Introduction
Chemotherapy for cancer is associated with a
constellation of adverse effects including toxicity in the
gastro-intestinal tract. Cytotoxic drugs may cause significant
injury and disruption of the gut mucosa with manifestations of
varying severity such as diarrhea or bacterial translocation
leading to local or disseminated infection (1). Patients with chemotherapy-induced
immunosuppression may present with neutropenic colitis which can be
a life-threatening condition particularly in the less common
occasion of full-thickness bowel necrosis and perforation (2).
Acute complications are very common in patients
receiving chemotherapy. Due to significant co-morbidity or various
concomitant parameters they may constitute challenging
inter-disciplinary cases at the emergency department. Our objective
is to present and discuss an unprecedented case of appendiceal
perforation and gangrene in a patient with metastatic small-cell
lung cancer and chemotherapy-induced neutropenic enterocolitis.
Case report
In September 2018 a 66-year-old woman with multiple
co-morbidity presented at the emergency department of our hospital
with a worsening abdominal pain of four days duration. The patient
was under chemotherapy with topotecan since June 2018 for a
primarily inoperable small-cell lung carcinoma with bone
metastases. The last dose of topotecan was administered two weeks
previously. Earlier chemotherapy regimens included carboplatin and
etoposide, first started in November 2017. Her comorbidities
included coronary artery disease, insulin-dependent type II
diabetes, hypertension, hypercholesterolemia, and liver steatosis.
Acetylsalicylic acid was used as an anti-aggregant medication. The
patient had exocrine pancreatic insufficiency and an established
diagnosis of chronic pancreatitis since 2008. Thereafter she
suffered from several episodes of acute-on-chronic pancreatitis
with the last episode in December 2017. In July 2018 she underwent
an endoscopic retrograde cholangiopancreatography (ERCP) in order
to relieve distal biliary obstruction caused by a chronic
pancreatic pseudocyst in the pancreatic head. At this procedure a
sphincterotomy of the sphincter of Oddi was performed and a 5-cm
long plastic biliary stent of 10-french diameter was successfully
deployed in the common bile duct. In addition, the surgical
anamnesis comprised an open cholecystectomy in 2003.
The initial clinical examination revealed guarding
on the right lower quadrant. The last bowel movement occurred four
days earlier. The patient had fever and mild hypotension. The
laboratory results showed leukocytopenia (white blood cell
count=1,7x10E9/l), neutropenia (neutrophil count=0,74x10E9/l),
profuse thrombocytopenia (platelet count=9x10E9/l), normocytic
anemia (hemoglobin=92 g/l), whereas the c-reactive protein (crp)
was high at 264 mg/l. The transaminase, bilirubin, glomerular
filtration rate, and amylase levels were normal and the alkaline
phosphatase level was slightly increased at a chronic basis (164
U/l) consistent to the presence of the stent and to the medication.
Blood cultures were negative. A computed tomography (CT) -scan of
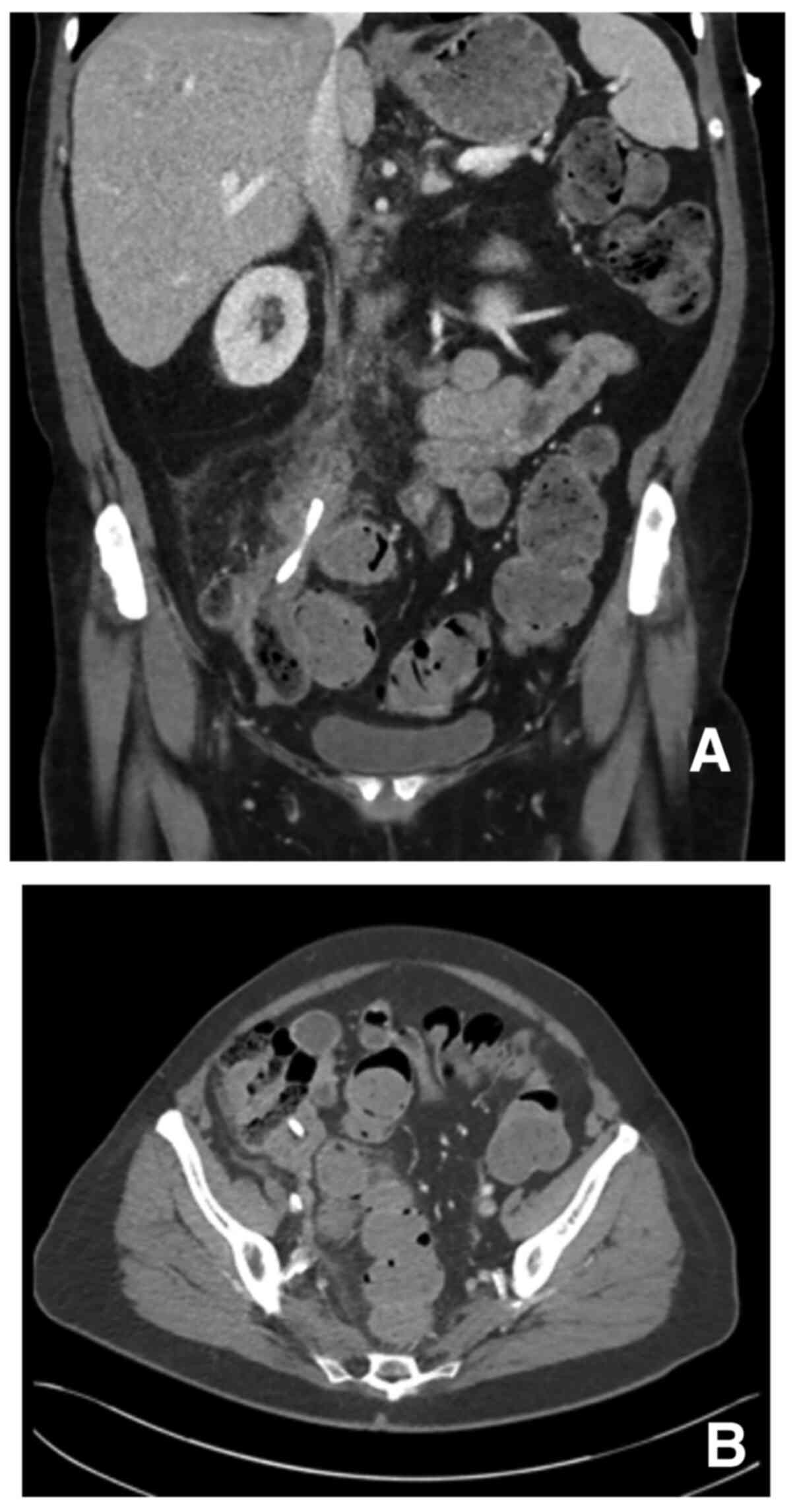
the abdomen showed that the biliary stent had migrated into the
lumen of the appendix (Figs. 1A and
B, and 2). The lumen was obstructed and the
appendix thickened due to inflammation (Fig. 1B). There was also peri-appendiceal
fat stranding without any extra-luminal air.
Immediate resuscitation with fluids and
broad-spectrum antibiotics (piperacillin/tazobactam) were initiated
and hemodynamic stability was restored. The patient was transfused
with platelets and a leukocyte-stimulating factor (filgrastim) and
corticosteroids were started. After transfusions the platelet count
increased to 80x10E9/l. A colonoscopy was attempted a few hours
after admission with the intent to relieve the appendix by removing
the stent and to continue with antibiotic therapy for the inflamed
appendix (unless deterioration would occur). The endoscopy was
abandoned due to poor visibility and an exploratory laparotomy was
performed using a 7-cm incision at the McBurney point. Laparoscopic
surgery was not performed due to high anesthesiologic risk.
Appendiceal inflammation was confirmed. The stent had perforated
the appendix at its apex with half of the stent being situated free
in the peritoneal cavity whereas the other half was inside the
appendix clogging its lumen. The wall of the caecum appeared
inflamed but there were no findings of frank peritonitis. The stent
was removed and appendicectomy was performed with local lavage and
drain placement.
The patient's immediate post-operative course was
uneventful. The crp reached a peak on the second postoperative day
(431 mg/l) and then it gradually dropped to 55 mg/l at discharge.
The drain was removed on the third postoperative day. The
leukocyte-stimulating factor was discontinued as the white blood
cell count was restored. She was discharged nine days after the
operation. Antibiotic therapy with cephalexin continued for seven
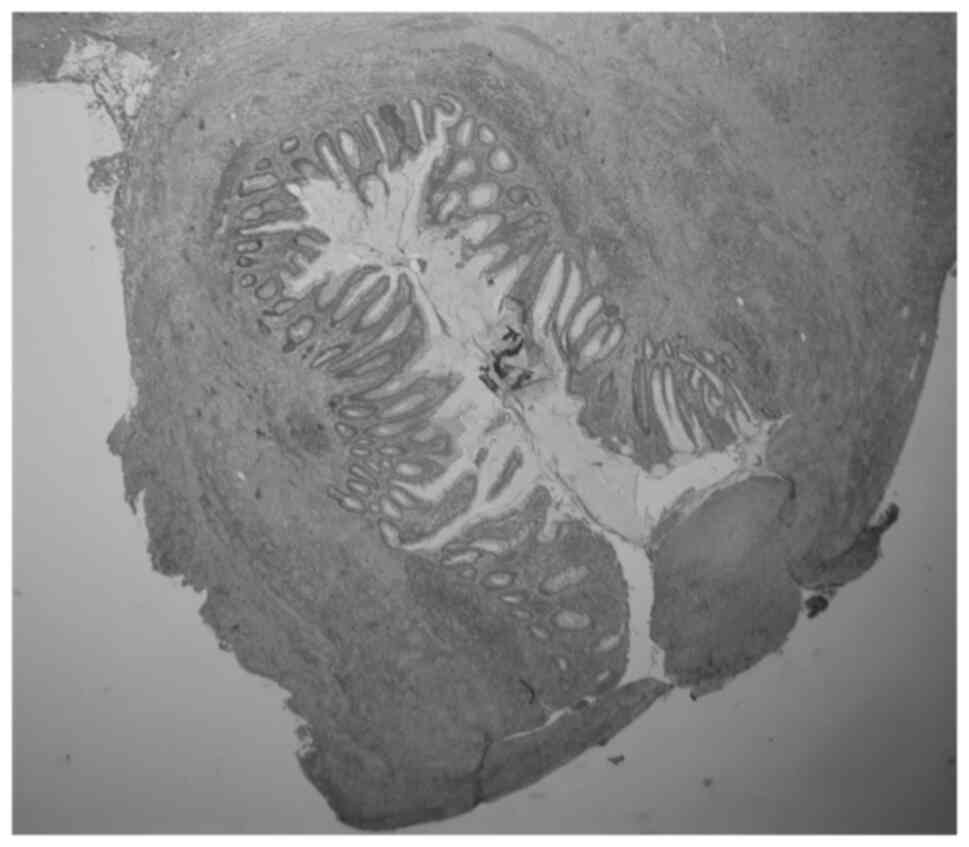
more days. The pathologic examination of the removed appendix
showed gangrenous changes with perforation and no neoplasia
(Fig. 3).
Ten days after discharge she returned to the
emergency department due to abdominal pain and fever. There was
local guarding at the right lower quadrant and the crp was 27 mg/l.
A CT-scan revealed a 2x3 -centimeter abscess located pre-sacrally
and medially to the caecum. The patient was treated with
broad-spectrum antibiotics alone (piperacillin/tazobactam) as the
location of the abscess precluded percutaneous drainage. Subsequent
CT-imaging showed near-total elimination of the infection. She was
transferred to a health center after 11 days of hospitalization
where a 5-day course of oral levofloxacin was continued.
The patient consented in written for the publication
of her case study.
Discussion
Biliary stent migration is a rather uncommon event
and bowel perforation due to the migrated stent is extremely rare
(3). Migrated plastic stents in
particular are normally eliminated from the gastro-intestinal tract
without any consequence (3). The
most common site of a perforation due to a stent is the duodenum
(4-6).
Sporadic cases of small- or large- bowel perforation have been
reported (7,8). Interestingly, such perforations have
occurred in conjunction with a separate predisposing factor such as
diverticula, adhesions, or incarcerated bowel in an incisional
hernia (7,8). To the best of our knowledge stent
migration into the appendix with perforation has not been reported
previously.
Various cytotoxic agents may cause colitis of
varying extent (1). In general,
neutropenic enterocolitis should be suspected in immunosuppressed
patients with abdominal pain, especially those receiving
chemotherapy (9). Typical features
in patients with neutropenic enterocolitis comprise a low
neutrophil count, fever, and abdominal pain due to inflammation
with bowel wall thickening of the ileocolonic region (2,9,10). The
histopathological examination of specimens with neutropenic
enterocolitis may include multiple alterations such as areas of
patchy necrosis, mucosal hemorrhage, ulceration, edema,
perforation, infiltrating organisms, and typically, depletion of
inflammatory cells (9).
The patient of our case was under topotecan
treatment which is a topoisomerase inhibitor employed as a
second-line chemotherapeutic agent for small-cell lung cancer
(10). A recent series of 64
patients with small-cell lung cancer who received 177 cycles of
topotecan reported significant hematologic toxicity including 25%
of neutropenia, 11% of thrombocytopenia, and 20.3% of grade 3 or 4
anemia (10). The greater majority
(80%) of hospitalizations were due to febrile neutropenia which
occurred in 17% of the patients (10). Although histopathology is the gold
standard for the final verification of neutropenic enterocolitis,
in real-life clinical settings diagnosis and decisions are based on
clinical, laboratory, and radiologic findings (2,9,10).
Diagnostic colonoscopy for mucosa inspection and biopsy acquisition
should be used very selectively - or even avoided - in patients
with clinical suspicion of neutropenic colitis as gas insufflation
may increase the risk of free perforation due to bowel wall
alterations and increased friability (2,9).
Acute appendicitis is part of the differential
diagnosis of neutropenic enterocolitis (2), however both entities were concurrently
present in the patient reported here. In this case it is still
challenging to discern a single factor as the cause of the
gangrenous changes in the appendix. Neutropenia may cause numerous
areas of mucosal patchy necrosis with bowel wall friability, but it
is rarely the reason of full-thickness necrosis or free perforation
(2,9). Indeed, conservative treatment with
aggressive resuscitation displays high-success rates and currently
surgery is reserved for more complicated cases (2). In our patient, it is very likely that
patchy ischemia in the surface of the appendiceal mucosa was
triggered by the neutropenic status and that further deterioration
leading to perforation ensued due to the increased pressure caused
by the obstruction of the lumen from the stent. Moreover, the
relatively reduced number of neutrophils in the appendix of this
patient possibly favored friability and bacterial growth.
Our patient had high surgical- and anesthesiologic-
risk by reason of the remarkable hematologic toxicity and
comorbidities. The initial therapeutic strategy was to assess the
feasibility of a short trial of conservative therapy with
broad-spectrum antibiotics, fluid resuscitation, correction of the
hematologic parameters, and relieving the appendix with endoscopic
removal of the stent. By judging the herein experience in
retrospect, primary surgery with appendicectomy (after aggressive
transfusions to restore the platelet count) would be suggestable
due to the high risk of gangrenous changes and perforation in the
ground of neutropenia, sepsis, and appendiceal-lumen obstruction
from a migrated biliary stent.
In conclusion, migration of a plastic biliary stent
in the gastro-intestinal tract is almost always innocuous but an
increased risk of perforation may exist in the case of concomitant
neutropenic enterocolitis. A CT-scan should always be obtained in
such patients in order to assess the position of the stent and to
evaluate signs of perforation. The combination of stent migration
into the appendix and neutropenia may itself be highly suggestive
of appendiceal ischemia and necrosis, despite the absence of
perforation in imaging. Primary treatment option should be urgent
appendicectomy after aggressive optimization of the hematologic
status.
Acknowledgements
The authors would like to acknowledge to Dr Johanna
Saukkonen (Department of Radiology, Kanta-Häme Central Hospital)
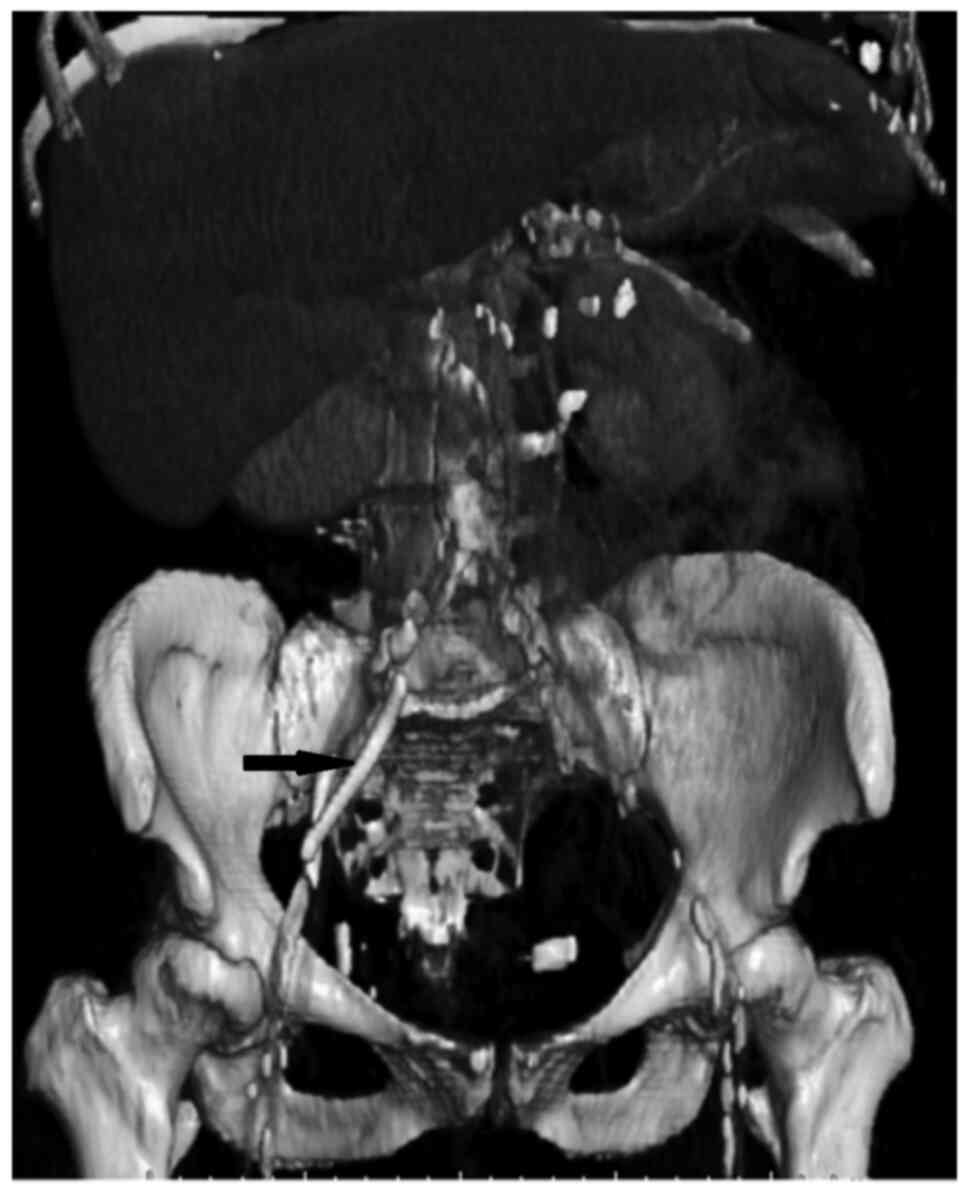
for reconstructing the 3-dimensional image that illustrated the
location of the migrated stent. The authors would also like to
thank Pathologists Mr. Kimmo Lähteenmäki and Ms. Marita Laurila
(both, Department of Pathology, Kanta-Häme Central Hospital) for
the histological images of the surgical specimen.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
PP, JK, AT, PS, GG, AP and AK conceived and designed
the current study. PP, JK and AK acquired the data. PP, JK, AT, PS,
GG, AP and AK analyzed and interpreted the data. PP, JK, AT, PS,
GG, AP and AK wrote, revised and edited the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided their written consent for the
publication of their data and associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Boussios S, Pentheroudakis G, Katsanos K
and Pavlidis N: Systemic treatment-induced gastrointestinal
toxicity: Incidence, clinical presentation and management. Ann
Gastroenterol. 25:106–118. 2012.PubMed/NCBI
|
|
2
|
Rodrigues FG, Dasilva G and Wexner SD:
Neutropenic enterocolitis. World J Gastroenterol. 23:42–47.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Dumonceau JM, Tringali A, Blero D, Deviere
J, Laugiers R, Heresbach D and Costamagna G: European Society of
Gastrointestinal Endoscopy. Biliary stenting: Indications, choice
of stents and results: European society of gastrointestinal
endoscopy (ESGE) clinical guideline. Endoscopy. 44:277–298.
2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Malgras B, Pierret C, Tourtier JP, Olagui
G, Nizou C and Duverger V: Double sigmoid colon perforation due to
migration of a biliary stent. J Visc Surg. 148:e397–e399.
2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Belyaev O, Müller CA and Uhl W: Double
sigmoid colon perforation by a migrated biliary stent. Acta Chir
Belg. 108:125–126. 2008.PubMed/NCBI
|
|
6
|
Anderson EM, Phillips-Hughes J and Chapman
R: Sigmoid colonic perforation and pelvic abscess complicating
biliary stent migration. Abdom Imaging. 32:317–319. 2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mady RF, Niaz OS and Assal MM: Migrated
biliary stent causing perforation of sigmoid colon and pelvic
abscess. BMJ Case Rep. 13(bcr2014206805)2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yilmaz Ö, Kiziltan R, Aydin O, Bayrak V
and Kotan Ç: A rare complication of biliary stent migration: Small
bowel perforation in a patient with incisional hernia. Case Rep
Surg. 2015(860286)2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Xia R and Zhang X: Neutropenic
enterocolitis: A clinico-pathological review. World J Gastrointest
Pathophysiol. 10:36–41. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Popovic F, Jakopovic M, Samarzija M,
Cucevic B, Kukulj S, Rogliç M and Plestina S: P1.07-013 treatment
related side effects of oral topotecan in small cell lung cancer:
Topic: Drug treatment alone and in combination with radiotherapy. J
Thorac Oncol. 12(S703)2017.
|