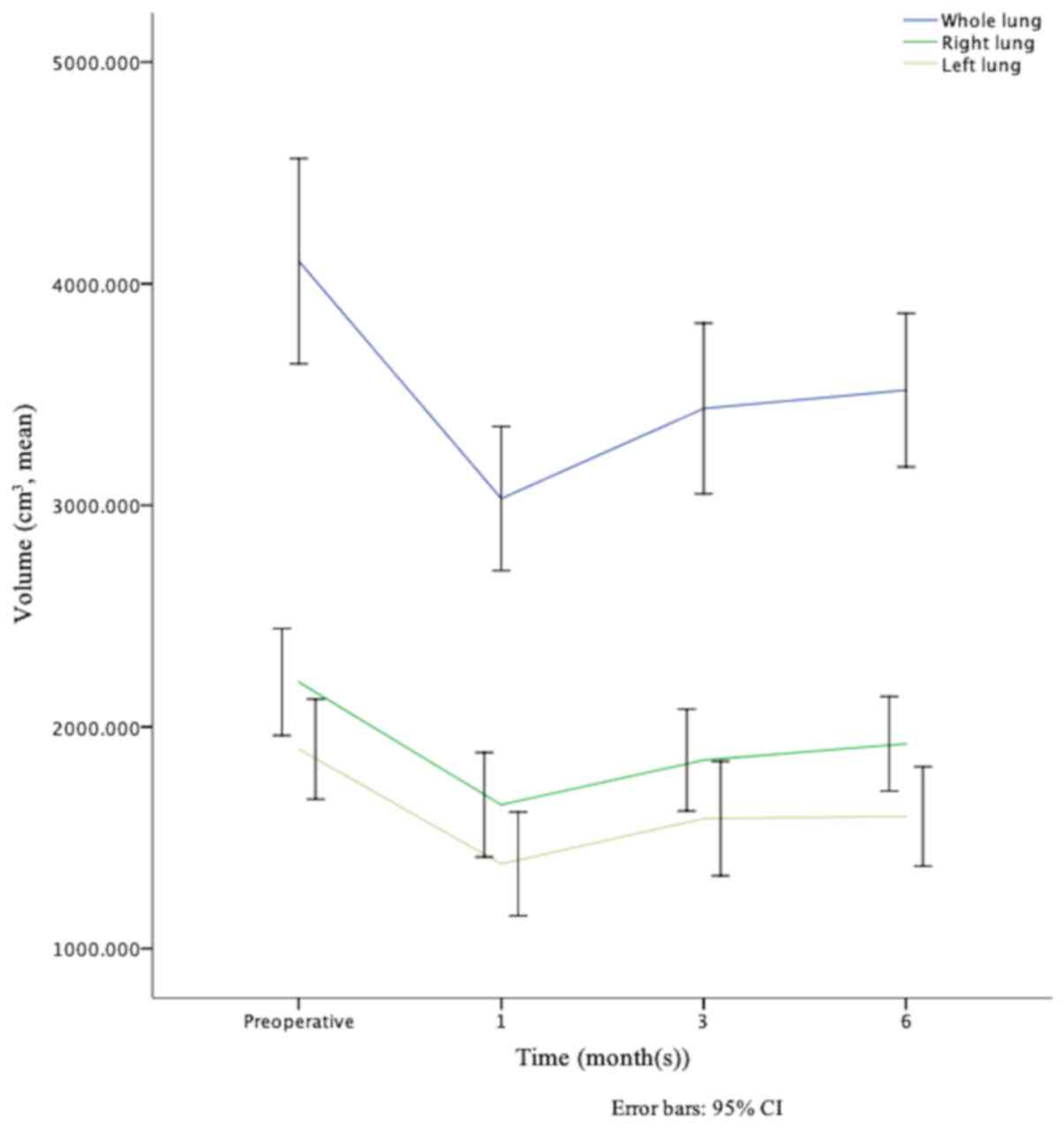
observation time points are shown in Table II. The mean T0 volume of the whole,
left and right lung was 4,101.884±1,328.220, 1,899.614±646.058 and
2,202.270±691.434, respectively. At T1, T2 and T3 months following
surgery the lung volume were 3,030.510±931.542, 1,381.809±670.565
and 1,648.701±676.186; 3,436.707±1,103.550, 1,586.401±739.232 and
1,850.306±658.665; and 3,519.711±992.889, 1,596.003±643.452 and
1,923.708±610.644 for whole, left and right lung, respectively. The
mean volume of the right lung was larger compared with that in the
left one, at each time point. In addition, the trendline of volume
changes in the whole, right and left lung were similar; it sharply
decreased during the first postoperative month, quickly increased
in the following 3 months, and slowly increased from the 3rd to the
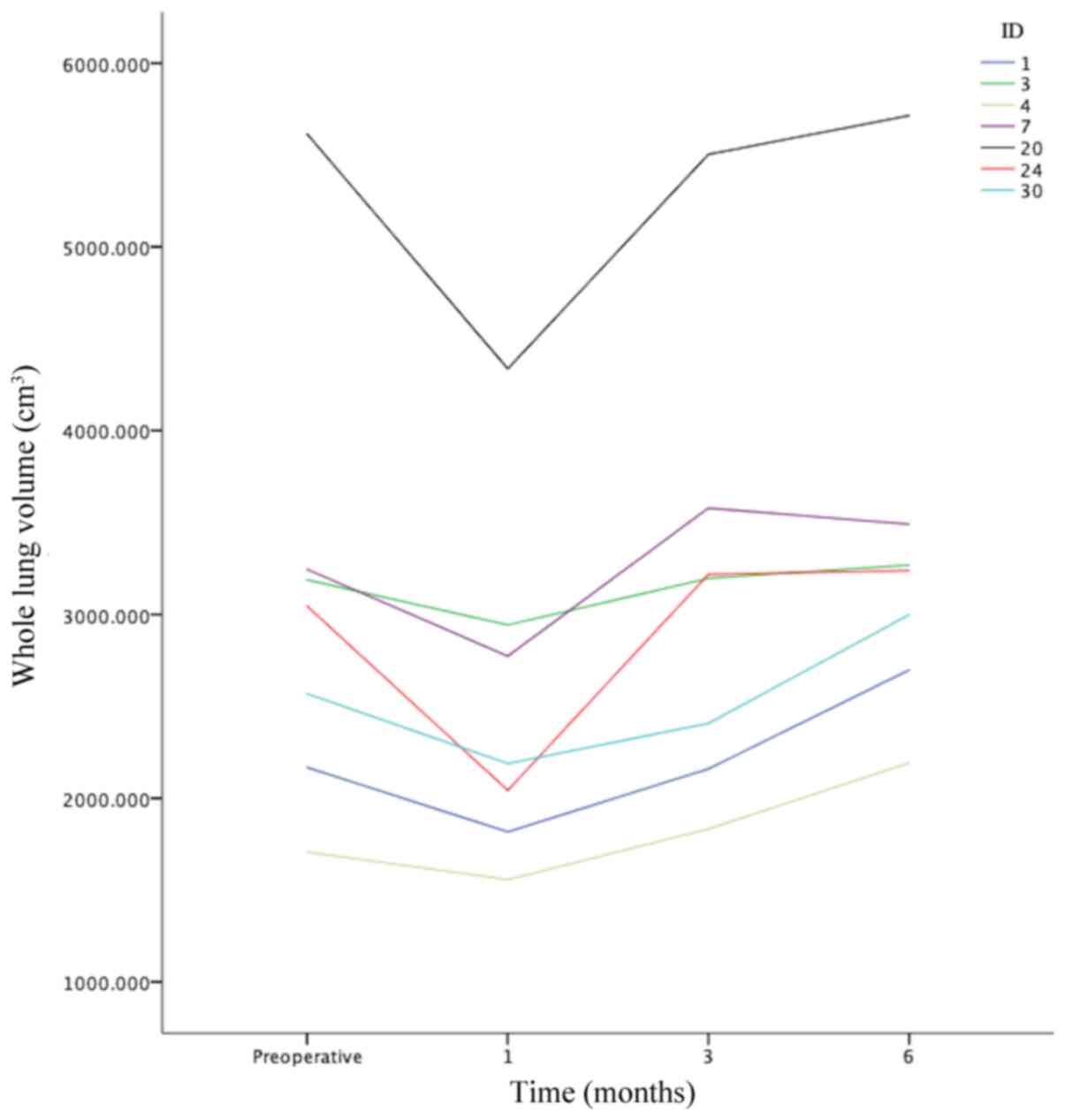
6th month (Fig. 1). In a total of
seven patients (20.588%), the whole lung volume at 6 months
following surgery was larger compared with that preoperatively
(Fig. 2). However, the differences
in the distribution of the observational variables were not
significant (all P>0.05) when the aforementioned seven patients
were compared with those whose whole lung volume at 6 months
following surgery was similar prior to surgery (Table III).
 | Table IIVolume of the whole, left and right
lungs at the 4 different time points. |
Table II
Volume of the whole, left and right
lungs at the 4 different time points.
| | Whole lung volume,
cm3 | Left lung volume,
cm3 | Right lung volume,
cm3 |
|---|
| Variable | T0 | T1 | T2 | T3 | T0 | T1 | T2 | T3 | T0 | T1 | T2 | T3 |
|---|
| Sex | | | | | | | | | | | | |
|
Female | 3,272.759±
810.066 | 2,477.130±
566.826 | 2,925.970±
567.092 | 3,043.140±
654.015 | 1,497.312±
395.527 | 1,086.578±
496.934 | 1,273.863±
475.406 | 1,317.724±
447.079 | 1,775.447±
431.080 | 1,390.552±
467.365 | 1,652.107±
436.681 | 1,725.416±
462.275 |
|
Male | 5,152.108±
1,095.435 | 3,731.458±
830.874 | 4,083.640±
1,286.341 | 4,123.368±
1,036.882 | 2,409.197±
533.703 | 1,755.768±
688.682 | 1,982.282±
834.880 | 1,948.489±
693.342 | 2,742.912±
572.334 | 1,975.691±
769.356 | 2,101.358±
810.313 | 2,174.879±
695.152 |
| Age, years | | | | | | | | | | | | |
|
Low | 4,155.538±
1,280.020 | 3,094.768±
961.623 | 3,359.832±
1,030.550 | 3,496.363±
796.523 | 1,899.322±
618.382 | 1,151.444±
537.880 | 1,322.485±
529.842 | 1,409.095±
467.056 | 2,256.216±
667.454 | 1,943.324±
694.713 | 2,037.347±
710.328 | 2,087.269±
566.783 |
|
High | 4,041.522±
1,420.182 | 2,958.221±
922.255 | 3,523.191±
1,208.512 | 3,545.978±
1,203.490 | 1,899.942±
696.332 | 1,640.969±
725.281 | 1,883.306±
840.502 | 1,806.275±
757.995 | 2,141.580±
734.480 | 1,317.251±
487.419 | 1,639.885±
541.661 | 1,739.703±
622.942 |
| BMI | | | | | | | | | | | | |
|
Low | 4,481.488±
1,494.895 | 3,199.363±
1,042.720 | 3,673.936±
1,321.679 | 3,689.551±
1,222.608 | 2,094.263±
722.249 | 1,333.293±
762.683 | 1,568.583±
855.659 | 1,564.583±
757.554 | 2,387.224±
783.281 | 1,866.070±
733.236 | 2,105.353±
764.965 | 2,124.968±
687.809 |
|
High | 3,674.829±
990.476 | 2,840.551±
777.277 | 3,169.824±
745.623 | 3,328.643±
633.396 | 1,680.633±
480.051 | 1,436.389±
569.210 | 1,606.445±
609.620 | 1,631.351±
507.880 | 1,994.196±
518.858 | 1,404.162±
525.447 | 1,563.379±
354.089 | 1,697.291±
425.326 |
| Smoking status | | | | | | | | | | | | |
|
No | 3,739.141±
1,250.955 | 2,735.445±
807.939 | 3,217.146±
879.126 | 3,244.740±
7,62.950 | 1,722.129±
604.613 | 1,199.674±
526.126 | 1,424.797±
533.893 | 1,432.506±
468.997 | 2,017.012±
655.011 | 1,535.771±
650.131 | 1,792.349±
666.148 | 1,812.234±
565.896 |
|
Yes | 5,280.799±
806.708 | 3,989.472±
620.692 | 4,150.280±
1,489.952 | 4,413.370±
1,172.613 | 2,476.442±
406.046 | 1,973.747±
779.455 | 2,111.612±
1070.479 | 2,127.369±
862.740 | 2,804.357±
423.970 | 2,015.726±
666.815 | 2,038.668±
638.268 | 2,286.001±
646.547 |
| Smoking in
males | | | | | | | | | | | | |
|
No | 5,005.034±
1,411.875 | 3,436.586±
985.468 | 4,007.479±
1,121.726 | 3,791.939±
814.952 | 2,332.345±
677.755 | 1,506.649±
511.953 | 1,834.476±
491.620 | 1,744.056±
402.359 | 2,672.689±
737.446 | 1,929.937±
926.171 | 2,173.004±
1,022.533 | 2,047.883±
777.546 |
|
Yes | 5,280.799±
806.708 | 3,989.472±
620.692 | 4,150.280±
1,489.952 | 4,413.370±
1,172.613 | 2,476.442±
406.046 | 1,973.747±
779.455 | 2,111.612±
1070.479 | 2,127.369±
862.740 | 2,804.357±
423.970 | 2,015.726±
666.815 | 2,038.668±
638.268 | 2,286.001±
646.547 |
| Surgical side | | | | | | | | | | | | |
|
Left | 3,986.829±
1,435.053 | 2,883.643±
957.540 | 3,105.051±
1,097.118 | 3,339.147±
865.230 | 1,830.101±
677.287 | 878.966±
276.732 | 1,045.696±
370.918 | 1,161.698±
308.242 | 2,156.729±
762.489 | 2,004.677±
699.511 | 2,059.355±
738.477 | 2,177.449±
570.135 |
|
Right | 4,216.938±
1,245.427 | 3,177.378±
909.428 | 3,768.363±
1,036.508 | 3,700.276±
1,102.435 | 1,969.127±
626.006 | 1,884.652±
559.936 | 2,127.106±
606.822 | 2,030.308±
598.418 | 2,247.810±
632.649 | 1,292.726±
429.463 | 1,641.258±
506.538 | 1,669.968±
554.263 |
| Mean total
volume | 4,101.884±
1,328.220 | 3,030.510±
931.542 | 3,436.707±
1,103.550 | 3,519.711±
992.888 | 1,899.614±
646.058 | 1,381.809±
670.565 | 1,586.401±
739.232 | 1,596.003±
643.452 | 2,202.270±
691.434 | 1,648.701±
676.186 | 1,850.306±
658.665 | 1,923.708±
610.644 |
 | Table IIIDemographic characteristics of 7
patients in which whole lung volume was larger at 6 months
postoperatively than preoperatively. |
Table III
Demographic characteristics of 7
patients in which whole lung volume was larger at 6 months
postoperatively than preoperatively.
| Variable | Whole lung volume
larger postoperatively than preoperatively | Whole lung volume
preoperatively than postoperatively | P-value |
|---|
| Sex | | | 0.104 |
|
Female | 6 | 13 | |
|
Male | 1 | 14 | |
| Age, years | | | 0.681 |
|
Low | 3 | 15 | |
|
High | 4 | 12 | |
| BMI | | | 0.214 |
|
Low | 2 | 16 | |
|
High | 5 | 11 | |
| Smoking status | | | >0.9999 |
|
No | 6 | 20 | |
|
Yes | 1 | 7 | |
| Surgical side | | | >0.9999 |
|
Left | 3 | 14 | |
|
Right | 4 | 13 | |
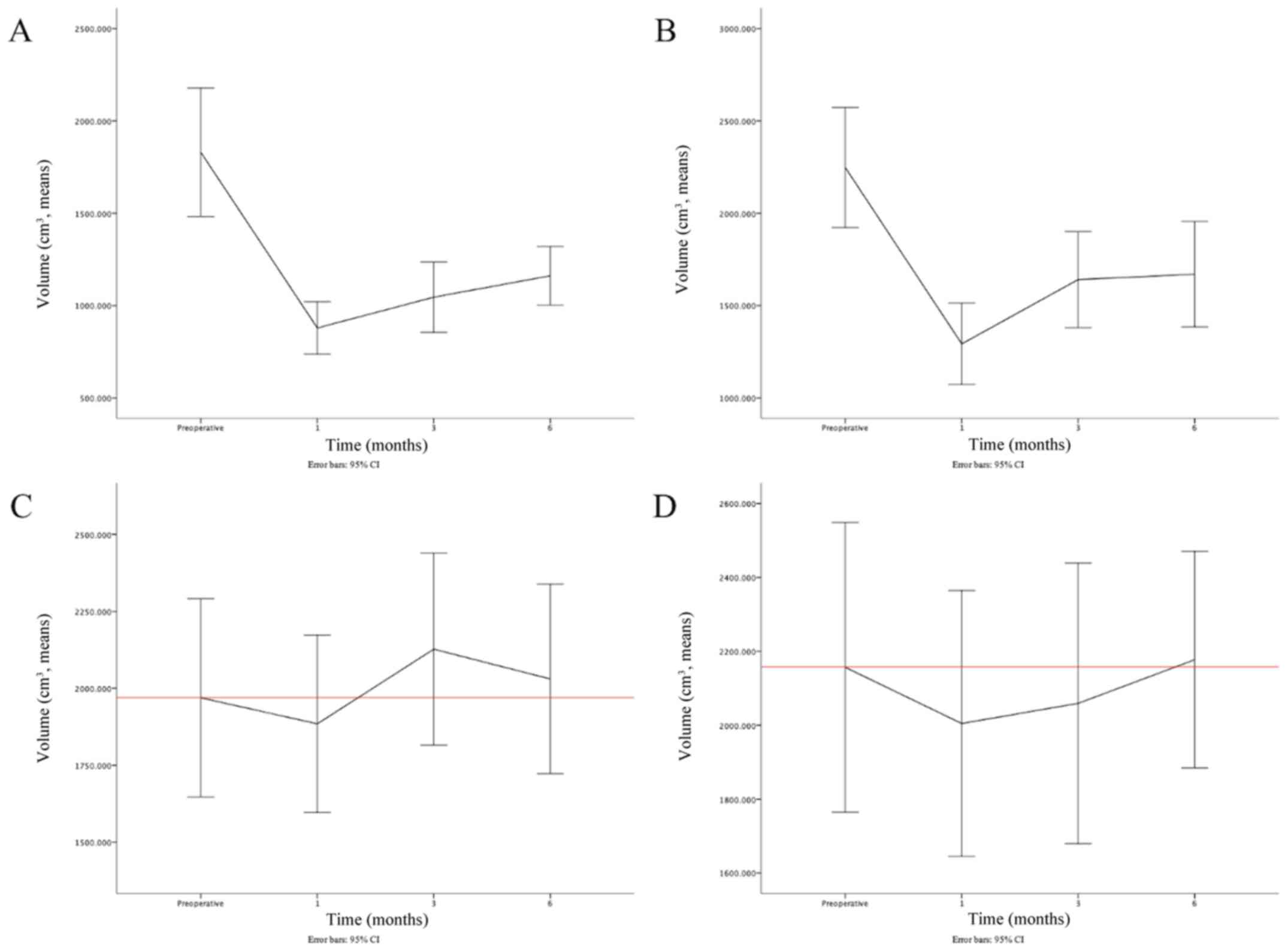
Changes in lung volume depends on
surgical side
When the surgical sides were taken into
consideration, the trendline of the ipsilateral lung volume was
consistent with the aforementioned results. The postoperative
changes on the left lung were consistent (Fig. 3A); however, the right lung rapidly
recovered from T1 to T2, and then slightly recovered from T2 to T3
(Fig. 3B). With respect to the
contralateral lung, the difference was notable. When the surgery
was performed on the right side, the volume of the left lung was
increased until T2, and then decreased slightly to just above the
baseline (Fig. 3C). On the
contrary, when the surgery was performed on the left side, the
trendline of the right lung volume was as aforementioned, except
that the mean volume at T3 increased to just above baseline
(Fig. 3D).
Results of mixed two-way ANOVA Whole,
left and right lung volume
The results of the mixed two-way ANOVA are shown in
Table IV. The differences in lung
volume for each variable on the whole, left and right lung at the
indicated observation time points (all P<0.05) were significant
compared with that for the main effect for the within-subject
effects. This finding indicated that the volume of the whole, left
and right lung was significantly changed over the course of time
from T0 to T3. However, the differences were not all significant
among diverse subgroups compared with the between-subject
effects.
 | Table IVResults of whole, right and left lung
volume changes analyzed using mixed two-way ANOVA. |
Table IV
Results of whole, right and left lung
volume changes analyzed using mixed two-way ANOVA.
| A, Whole lung
volume |
|---|
| | Within-subject
effects (at different time points) | Between-subject
effects (among diverse subgroups) |
|---|
| | Main effect | Interaction with
time | Intercept | Main effect |
|---|
| Variable | P-value | η2 | P-value | η2 | P-value | η2 | P-value | η2 |
|---|
| Sex | <0.001 | 0.541 | 0.001 | 0.157 | <0.001 | 0.957 | <0.001 | 0.437 |
| Age | <0.001 | 0.488 | 0.503 | 0.024 | <0.001 | 0.924 | 0.979 | <0.001 |
| BMI | <0.001 | 0.490 | 0.154 | 0.053 | <0.001 | 0.928 | 0.152 | 0.063 |
| Smoking status | <0.001 | 0.460 | 0.144 | 0.054 | <0.001 | 0.934 | 0.002 | 0.265 |
| Smoking in
males | <0.001 | 0.580 | 0.614 | 0.045 | <0.001 | 0.956 | 0.444 | 0.046 |
| Surgical side | <0.001 | 0.494 | 0.226 | 0.044 | <0.001 | 0.927 | 0.277 | 0.037 |
| B, Left lung
volume |
| | Within-subject
effects (at different time points) | Between-subject
effects (among diverse subgroups) |
| | Main effect | Interaction with
time | Intercept | Main effect |
| Variable | P-value | η2 | P-value | η2 | P-value | η2 | P-value | η2 |
| Sex |
<0.001a | 0.339a | 0.269a | 0.040a | <0.001 | 0.919 | <0.001 | 0.345 |
| Age |
<0.001a | 0.345a | 0.005a | 0.161a | <0.001 | 0.876 | 0.223 | 0.092 |
| BMI |
<0.001a | 0.342a | 0.007a | 0.152a | <0.001 | 0.875 | 0.813 | 0.002 |
| Smoking |
<0.001a | 0.254a | 0.860a | 0.003a | <0.001 | 0.895 | 0.002 | 0.257 |
| Smoking in
male | 0.005a | 0.364a | 0.597a | 0.034a | <0.001 | 0.926 | 0.331 | 0.073 |
| Surgical sides |
<0.001a | 0.477a |
<0.001a | 0.484a | <0.001 | 0.922 | <0.001 | 0.403 |
| C, Right lung
volume |
| | Within-subject
effects (at different time points) | Between-subject
effects (among diverse subgroups) |
| | Main effect | Interaction with
time | Intercept | Main effect |
| Variable | P-value | η2 | P-value | η2 | P-value | η2 | P-value | η2 |
| Sex |
<0.001a | 0.397a | 0.004a | 0.147a | <0.001 | 0.936 | 0.002 | 0.267 |
| Age | <0.001 | 0.380 | 0.011 | 0.109 | <0.001 | 0.920 | 0.070 | 0.099 |
| BMI |
<0.001a | 0.346a | 0.762a | 0.010a | <0.001 | 0.924 | 0.024 | 0.149 |
| Smoking |
<0.001a | 0.368a | 0.043a | 0.088a | <0.001 | 0.908 | 0.038 | 0.128 |
| Smoking in
male | <0.001 | 0.478 | 0.595 | 0.047 | <0.001 | 0.929 | 0.819 | 0.004 |
| Surgical sides | <0.001 | 0.423 | <0.001 | 0.289 | <0.001 | 0.921 | 0.058 | 0.108 |
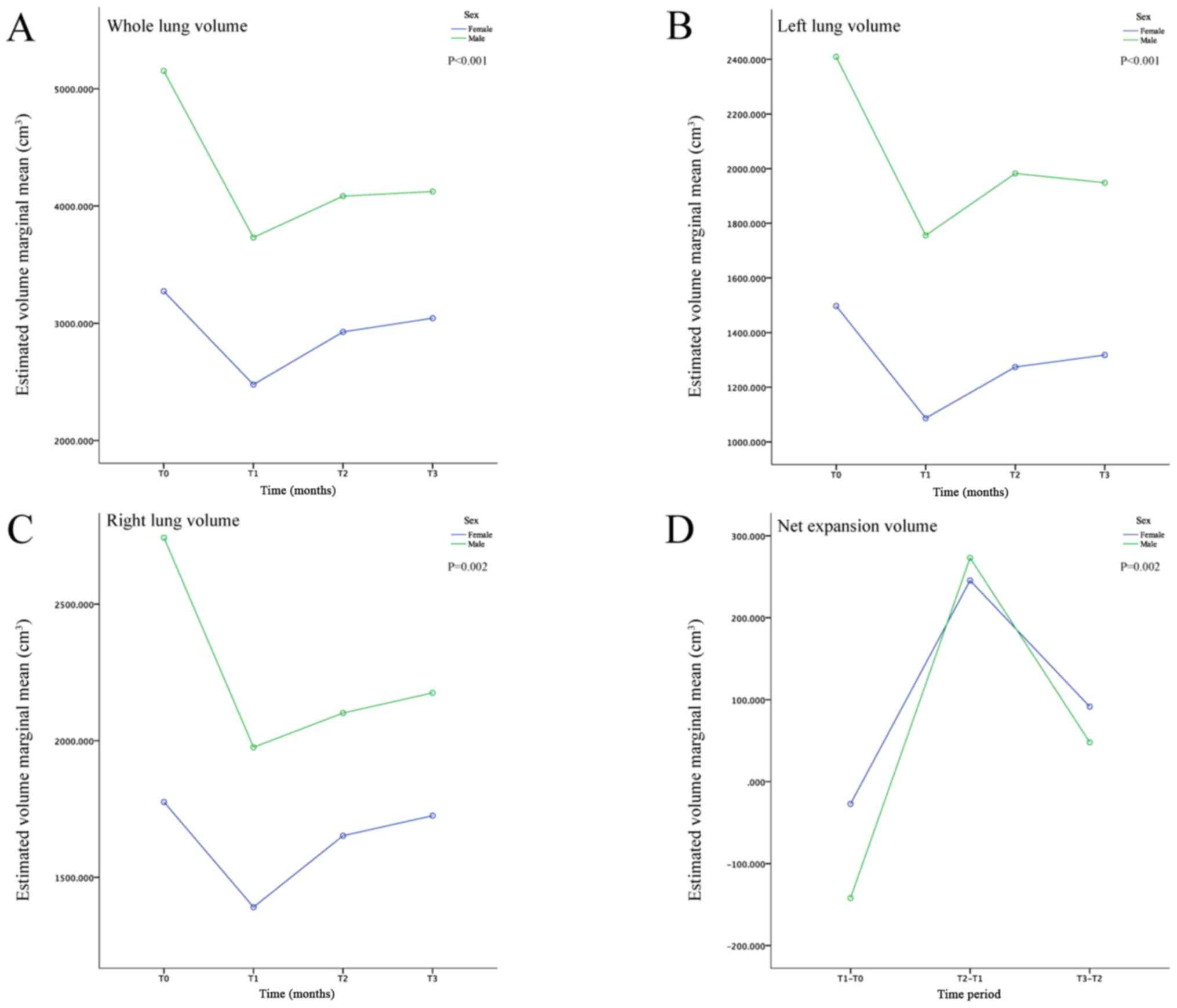
Sex
The differences on the whole, left and right lung
volume were significant between the sex subgroups compared with the
main effect for the between-subject effects (all P<0.05). In
addition, the whole, left and right lung volume in males was
notably increased compared with females among the different
observation time points (Table II;
Fig. 4A-C). Furthermore, the
interactive effects of time and sex on the whole and right lung
volume was significant (both P<0.05) (interaction with time
under within-subject effects; Table
IV), but not on that on the left lung volume, indicating that
the changing trends on the whole and right lung volume were
significantly different between males and females over time (from
T0 to T3).
Age
The interactive effects of age and time were
significantly different on the volume of the left and right lung
(both P<0.05) (interaction with time under within-subject
effects); however, the differences on the whole, left and right
lung volume were not significant between the age subgroups (all
P>0.05) (main effect under between-subject effects) (Table IV). These findings indicated that
the whole, left and right lung volume was similar between the low-
and high-age groups at each observation time point, and that the
changing trend of the whole lung volume, but not of that of the
left and right lung, was the same between the age subgroups.
BMI
The differences on the right lung volume were
significant between the BMI subgroups (P<0.05; main effect under
between-subject effects; Table
IV). However, no significant differences were observed in the
whole and left lung volume (both P>0.05) (main effect under
between-subject effects).
Smoking status
The differences on the whole, left and right lung
volume were significant between smoking status and the
between-subject effects (all P<0.05) (main effect under
between-subject effects; Table
IV); however, the differences were not significant when
compared with the male subgroup (all P>0.05) (main effect under
between-subjects effects), since all smokers were males. This
finding suggested that the whole, left and right lung volume was
similar at each time point regardless of the smoking status.
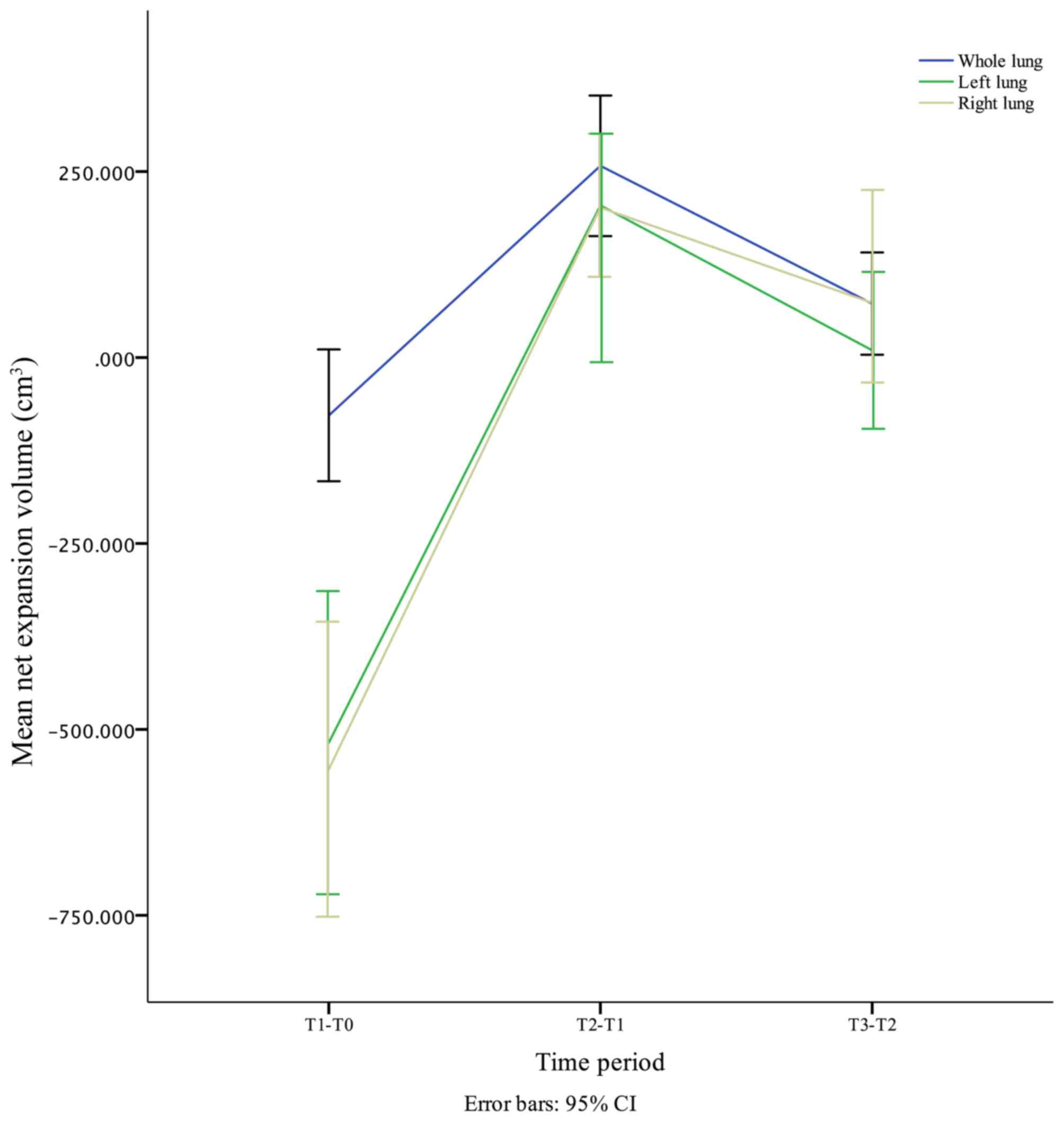
Net expansion volume
The net expansion volume of the whole, left and
right lung is presented in Table V.
In general, the mean net expansion volume of the whole, left and
right lung was sharply decreased during the first postoperative
month, quickly increased in the next three months, and slowly
decreased from the 3rd to the 6th month (Fig. 5). These changes were in accordance
with the volume changes. The differences in age, BMI and surgical
side in the left lung, as well as sex and surgical side in the
right lung, were significant. However, these observational
variables were not significant in the whole lung (Table VI). The combination of the
aforementioned results indicated that the differences in the
postoperative whole lung volume could be mainly attributed to the
differences observed preoperatively regardless of the observational
variables. For example, the lung volume in males was larger
compared with that in females preoperatively and postoperatively,
as the postoperative net expansion volume was similar between males
and females (Fig. 4D).
 | Table VNet expansion volume changes of
whole, left and right lung. |
Table V
Net expansion volume changes of
whole, left and right lung.
| | Whole lung volume,
cm3 | Left lung volume,
cm3 | Right lung volume,
cm3 |
|---|
| Variable | T1-T0 | T2-T1 | T3-T2 | T1-T0 | T2-T1 | T3-T2 | T1-T0 | T2-T1 | T3-T2 |
|---|
| Sex | | | | | | | | | |
|
Female | -27.142± | 245.440± | 91.538± | -410.734± | 187.285± | 43.861± | -384.895± | 261.556± | 73.309± |
| | 177.701 | 164.166 | 155.426 | 452.226 | 175.553 | 242.817 | 429.201 | 217.434 | 240.729 |
|
Male | -142.024± | 273.073± | 48.058± | -653.429± | 226.514± | -33.793± | -767.221± | 125.667± | 73.521± |
| | 321.508 | 370.911 | 243.555 | 710.643 | 372.277 | 369.170 | 660.695 | 620.850 | 8,499.807 |
| Age, years | | | | | | | | | |
|
Low | -32.202± | 182.494± | 71.667± | -747.878± | 171.041± | 86.610± | -312.892± | 94.024± | 49.921± |
| | 218.993 | 263.741 | 243.561 | 569.819 | 277.913 | 251.492 | 418.738 | 527.546 | 487.915 |
|
High | -129.151± | 342.160± | 73.1311± | -258.973± | 242.337± | -77.031± | -824.329± | 322.633± | 99.819± |
| | 286.504 | 260.654 | 134.823 | 496.693 | 277.109 | 338.407 | 603.099 | 286.154 | 177.439 |
| BMI | | | | | | | | | |
|
Low | -133.694± | 298.775± | 64.968± | -760.970± | 235.290± | -4.00± | -521.154± | 239.283± | 19.615± |
| | 217.425 | 270.518 | 193.242 | 628.158 | 248.284 | 305.399 | 604.786 | 475.435 | 441.113 |
|
High | -14.972± | 211.344± | 80.668± | -244.244± | 170.056± | 24.906± | -590.034± | 159.217± | 133.913± |
| | 283.210 | 271.875 | 207.242 | 389.484 | 308.100 | 308.459 | 541.242 | 408.671 | 273.481 |
| Smoking status | | | | | | | | | |
|
No | -59.844± | 255.604± | 57.093± | -522.454± | 225.123± | 7.709± | -481.241± | 256.578± | 19.885± |
| | 250.658 | 201.136 | 185.427 | 621.998 | 240.067 | 259.168 | 457.850 | 263.210 | 349.929 |
|
Yes | -136.265± | 264.218± | 121.959± | -502.695± | 137.866± | 15.756± | -788.631± | 22.942± | 247.333± |
| | 272.478 | 448.125 | 237.795 | 473.683 | 381.821 | 437.564 | 830.976 | 787.057 | 407.546 |
| Smoking in
males | | | | | | | | | |
|
No | -148.605± | 283.193± | -36.400± | -825.69± | 327.827± | -90.420± | -742.752± | 243.067± | -125.121± |
| | 393.037 | 294.107 | 238.403 | 922.864 | 361.716 | 295.997 | 460.026 | 382.808 | 550.264 |
|
Yes | -136.265± | 264.218± | 121.959± | -502.695± | 137.866± | 15.756± | -788.631± | 22.942± | 247.333± |
| | 272.478 | 448.125 | 237.795 | 473.683 | 381.821 | 437.564 | 830.976 | 787.057 | 407.546 |
| Surgical sides | | | | | | | | | |
|
Left | -30.281± | 166.730± | 116.001± | -951.135± | 166.730± | 116.001± | -152.052± | 54.67± | 118.094± |
| | 186.002 | 248.420 | 217.314 | 461.452 | 248.420 | 217.314 | 330.877 | 531.445 | 500.732 |
|
Right | -125.370± | 348.532± | 28.710± | -84.475± | 242.454± | -96.797± | -955.085± | 348.532± | 28.710± |
| | 305.753 | 268.004 | 169.700 | 301.425 | 303.290 | 342.721 | 462.288 | 268.004 | 169.700 |
| Total volume | -77.825± | 257.631± | 72.356± | -517.805± | 204.592± | 9.602± | -553.568± | 201.605± | 73.402± |
| | 253.829 | 270.665 | 197.034 | 583.746 | 275.675 | 302.507 | 568.154 | 440.459 | 370.928 |
 | Table VIResults of the net expansion volume
changes in whole, right and left lung volume analyzed using mixed
two-way ANOVA. |
Table VI
Results of the net expansion volume
changes in whole, right and left lung volume analyzed using mixed
two-way ANOVA.
| A, Whole lung
volume |
|---|
| | Within-subject
effects (at different time points) | Between-subject
effects (among diverse subgroups) |
|---|
| | Main effect | Interaction with
time | Intercept | Main effect |
|---|
| Variable | P-value | η2 | P-value | η2 | P-value | η2 | P-value | η2 |
|---|
| Sex |
<0.001a | 0.288a | 0.525a | 0.017a | <0.001 | 0.462 | 0.171 | 0.058 |
| Age, years |
<0.001a | 0.298a | 0.166a | 0.057a | <0.001 | 0.472 | 0.504 | 0.014 |
| BMI |
<0.001a | 0.277a | 0.300a | 0.036a | <0.001 | 0.469 | 0.625 | 0.008 |
| Smoking status | 0.001a | 0.241a | 0.605a | 0.012a | <0.001 | 0.384 | 0.979 | <0.001 |
| Smoking in
males | 0.033a | 0.267a | 0.703a | 0.018a | 0.081 | 0.216 | 0.424 | 0.050 |
| Surgical side |
<0.001a | 0.296a | 0.075a | 0.085a | <0.001 | 0.466 | 0.995 | <0.001 |
| B, Left lung
volume |
| | Within-subject
effects (at different time points) | Between-subject
effects (among diverse subgroups) |
| | Main effect | Interaction with
time | Intercept | Main effect |
| Variable | P-value | η2 | P-value | η2 | P-value | η2 | P-value | η2 |
| Sex | <0.001 | 0.424 | 0.449 | 0.025 | 0.001 | 0.280 | 0.131 | 0.070 |
| Age, years | <0.001 | 0.435 | 0.009 | 0.136 | 0.002 | 0.262 | 0.029 | 0.140 |
| BMI | <0.001 | 0.430 | 0.016 | 0.121 | 0.001 | 0.274 | 0.007 | 0.206 |
| Smoking status | <0.001 | 0.321 | 0.906 | 0.003 | 0.007 | 0.209 | 0.789 | 0.002 |
| Smoking in
males | 0.001 | 0.431 | 0.472 | 0.056 | 0.020 | 0.351 | 0.510 | 0.034 |
| Surgical side | <0.001 | 0.534 | <0.001 | 0.390 | <0.001 | 0.387 | <0.001 | 0.477 |
| C, Right lung
volume |
| | Within-subject
effects (at different time points) | Between-subject
effects (among diverse subgroups) |
| | Main effect | Interaction with
time | Intercept | Main effect |
| Variable | P-value | η2 | P-value | η2 | P-value | η2 | P-value | η2 |
| Sex |
<0.001a | 0.386a | 0.338a | 0.033a | <0.001 | 0.355 | 0.001 | 0.279 |
| Age, years |
<0.001a | 0.417a | 0.015a | 0.134a | 0.002 | 0.266 | 0.174 | 0.057 |
| BMI |
<0.001a | 0.373a | 0.669a | 0.011a | 0.003 | 0.248 | 0.842 | 0.001 |
| Smoking status |
<0.001a | 0.352a | 0.176a | 0.054a | 0.001 | 0.300 | 0.118 | 0.075 |
| Smoking in
males | 0.009a | 0.361a | 0.465a | 0.050a | 0.001 | 0.593 | 0.692 | 0.012 |
| Surgical side |
<0.001a | 0.449a |
<0.001a | 0.279a | <0.001 | 0.345 | <0.001 | 0.378 |
Surgical side
The results showed that the changing trend of volume
and net expansion volume was significant in the ipsilateral lung
compared with that in the contralateral lung (all P<0.05) (main
effect under the between-subjects effects; Tables VII and VIII). It indicated that the ipsilateral
lung experienced more dramatic change of volume than contralateral
after lobectomy.
 | Table VIIVolume and net expansion volume
changes of the ipsilateral and contralateral lung. |
Table VII
Volume and net expansion volume
changes of the ipsilateral and contralateral lung.
| | Lung volume,
cm3 | Net expansion
volume, cm3 |
|---|
| Surgical side | T0 | T1 | T2 | T3 | T1-T0 | T2-T1 | T3-T2 |
|---|
| Ipsilateral | 2,038.956±
679.271 | 1,085.846±
413.099 | 1,343.477±
531.478 | 1,415.832±
511.427 | -953.110±
454.823 | 257.631±
270.665 | 72.356±
197.034 |
| Contralateral | 2,062.928±
693.509 | 1,944.664±
626.872 | 2,093.230±
666.431 | 2,103.879±
580.349 | -118.264±
313.543 | 148.566±
436.599 | 10.649±
436.361 |
 | Table VIIIDifferences in volume and net
expansion between ipsilateral and contralateral lungs according to
surgical sides analyzed using mixed two-way ANOVA. |
Table VIII
Differences in volume and net
expansion between ipsilateral and contralateral lungs according to
surgical sides analyzed using mixed two-way ANOVA.
| | Within-subject
effects (at different time points) | Between-subject
effects (among diverse subgroups) |
|---|
| | Main effect | Interaction with
time | Intercept | Main effect |
|---|
| Variable | P-value | η2 | P-value | η2 | P-value | η2 | P-value | η2 |
|---|
| Volume | <0.001 | 0.435 | <0.001 | 0.36 | <0.001 | 0.913 | <0.001 | 0.221 |
| Net expansion |
<0.001a | 0.427a |
<0.001a | 0.295a | <0.001 | 0.364 | <0.001 | 0.427 |
Discussion
To the best of our knowledge, the present study
reported for the first time the perioperative changes on lung
volume. The main finding was that the whole, right and left lung
exhibited a similar trendline of volume changes (Fig. 1, Fig.
2 and Fig. 3). More
specifically, the whole, right and left lung volumes were notably
decreased during the first postoperative month (all P<0.05),
increased quickly in the next three months (all P<0.05) then
increased more slowly from the 3rd to the 6th month (all
P>0.05). In addition, the differences in all 3 lung volumes were
all significant among the four observational time points (all
P<0.05) (Table II). The results
indicated that the postoperative compensatory expansion of the
bilateral lung, and consequently of the whole lung, occurred before
the first month following surgery and lasted ≥6 months
postoperatively. Furthermore, the results showed that the
postoperative lung volume did not change linearly. These findings
are consistent with previous studies. For example, Nagamatsu et
al (22) demonstrated that the
forced expiratory volume in 1.0 second (FEV1) per square meter
(FEV1/m2), a main parameter of PF, was sharply decreased
during the first postoperative month (P<0.001), then quickly
increased for the next three months (P<0.001), and increased
slowly for the next 3 months. Additional studies also revealed that
FEV1 (23-25),
vital capacity (VC) (24), forced
vital capacity (FVC) (25) and
diffusion capacity of the lung for carbon monoxide
(DLCO) (23,24) were decreased in the early
postoperative phase, after which they gradually improved, but not
linearly. Further studies are required for identifying similar
trendlines between the perioperative lung volume observed in the
present study and PFTs used in previous studies; however,
volumetric CT could be used to analyze perioperative PF or in
combination with preoperative PFTs to predict the actual recovery
of lung function following surgery. Furthermore, when the
postoperative PF was predicted the predictive time points should
also be also taken into consideration (22-25).
In addition, the results from the present study
demonstrated that there were seven patients (20.588%) with an
increased whole lung volume 6th months following surgery compared
with that preoperatively (Fig. 2).
This could be attributed to preoperative obstructive pneumonia in
central lung cancer or an enormous space occupying lesion in
peripheral lung cancer. According to widely used algorithms, which
were developed to predict postoperative PF based on the
preoperative evaluated and resected segment, the postoperative PF
should be inferior to the preoperative one (6-8).
However, previous studies have already verified that it is
imprecise to predict the postoperative function using these
algorithms (9-11).
This observation is reasonable and inevitable, since all of these
algorithms are based on linear regression and ignore the variation
of the postoperative lung volume re-expansion over time and the
effects of compensatory expansion on PF recovery. Furthermore, the
PFTs require the effort of the patient, which sometimes vary with
time and the patient (24).
However, the results of the current study and previous studies
confirmed that the postoperative lung volume or PF did not change
linearly (22-25).
When the surgical side was taken into consideration,
postoperative compensatory expansion was not only observed in the
ipsilateral residual lung, but also in the contralateral intact
lung (Fig. 3). Ueda et al
(26) and Choe et al
(17) used volumetric CT to observe
the changes in the regional lung volume after lobectomy or
segmentectomy. The authors revealed that not only did the
ipsilateral residual lung lobe increase significantly, but also the
contralateral lung. Ueda et al (26) suggested that the compensatory
expansion of the bilateral residual lung following major resection,
particularly after lobectomy, could contribute to improved
postoperative residual pulmonary function, and that increased
postoperative functional lung volume was significantly correlated
with improved postoperative PF (R=0.6; P<0.001).
Unlike previous studies (22-25),
which investigated the changes in lung volume at only two different
time points, the present study consistently recorded the
perioperative changes. In addition, it was also found that the
changing trends of the net compensatory expansion volume of the
residual lung, which expanded rapidly between the 1st and 3rd
postoperative month and then slowly for the next 3 months, were not
linear in both the ipsilateral residual and contralateral intact
lung (Fig. 5). This trend was
similar with that observed in the whole lung, as aforementioned,
and could be the root cause of the latter. With respect to the
intactness of the contralateral lung, the only interpretation of
the compensatory expansion could be the toward shift of the
mediastinum ipsilateral chest, due to the ipsilateral chest volume
loss attributed to lobectomy. The differences in the net
compensatory expansion volume in the left and right lung were
significant between the surgical sides (P<0.001; Table VI), and the ipsilateral and
contralateral lung (P<0.001; Table VIII); however, no significant
differences were observed in the whole lung (P=0.995; Table VI; Fig.
5). This phenomenon could be attributed to the neutralization
of the differences in the surgical side by summation when
calculating the whole lung volume. Choe et al (17) also demonstrated that the differences
on the postoperative whole lung volume were not significant between
the surgical sides, although a significant difference on the
compensatory expansion volume between the contralateral and
ipsilateral lung was observed.
Furthermore, the current study showed that sex
exerted a significant influence on postoperative early lung volume
recovery. The trendline changes of the whole, left and right lung
volume were similar between males and females; however, the
perioperative mean lung volume in males was significantly larger
compared with that in females (P<0.05; Tables II and IV; Fig.
4). This finding could be due to the preoperative existing
differences on lung volume. This was also in line with the study by
Khieya (27) who reported that
males were taller and more muscular with respect to body
proportions, and not due to sex itself. This finding could also be
verified by the absence of a significant difference in the net
expansion volume of the whole and left lung between males and
females (Table VI), and the fact
that the adult lung does not generally have the ability to
regenerate new alveolar septal tissues (12). Kim et al (28) showed that the differences on the
preservation of FEV1 following VATS were not significant between
male and female patients with NSCLC. In addition, Takahashi et
al (29) confirmed that the sex
was not associated with improvement of postoperative PF following
lobectomy.
The results of the present study also demonstrated
that age, BMI and smoking status had no effect on postoperative
lung volume and net expansion of the whole lung following VATS
lobectomy. Measuring lung volume alone cannot adequately describe
lung function; however, variations on lung volume may reflect
changes in its function. It is widely accepted that obesity, old
age and smoking increases the risk of postoperative pulmonary
complications (30); however, their
effect on lung function remains controversial. Matsumoto et
al (31) suggested that smoking
did not affect the decreased volume of VC and FEV1 following
pulmonary lobectomy. In addition, Kim et al (28) confirmed that the differences on FEV1
preservation following VATS were not significant between smokers
and non-smokers, while Takahashi et al (29) showed that smoking was negatively
correlated with improvement of postoperative PF following
lobectomy.
Khieya (27)
investigated 317 healthy individuals in the Southern Angami
population, and found that BMI was not statistically associated
with FEV1, FVC, peak expiratory flow and FEV1/FVC. Furthermore,
Kobayashi et al (32)
retrospectively reviewed the medical records of 445 patients who
underwent surgery for lung cancer between 2001 and 2009, and
verified that BMI had no effect on PF at years 1 and 5 year
following lobectomy. Previous studies also confirmed that BMI had
no effect on postoperative PFTs (28,29).
Several studies have demonstrated that age was
negatively correlated with PFTs (5,27,28,33).
However, a study showed that the differences on PFTs were not
significant among the low, medium and high age groups (34). Takahashi et al (29) also found that age was not associated
with improvement of postoperative PF following lobectomy. However,
further studies are required to identify the effect of age, BMI and
smoking on postoperative lung function following VATS
lobectomy.
There are some limitations in the present
preliminary study. Firstly, there was no investigation into the
association between lung volume on CT scan and PFTs, since PFTs
were not routinely performed following surgery. Secondly, due to
the small number of patients, the changes in lung volume, with
respect to the resected lobes were not analyzed. Therefore, these
analyses will be performed in our future studies. Thirdly, the
preoperative PFT was only performed in the patients. As this is a
preliminary study, the aim was to investigate perioperative lung
volume changes, and the PFT is currently being performed at all the
follow-up time points in the ongoing study. Finally, a longer
observation time is also required to reveal the lung volume changes
one year or more following surgery.
Regardless of these limitations, the current study
showed that the early changes on postoperative lung volume were not
linear, since the lung volume was significantly decreased during
the first postoperative month, rapidly increased in the next 3
months, then slowly increased from 3-6 months. In addition, the
results demonstrated that sex, age, BMI, smoking status and
surgical sides had no effect on the postoperative volume and net
expansion of the whole lung following VATS lobectomy.
Supplementary Material
Technical images using volumetric
computed tomography. The red box indicates the preoperative volume
of each lobe. Light blue indicates the RU, pink indicates the RM,
green indicates the RL, cyan indicates the LU and yellow indicates
the LL. RU, right upper lobe; RM, right middle lobe; RL, right
lower lobe; LU, left upper lobe; LL, left lower lobe.
Acknowledgements
Not applicable.
Funding
This study was partly funded by the Wu Jieping Medical
Foundation (grant no. 320.6750.18470).
Availability of data and materials
The datasets used and/or analyzed in the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XD and HL was involved in the conception and design
of the study. CC provided administrative support. LL, MZ, ZT, JZ,
PL and HX recruited the patients. LL, MZ, ZT, PL and HX collected
and organized the data. XD and HL confirm the authenticity of all
the raw data. All authors analyzed and interpreted the data, wrote
the manuscript and provided final approval of the manuscript.
Ethics approval and consent to
participate
This study was approved the Human Ethics Committee
of The Affiliated Hospital of Guizhou Medical University (Guizhou,
China; approval no. 2020-244). Medical record review was performed
in accordance with the institutional ethics review board
guidelines. Informed consent was provided by all individual
participants included in the study.
Patient consent for publication
Patients signed informed consent regarding
publishing their data and photographs.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Selzer A and Sarkiss M: Preoperative
pulmonary evaluation. Med Clin North Am. 103:585–599.
2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lee H, Kim HK, Kang D, Kong S, Lee JK, Lee
G, Shin S, Cho J, Zo JI, Shim YM and Park HY: Prognostic value of
6-Min walk test to predict postoperative cardiopulmonary
complications in patients with non-small cell lung cancer. Chest.
157:1665–1673. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lakshminarasimhachar A and Smetana GW:
Preoperative evaluation: Estimation of pulmonary risk. Anesthesiol
Clin. 34:71–88. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ferguson MK, Watson S, Johnson E and
Vigneswaran WT: Predicted postoperative lung function is associated
with all-cause long-term mortality after major lung resection for
cancer. Eur J Cardiothorac Surg. 45:660–664. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Juhl B and Frost N: A comparison between
measured and calculated changes in the lung function after
operation for pulmonary cancer. Acta Anaesthesiol Scand Suppl.
57:39–45. 1975.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Egeblad K, Aunsholt NA, Funder V and
Nielsen PH: A simple method for predicting pulmonary function after
lung resection. Scand J Thorac Cardiovasc Surg. 20:103–107.
1986.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nakahara K, Monden Y, Ohno K, Miyoshi S,
Maeda H and Kawashima Y: A method for predicting postoperative lung
function and its relation to postoperative complications in
patients with lung cancer. Ann Thorac Surg. 39:260–265.
1985.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ontiveros N, Eapen-John D, Song J, Li L,
Sheshadri A, Tian X, Ghosh N, Vaporciyan AA, Correa M, Walsh G, et
al: Predicting postoperative lung function following lobectomy.
Chest. 152(A640)2017.
|
|
10
|
Varela G, Brunelli A, Rocco G, Marasco R,
Jiménez MF, Sciarra V, Aranda JL and Gatani T: Predicted versus
observed FEV1 in the immediate postoperative period after pulmonary
lobectomy. Eur J Cardiothorac Surg. 30:644–648. 2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Brunelli A, Refai M, Salati M, Xiumé F and
Sabbatini A: Predicted versus observed FEV1 and DLCO after major
lung resection: A prospective evaluation at different postoperative
periods. Ann Thorac Surg. 83:1134–1139. 2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bremer JL: The fate of the remaining lung
tissue after lobectomy or pneumonectomy. J Thorac Surg. 6:336–343.
1937.
|
|
13
|
Wakamatsu I, Matsuguma H, Nakahara R and
Chida M: Factors associated with compensatory lung growth after
pulmonary lobectomy for lung malignancy: An analysis of lung weight
and lung volume changes based on computed tomography findings. Surg
Today. 50:144–152. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Vinogradskiy Y, Jackson M, Schubert L,
Jones B, Castillo R, Castillo E, Guerrero T, Mitchell J, Rusthoven
C, Miften M and Kavanagh B: Assessing the use of 4DCT-ventilation
in pre-operative surgical lung cancer evaluation. Med Phys.
44:200–208. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Suzuki H, Oishi H, Noda M, Watanabe T,
Matsuda Y, Tominaga J, Sado T, Sakurada A, Kurosawa H, Takase K and
Okada Y: Correlation between the native lung volume change and
postoperative pulmonary function after single lung transplantation
for lymphangioleiomyomatosis: Evaluation of lung volume by
three-dimensional computed tomography volumetry. PLoS One.
14(e0210975)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gu S, Leader J, Zheng B, Chen Q, Sciurba
F, Kminski N, Gur D and Pu J: Direct assessment of lung function in
COPD using CT densitometric measures. Physiol Meas. 35:833–845.
2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Choe J, Lee SM, Chae EJ, Kim YH, Kim N and
Seo JB: Evaluation of postoperative lung volume and perfusion
changes by dual-energy computed tomography in patients with lung
cancer. Eur J Radiol. 90:166–173. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fourdrain A, De Dominicis F, Lafitte S,
Iquille J, Prevot F, Lorne E, Monconduit J, Bagan P and Berna P:
Quantitative computed tomography to predict postoperative FEV1
after lung cancer surgery. J Thorac Dis. 9:2413–2418.
2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kobayashi K, Saeki Y, Kitazawa S,
Kobayashi N, Kikuchi S, Goto Y, Sakai M and Sato Y:
Three-dimensional computed tomographic volumetry precisely predicts
the postoperative pulmonary function. Surg Today. 47:1303–1311.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Oswald NK, Halle-Smith J, Mehdi R,
Nightingale P, Naidu B and Turner AM: Predicting postoperative lung
function following lung cancer resection: A systematic review and
meta-analysis. EClinicalMedicine. 15:7–13. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sihoe ADL: The evolution of minimally
invasive thoracic surgery: Implications for the practice of
uniportal thoracoscopic surgery. J Thorac Dis. 6 (Suppl
6):S604–S617. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Nagamatsu Y, Maeshiro K, Kimura NY, Nishi
T, Shima I, Yamana H and Shirouzu K: Long-term recovery of exercise
capacity and pulmonary function after lobectomy. J Thorac
Cardiovasc Surg. 134:1273–1278. 2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kim HK, Lee YJ, Han KN and Choi YH:
Pulmonary function changes over 1 year after lobectomy in lung
cancer. Respir Care. 61:376–382. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yokoba M, Ichikawa T, Harada S, Naito M,
Sato Y and Katagiri M: Postoperative pulmonary function changes
according to the resected lobe: A 1-year follow-up study of
lobectomized patients. J Thorac Dis. 10:6891–6902. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kim SJ, Lee YJ, Park JS, Cho YJ, Cho S,
Yoon HI, Kim K, Lee JH, Jheon S and Lee CT: Changes in pulmonary
function in lung cancer patients after video-assisted thoracic
surgery. Ann Thorac Surg. 99:210–217. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ueda K, Tanaka T, Hayashi M, Li TS, Tanaka
N and Hamano K: Computed tomography-defined functional lung volume
after segmentectomy versus lobectomy. Eur J Cardiothorac Surg.
37:1433–1437. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Khieya T: An Evaluation of the pulmonary
function tests and their association with age and body mass index
(BMI) in the southern angami population. J North East Indian Cult.
3:82–91. 2017.
|
|
28
|
Kim SJ, Ahn S, Lee YJ, Park JS, Cho YJ,
Cho S, Yoon HI, Kim K, Lee JH, Jheon S and Lee CT: Factors
associated with preserved pulmonary function in non-small-cell lung
cancer patients after video-assisted thoracic surgery. Eur J
Cardiothorac Surg. 49:1084–1090. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Takahashi Y, Matsutani N, Morita S, Dejima
H, Nakayama T, Uehara H and Kawamura M: Predictors of long-term
compensatory response of pulmonary function following major lung
resection for non-small cell lung cancer: Long-term lung function
after lobectomy. Respirology. 22:364–371. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Smetana GW: Preoperative pulmonary
evaluation. N Engl J Med. 340:937–944. 1999.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Matsumoto R, Takamori S, Yokoyama S,
Hashiguchi T, Murakami D, Yoshiyama K, Nishi T, Kashihara M,
Mitsuoka M, Hayashida R, et al: Lung function in the late
postoperative phase and influencing factors in patients undergoing
pulmonary lobectomy. J Thorac Dis. 10:2916–2923. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kobayashi N, Kobayashi K, Kikuchi S, Goto
Y, Ichimura H, Endo K and Sato Y: Long-term pulmonary function
after surgery for lung cancer. Interact Cardiovasc Thorac Surg.
24:727–732. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Behera A, Behera BK, Dash S and Mishra S:
Variation of pulmonary function tests with relation to increasing
age in healthy adults. Int J Health Sci Res. 4:136–141. 2014.
|
|
34
|
Britto RR, Zampa CC, Oliveira TA, de
Oliveira TA, Prado LF and Parreira VF: Effects of the aging process
on respiratory function. Gerontology. 55:505–510. 2009.PubMed/NCBI View Article : Google Scholar
|