Introduction
Human malignant melanoma (MM) is a life-threatening
and highly aggressive tumour with a pronounced propensity to
metastasize. The incidence and mortality rates of MM have been
increasing worldwide. In Germany, there were an estimated 21,410
new MM cases and 3,042 deaths in 2016, whereas the reported MM
incidence among women and men was reported to increase by >4%
annually between 2003 and 2013(1).
Although the 5-year survival rate for early-stage MM is >90% and
drug therapy for metastatic MM has rapidly progressed in recent
years, the 3-year overall survival decreases to <60% for
advanced-stage MM with distant metastasis (2). Therefore, it is crucial to identify
diagnostic markers to enable early identification of disease
progression, which may be pivotal in improving survival outcomes
for patients with advanced-stage MM. However, only a few clinically
useful serological markers are currently available, and the
prognostic outcome is mainly determined by tumour thickness, lymph
node involvement, ulceration status of the excised primary tumour
and identification of distant metastasis. To date, the only
biomarker used in the American Joint Committee on Cancer (AJCC)
staging system is lactate dehydrogenase (LDH), the most validated
and strongest independent prognostic factor for MM with distant
metastasis (3). In addition to LDH,
S100 calcium-binding protein B (S100B) is another prognostic and
diagnostic serum marker in MM patients with distant metastasis for
monitoring tumour response to treatment and identifying recurrent
disease (4). Several other
diagnostic serum factors have been investigated in MM with distant
disease, such as melanoma inhibitory activity protein, galectin-3,
vascular endothelial growth factor (VEGF) and matrix
metalloproteinase-9, but none have reached clinical relevance
(5). Therefore, there are currently
no validated serum markers for identifying MM progression at its
earlier stages.
2-Methoxyestradiol (2-ME) is an endogenous
metabolite of 17β-estradiol, which is generated by
catechol-O-methyltransferase (6).
Recent studies have revealed that the serum levels of 2-ME are low
in non-pregnant women and physiologically increase during
pregnancy, reaching a maximum during the last trimester, whereas
2-ME level is suppressed in women with preeclampsia (7). A number of studies suggested that 2-ME
is critical for placental homeostasis by regulation of
cytotrophoblast differentiation and oxygen tension during normal
pregnancy, whereas impaired 2-ME levels have been implicated in the
pathophysiology of preeclampsia (8). Interestingly, beside these
physiological effects in pregnancy, it has been found that 2-ME
exerts anticancer effects by inducing growth arrest or
caspase-dependent and -independent apoptosis in a number of tumour
cell lines (e.g., melanoma, bladder cancer, leukaemia and ovarian
cancer) in vitro and in mouse experiments in vivo
(9-12).
Additionally, 2-ME has been shown to inhibit angiogenesis by
modulating angiogenesis-related genes, such as hypoxia inducible
factor-1α (HIF-1α) and VEGF (13,14).
Therefore, clinical trials with 2-ME in cancer treatment are
underway (15). Furthermore, it has
been found that 2-ME levels were reduced in patients with
endometrial cancer compared with those in healthy controls
(16). In melanoma cell 2-ME was
able to inhibit cell growth and to induce apoptosis (17).
In view of this data and with the knowledge, that
angiogenesis plays a crucial role in melanoma progression it was
hypothesized that 2-ME serum levels could be reduced in patients
with malignant melanoma. Therefore, the aim of the present pilot
study was to analyse 2-ME serum levels in melanoma patients
compared to healthy volunteers (negative control) and pregnant
woman (positive control). We analysed whether 2-ME serum levels of
patients with MM are correlated with the tumour stage and if they
can be used as prognostic/diagnostic serum markers during disease
progression. The evaluation of 2-ME as a treatment option in
melanoma patients was not addressed in this study.
Materials and methods
Ethics statement
All patients and healthy controls provided written
informed consent prior to enrolment and publication of any data
according to the principles outlined in the Declaration of
Helsinki. The study protocol was approved by the Institutional
Ethics Committee Frankfurt (ethic vote SDO-01-2013) and Mainz
[ethic vote 837.270.14 (9509)].
Study population
A total of 78 treated and subsequently followed-up
patients with histologically confirmed stage I-IV MM recruited
between May 2014 and August 2016 from the Departments of
Dermatology of the University Hospitals in Frankfurt and Mainz
(Germany) were included in the present study. The patients were
staged according to the 8th edition of the AJCC staging system
(3). The following data on the
characteristics of each patient were collected where available:
Sex, age, Breslow's tumour thickness, disease stage according to
the AJCC staging system, serum LDH and S100B levels. Details on the
clinical and histopathological characteristics of all enrolled
patients are provided in Table
I.
 | Table IClinicopathological characteristics of
patients with melanoma (n=78). |
Table I
Clinicopathological characteristics of
patients with melanoma (n=78).
| Clinical
variable | Value |
|---|
| Sex, n | |
|
Male | 52 |
|
Female | 26 |
| Median age (range),
years | 61 (30-87) |
| Breslow thickness of
primary tumor, n | |
|
Tx | 1 |
|
T0 (no
evidence of primary tumor) | 11 |
|
T1 (≤1
mm) | |
|
T1a
(<0.8 mm, not ulcerated) | 7 |
|
T1b
(≥0.8 mm) | 5 |
|
T2 (1.1-2.0
mm) | 21 |
|
T3 (2.1-4.0
mm) | 22 |
|
T4 (>4
mm) | 11 |
| Clinical stage,
n | |
|
I | 7 |
|
II | 12 |
|
III | 25 |
|
IV | 34 |
| Lactate
dehydrogenase, n | |
|
<248
U/l | 46 |
|
≥248
U/l | 24 |
|
Unknown | 8 |
| S100 calcium-binding
protein B, n | |
|
<0.105
µg/l | 57 |
|
≥0.105
µg/l | 13 |
|
Unknown | 8 |
Control samples
Serum samples were collected from 10 healthy blood
donors (4 women and 6 men with a mean age of 41 years; range, 30-53
years) at the Department of Dermatology of the University Hospital
Frankfurt (Germany) and used as controls for this study.
Additionally, serum samples were collected from 15 healthy women in
the second trimester of pregnancy, (mean age, 32 years; range,
26-40 years) at the Department of Gynaecology and Obstetrics of the
University Hospital Frankfurt (Germany) and used as positive
controls for 2-ME serum levels.
Sample collection
Venous blood samples were obtained and separated
within 1 h by centrifugation at 1,000 x g for 15 min at 4˚C. All
collected serum samples were stored at -80˚C until analysis.
Lipaemic or haemolysed samples were eliminated from the study.
Measurement of 2-ME serum levels
Serum samples were assayed with the 2-ME ELISA kit
cat. no. 582261 (Cayman Chemical Company) according to the
manufacturer's instructions. The optical density was measured using
an ELISA reader (ELISA-Reader ASYS Expert 96; Deelux Labortechnik
GmbH).
Statistical analysis
Statistical analyses were performed using Microsoft
Excel and SigmaPlot 12.0 software (Systat Software, Inc.).
P<0.05 was considered to indicate statistically significant
differences. To test for normality distribution, the Shapiro-Wilk
test was performed. The Wilcoxon Mann-Whitney U test was used to
test for differences between two groups. For differences between
more than two groups, the Kruskal-Wallis test was applied.
Subsequently, to test for differences between groups, Dunn's post
hoc analysis was employed. Correlation coefficients were determined
by Spearman's correlation analysis.
Results
Comparison of 2-ME serum levels
between controls and patients with MM
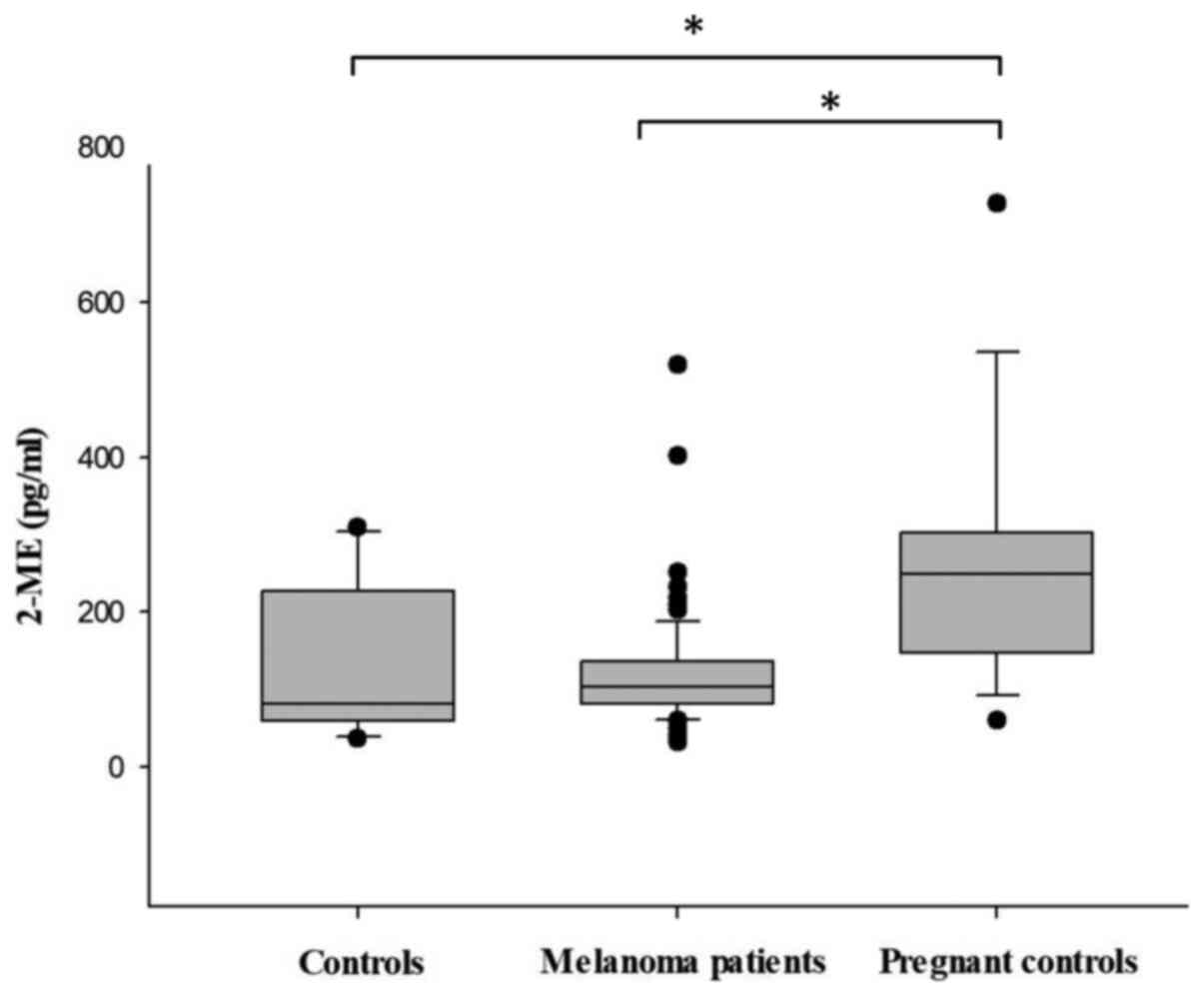
First, the 2-ME serum levels of the healthy
volunteers (negative control group) and healthy women in second
trimester of pregnancy (positive control group) were evaluated at
baseline. As expected, it was observed that healthy pregnant women
exhibited significantly higher mean concentrations of 2-ME in
comparison with the healthy control group (258.4 vs. 138.7 pg/ml,
respectively; P<0.05). Furthermore, the 2-ME serum levels of the
78 recruited MM patients were evaluated. No significant difference
in the serum levels of 2-ME was observed between MM patients and
the healthy control group (121.7 vs. 138.7 pg/ml, respectively;
P>0.05), whereas the serum levels of healthy pregnant women
(positive control) were significantly higher compared with those of
patients with MM (258.4 vs. 121.7 pg/ml, respectively; P<0.05).
Taken together, these findings demonstrated that serum 2-ME is not
suitable for distinguishing between MM patients and healthy
controls (Fig. 1).
Association between serum 2-ME levels
and clinical prognostic factors in MM
Further analysis revealed that the serum levels of
2-ME were not correlated with tumour thickness in MM patients
(r=-0.188, P>0.05). Evaluation of 2-ME serum levels in relation
to the tumour stages of the TNM classification (T0-T4) showed no
significant differences between groups (P>0.05) (Table II). Further evaluation of tumour
substages was not performed due to the limited sample size.
Furthermore, a possible association between the serum levels of
2-ME and S100B in the recruited MM patients was investigated. It
was observed that 2-ME could not differentiate between patients
with normal S100B levels (≤0.105 µg/l; n=57) and those with
elevated S100B levels (≥0.105 µg/l; n=13) (124.4 vs. 111.9 pg/ml,
respectively; r=-0.0485; P>0.05). Additionally, a possible
correlation between the serum levels of 2-ME and LDH was
investigated in the patients. The results of the analysis
demonstrated that 2-ME was unable to distinguish between MM
patients with normal serum LDH levels (<248 U/l; n=46) and those
with elevated serum LDH levels (≥248 U/l; n=24) (128.1 vs. 110.5
pg/ml, respectively; r=-0.0478; P>0.05). Finally, the 2-ME serum
level distribution was analysed in MM patients with AJCC stage I
(113.6 pg/ml; n=8), II (122.2 pg/ml; n=11), III (132.2 pg/ml; n=25)
and IV (116.8 pg/ml; n=34). The results revealed no significant
correlation between the concentration of 2-ME and AJCC stage
(r=-0.0417; P>0.05). Therefore, none of the known clinical
parameters, such as Breslow tumour thickness, S100B and LDH serum
levels and AJCC stage, were found to be significantly correlated
with the serum 2-ME levels in patients with MM (Table II).
 | Table II2-ME levels and the clinical
characteristics of patients with melanoma. |
Table II
2-ME levels and the clinical
characteristics of patients with melanoma.
| Clinical
characteristics | Cases, n | Mean 2-ME serum
levels, pg/ml | P-value | Spearman correlation
coefficient |
|---|
| Patients | | | <0.05a,b | |
|
Pregnant
controls | 15 | 258.4 | | |
|
Non-pregnant
controls | 10 | 138.7 | | |
|
Patients
with MM | 78 | 121.7 | | |
| Tumor thickness
(mm) | | | >0.05b | -0.188 |
|
T0 (no
evidence of primary tumor) | 11 | 129.3 | | |
|
T1 (≤1) | 12 | 148.1 | | |
|
T2
(1.1-2.0) | 21 | 102.0 | | |
|
T3
(2.1-4.0) | 22 | 136.2 | | |
|
T4
(>4) | 11 | 93.3 | | |
| S100 calcium-binding
protein B, µg/l | | | >0.05c | -0.049 |
|
<0.105 | 57 | 124.4 | | |
|
≥0.105 | 13 | 111.9 | | |
| Lactate
dehydrogenase, U/l | | | >0.05c | -0.048 |
|
<248 | 46 | 128.1 | | |
|
≥248 | 24 | 110.5 | | |
| Clinical stage | | | >0.05b | -0.042 |
|
I | 7 | 113.6 | | |
|
II | 12 | 122.2 | | |
|
III | 25 | 132.2 | | |
|
IV | 34 | 116.8 | | |
Discussion
MM is a potentially life-threatening disease with a
poor prognosis in the advanced stages (2). Improvement of survival outcomes of
patients with MM largely depends on early detection. To date, there
are only two clinically approved serum markers for the monitoring
of patients with MM: S100B and LDH. Unfortunately, the prognostic
value of both markers is limited and therefore, there is a great
need to identify new prognostic biomarkers.
From preclinical data, 2-ME appeared to be a
promising molecule to investigate as a prognostic marker in
melanoma. It has been demonstrated that 2-ME exerts anti-angiogenic
effects through downregulation of VEGF and HIF-1α and induces
apoptosis in endothelial cells (13,14,18).
It has been reported that 2-ME inhibits cell proliferation by
inducing apoptosis and promoting G2/M phase arrest of MM cells
(11,17). Additionally, exogenic applied 2-ME
has been shown to be effective against MM growth, independent of
the BRAF mutational status, and significantly decreased cell
proliferation in a 3D skin reconstruction model (11). Based on these data and findings from
a recent study on patients with endometrial carcinoma who exhibited
reduced endogenous levels of 2-ME compared to healthy subjects, we
decided to assess 2-ME serum levels in patients with MM compared to
healthy volunteers (negative control) and pregnant woman (positive
control) (16). Our intention was
to evaluate the potential of endogenously generated 2-ME, as a
prognostic serum marker and therefore patients were not exposed to
exogenous 2-ME. As expected, the 2-ME serum levels were elevated in
pregnant healthy women compared to healthy volunteers and MM
patients. The 2-ME serum levels of the MM patients were slightly,
but not significantly, lower compared with those of healthy
controls. In the present study, 2-ME levels were not found to be
correlated with any of the analysed prognostic relevant
clinicopathological parameters, such as tumour thickness, AJCC
stage, S100B and LDH serum levels.
Although a relatively large number of 78 patients
with malignant melanoma in different tumour stages were examined in
this study, the tumour subgroups were in part relatively small, so
that a further subdivision was not possible. Since no trend could
be derived from the analyses so far, it is likely, that further
subgroup analyses would not have yielded any additional findings. A
further limitation is that the serum levels were determined only at
one time point of the disease, regardless of the state of
treatment. Future studies should address several time points of the
disease and additionally evaluate treatments and tumour burden in
order to rule out possible confounders.
In conclusion, these results are the first to
indicate that endogenous 2-ME serum levels are not reduced in
patients with MM. Therefore, in contrast to the potential treatment
effects of exogenously administered 2-ME, endogenous 2-ME serum
levels cannot be used as diagnostic or prognostic marker in MM. In
our opinion, further investigations should focus on the therapeutic
applications of systemically administered 2-ME in patients with MM,
rather than the prognostic potential of 2-ME serum levels.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
IH, RK, SK, CL, FL and MM conceived and designed the
experiments. IH, JK, NZ and MD performed the experiments. IH, JK
and FW analysed the data. MD, IH, JK, FW, SK, CL, NZ and FL
contributed to data/materials/analysis tools. IH, JK and MM
assessed the authenticity of the raw data and wrote the paper. All
authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
All patients and healthy controls in the present
study provided written informed consent prior to enrolment and
publication of any data according to the principles outlined in the
Declaration of Helsinki. The study protocol was approved by the
Institutional Ethics Committee Frankfurt (ethic vote SDO-01-2013)
and Mainz [ethic vote 837.270.14 (9509)].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Barnes B, Kraywinkel K, Nowossadeck E,
Schönfeld I, Starker A, Wienecke A and Wolf U: Bericht zum
Krebsgeschehen in Deutschland 2016. Robert Koch-Institut, Berlin,
2016.
|
|
2
|
Malissen N and Grob JJ: Metastatic
melanoma: Recent therapeutic progress and future perspectives.
Drugs. 78:1197–1209. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Amin MB, Edge S, Greene F, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC (eds), et al: AJCC Cancer Staging Manual. Springer
International Publishing, New York, NY, 2017.
|
|
4
|
Mocellin S, Zavagno G and Nitti D: The
prognostic value of serum S100B in patients with cutaneous
melanoma: A meta-analysis. Int J Cancer. 123:2370–2376.
2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Karagiannis P, Fittall M and Karagiannis
SN: Evaluating biomarkers in melanoma. Front Oncol.
4(383)2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Perez-Sepulveda A, Espana-Perrot PP,
Norwitz ER and Illanes SE: Metabolic pathways involved in
2-methoxyestradiol synthesis and their role in preeclampsia. Reprod
Sci. 20:1020–1029. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shen Z, Wu Y, Chen X, Chang X, Zhou Q,
Zhou J, Ying H, Zheng J, Duan T and Wang K: Decreased maternal
serum 2-methoxyestradiol levels are associated with the development
of preeclampsia. Cell Physiol Biochem. 34:2189–2199.
2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Parada-Bustamante A, Valencia C, Reuquen
P, Diaz P, Rincion-Rodriguez R and Orihuela PA: Role of
2-methoxyestradiol, an endogenous estrogen metabolite, in health
and disease. Mini Rev Med Chem. 15:427–438. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Huang XX, Wang RX, Lin Q, Chen W, Pan Y,
Wang SQ, Weng ZL and Huang WP: Inhibitory effects of
2-methoxyestradiol on cell growth and invasion in human bladder
cancer T-24 cells. Pharmazie. 72:87–90. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhe N, Chen S, Zhou Z, Liu P, Lin X, Yu M,
Cheng B, Zhang Y and Wang J: HIF-1α inhibition by
2-methoxyestradiol induces cell death via activation of the
mitochondrial apoptotic pathway in acute myeloid leukemia. Cancer
Biol Ther. 17:625–634. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Massaro RR, Faiao-Flores F, Rebecca VW,
Sandri S, Alves-Fernandes DK, Pennacchi PC, Smalley KS and
Maria-Engler SS: Inhibition of proliferation and invasion in 2D and
3D models by 2-methoxyestradiol in human melanoma cells. Pharmacol
Res. 119:242–250. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kato S, Sadarangani A, Lange S, Delpiano
AM, Vargas M, Brañes J, Carvajal J, Lipkowitz S, Owen GI and Cuello
MA: 2-methoxyestradiol mediates apoptosis through caspase-dependent
and independent mechanisms in ovarian cancer cells but not in
normal counterparts. Reprod Sci. 15:878–894. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ricker JL, Chen Z, Yang XP, Pribluda VS,
Swartz GM and Van Waes C: 2-methoxyestradiol inhibits
hypoxia-inducible factor 1alpha, tumor growth, and angiogenesis and
augments paclitaxel efficacy in head and neck squamous cell
carcinoma. Clin Cancer Res. 10:8665–8673. 2004.PubMed/NCBI View Article : Google Scholar
|
|
14
|
El Naga RN, El-Demerdash E, Youssef SS,
Abdel-Naim AB and El-Merzabani M: Cytotoxic effects of
2-methoxyestradiol in the hepatocellular carcinoma cell line HepG2.
Pharmacology. 84:9–16. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Solum EJ, Akselsen OW, Vik A and Hansen
TV: Synthesis and pharmacological effects of the anti-cancer agent
2-methoxyestradiol. Curr Pharm Des. 21:5453–5466. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhao H, Jiang Y, Liu Y, Yun C and Li L:
Endogenous estrogen metabolites as biomarkers for endometrial
cancer via a novel method of liquid chromatography-mass
spectrometry with hollow fiber liquid-phase microextraction. Horm
Metab Res. 47:158–164. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Dobos J, Timar J, Bocsi J, Burián Z, Nagy
K, Barna G, Peták I and Ladányi A: In vitro and in vivo antitumor
effect of 2-methoxyestradiol on human melanoma. Int J Cancer.
112:771–776. 2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Newman SP, Leese MP, Purohit A, James DR,
Rennie CE, Potter BV and Reed MJ: Inhibition of in vitro
angiogenesis by 2-methoxy- and 2-ethyl-estrogen sulfamates. Int J
Cancer. 109:533–540. 2004.PubMed/NCBI View Article : Google Scholar
|