Introduction
Bone-modifying agents (BMAs), including
bisphosphonate and anti-receptor activator of NF-κB ligand (RANKL)
antibody, are effective in treating bone metastases derived from
various cancer types (1,2). Many oncology practice guidelines
recommend combination therapy, including BMAs and anticancer
agents, to treat patients with bone metastases in order to prevent
skeletal-related events (3,4). A main contraindication for BMAs is
tooth extraction. All patients need to receive oral care prior to
treatment with BMAs, as osteonecrosis of the jaw is a known adverse
event of these agents (5).
Conversely, incidence of osteonecrosis due to BMAs identified at
other anatomical locations is not well known, and some of the
evidence has been conflicting. Mont et al found that
osteoporosis was not a risk factor for osteonecrosis of the femoral
head (ONFH) (6), while Gangji et
al reported that non-traumatic ONFH is associated with low bone
mineral density (7), a disease
treated mainly with BMAs. Thus, the incidence and mechanisms of
ONFH in cancer patients with bone metastasis who received BMAs
should be further explored. The incidence of atypical femoral
fractures in elderly breast cancer patients is similar to that seen
in non-breast cancer patients (8).
A recent report demonstrated that zoledronate did not prevent
collapse of the femoral head, and did not reduce the need for hip
arthroplasty (9). Mechanisms
underlying the mode of action for BMAs on the bone are still
controversial (10,11). Here, the case of a breast cancer
patient undergoing long-term treatment with either zoledronic acid
or anti-RANKL antibody, who experienced bilateral ONFH, is
presented.
Case report
A 57-year-old woman, first diagnosed with stage IV
cancer in 2012, had no history of heavy alcohol consumption, other
comorbidities, or family history of cancer. Positron emission
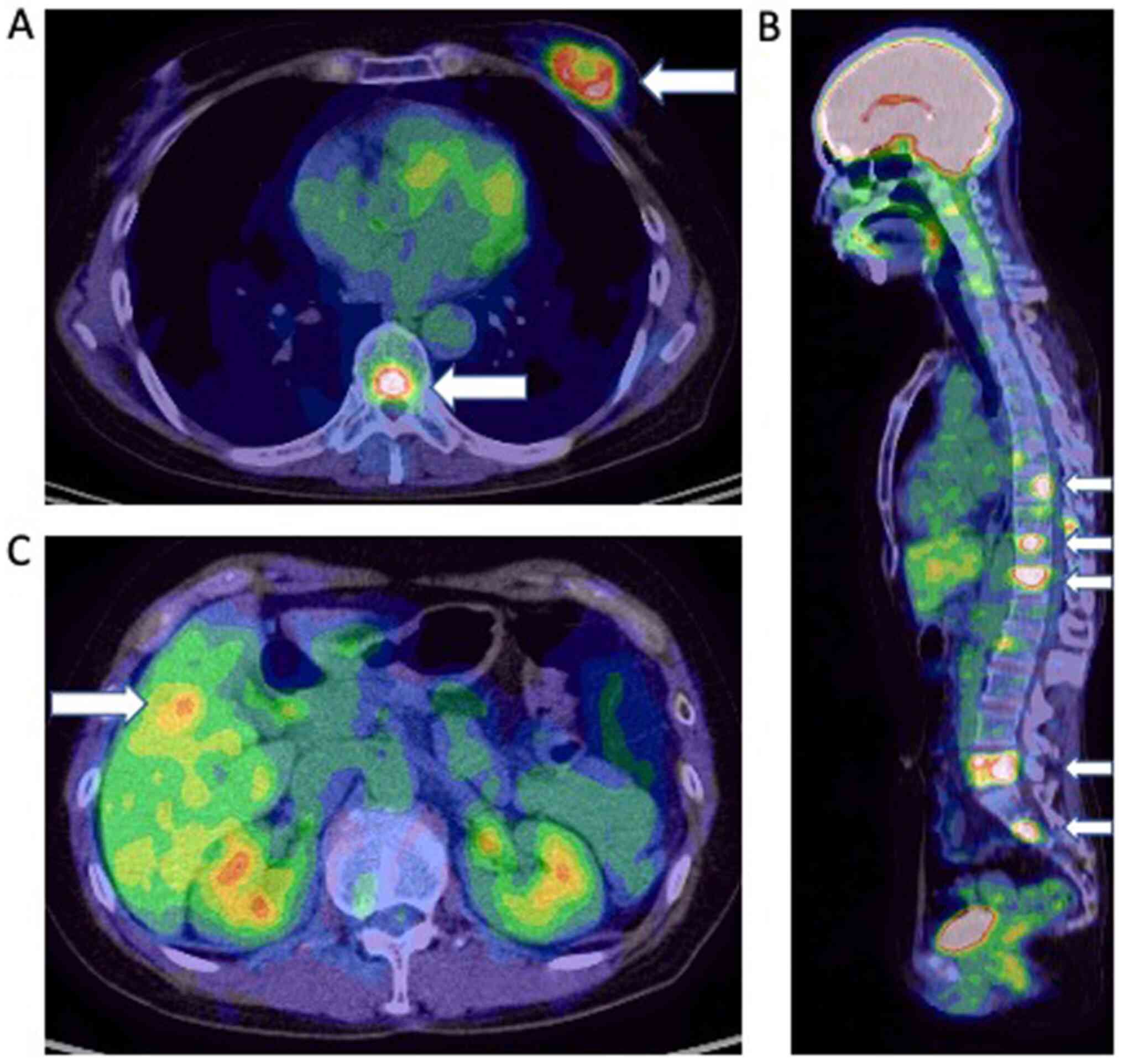
tomography-computed tomography (PET-CT) showed a left breast tumor
with numerous metastatic tumors in the axillary lymph nodes, bone,
liver, and lung (cT2N1M1) (Fig. 1).
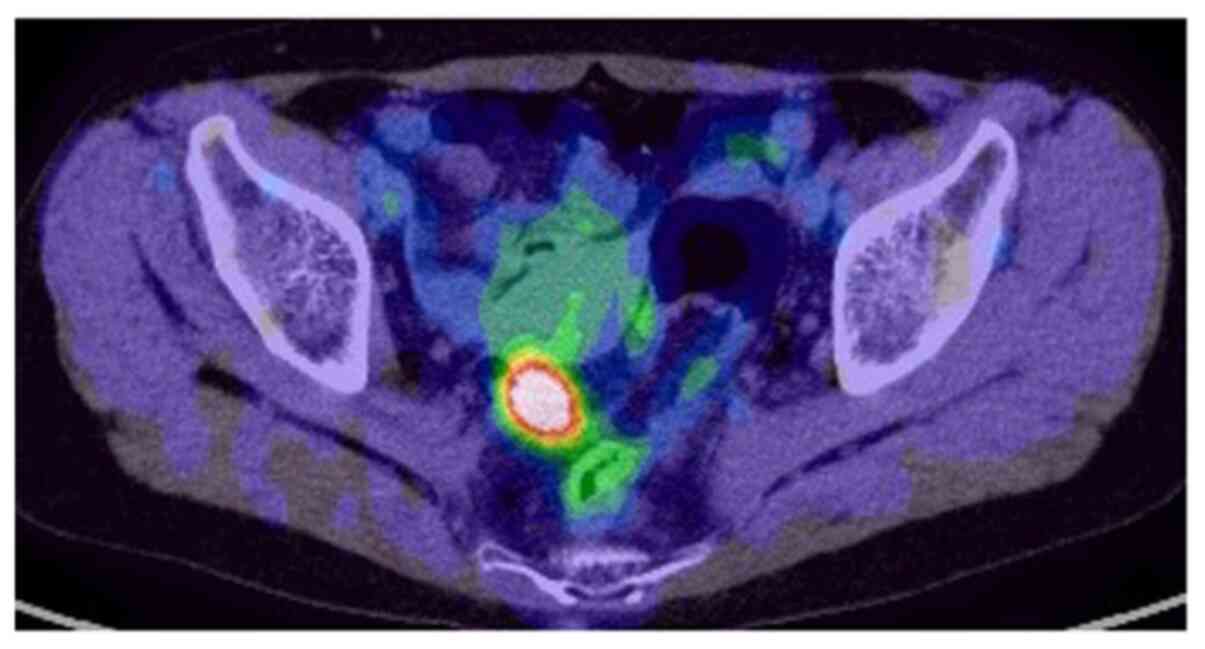
Additionally, PET-CT results suggested colon cancer in the sigmoid
region with no regional lymph node metastasis (Fig. 2). Colonoscopy aided in the diagnosis
of type 2 colon cancer (T1N0M0), and a biopsy was performed. The
pathology reports revealed invasive ductal carcinoma (estrogen
receptor-, progesterone receptor-, and HER2-negative) of the left
breast and adenocarcinoma of the sigmoid colon (i.e., dual cancer).
Considering the TN stage, the distant metastases observed upon
initial diagnosis were logically assumed to be from the breast
cancer and not the colon cancer. It was decided that systemic
therapy for breast cancer was a priority, and if a good response
was obtained, the colon cancer would be subsequently treated with
surgery. The patient received six cycles of epirubicin (90
mg/m2) combined with cyclophosphamide (600
mg/m2) and zoledronic acid beginning in August 2012.
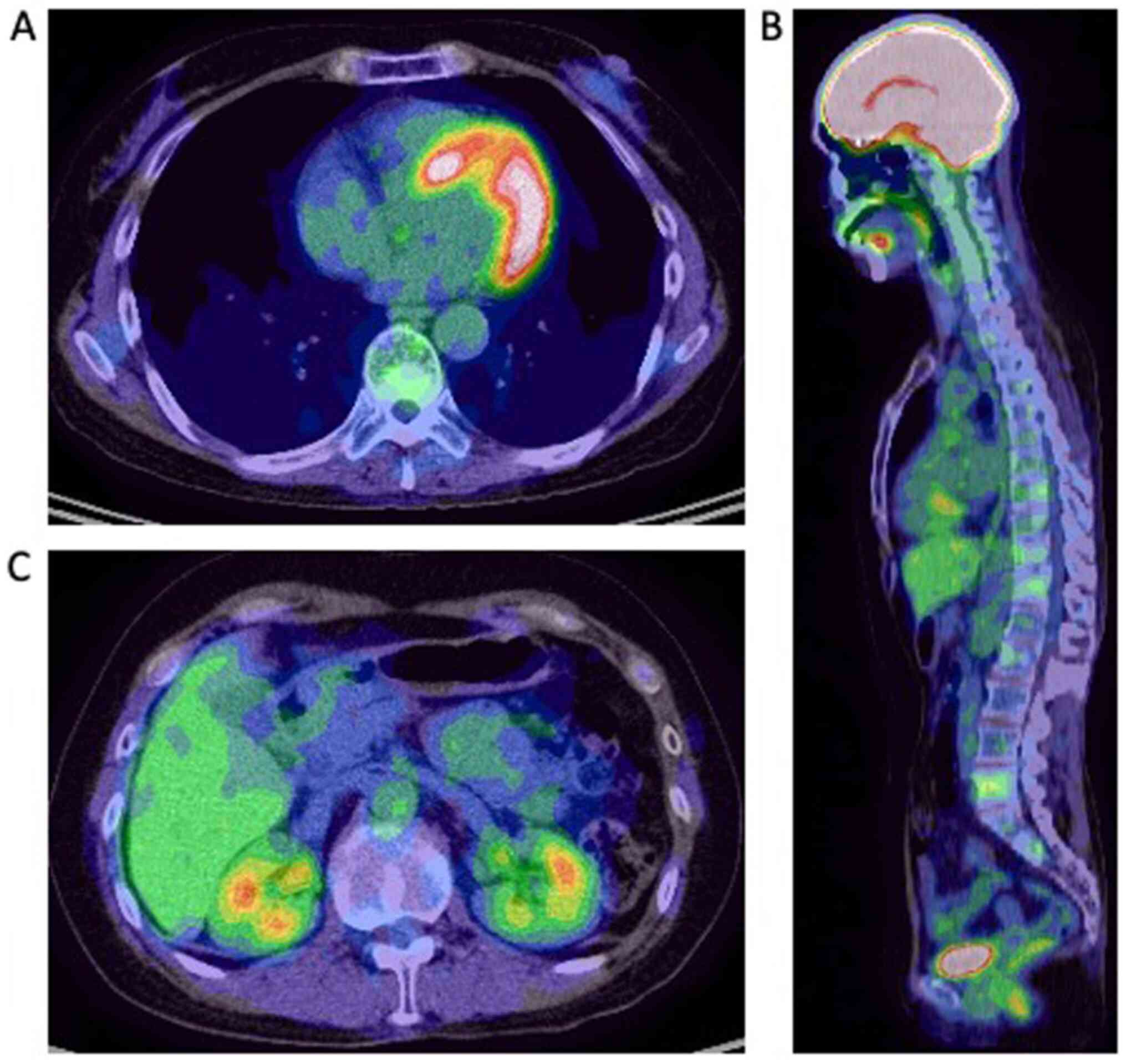
Results of a follow-up PET-CT (Fig.
3) showed that the active distant metastases disappeared. The
patient then underwent a laparoscopy-assisted colectomy. After the
colectomy, PET-CT indicated left breast cancer with no distant
metastases. The patient subsequently underwent left mastectomy with
axillary lymph node dissection. Analysis of the resected tumor
revealed triple-negative breast cancer. The patient received seven
cycles of docetaxel (75 mg/m2) and zoledronic acid.
In August 2013, PET-CT revealed complete response.
Docetaxel treatment was discontinued, but the patient continued to
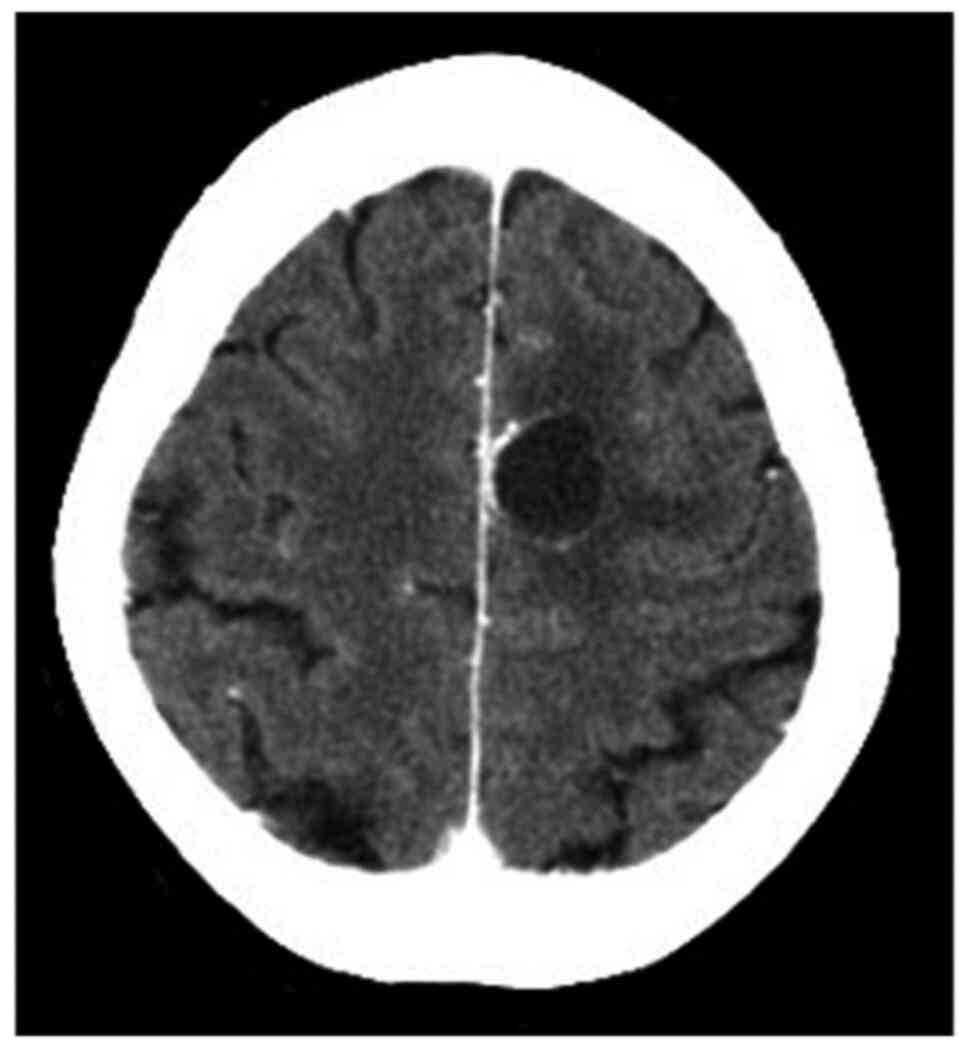
receive zoledronic acid after chemotherapy. In February 2014, a
brain metastasis was diagnosed during BMA treatment (Fig. 4). The brain tumor was treated with
32.5 Gy of irradiation. In addition, 1-4 mg of betamethasone was
administered daily for 14 days (total=44 mg).
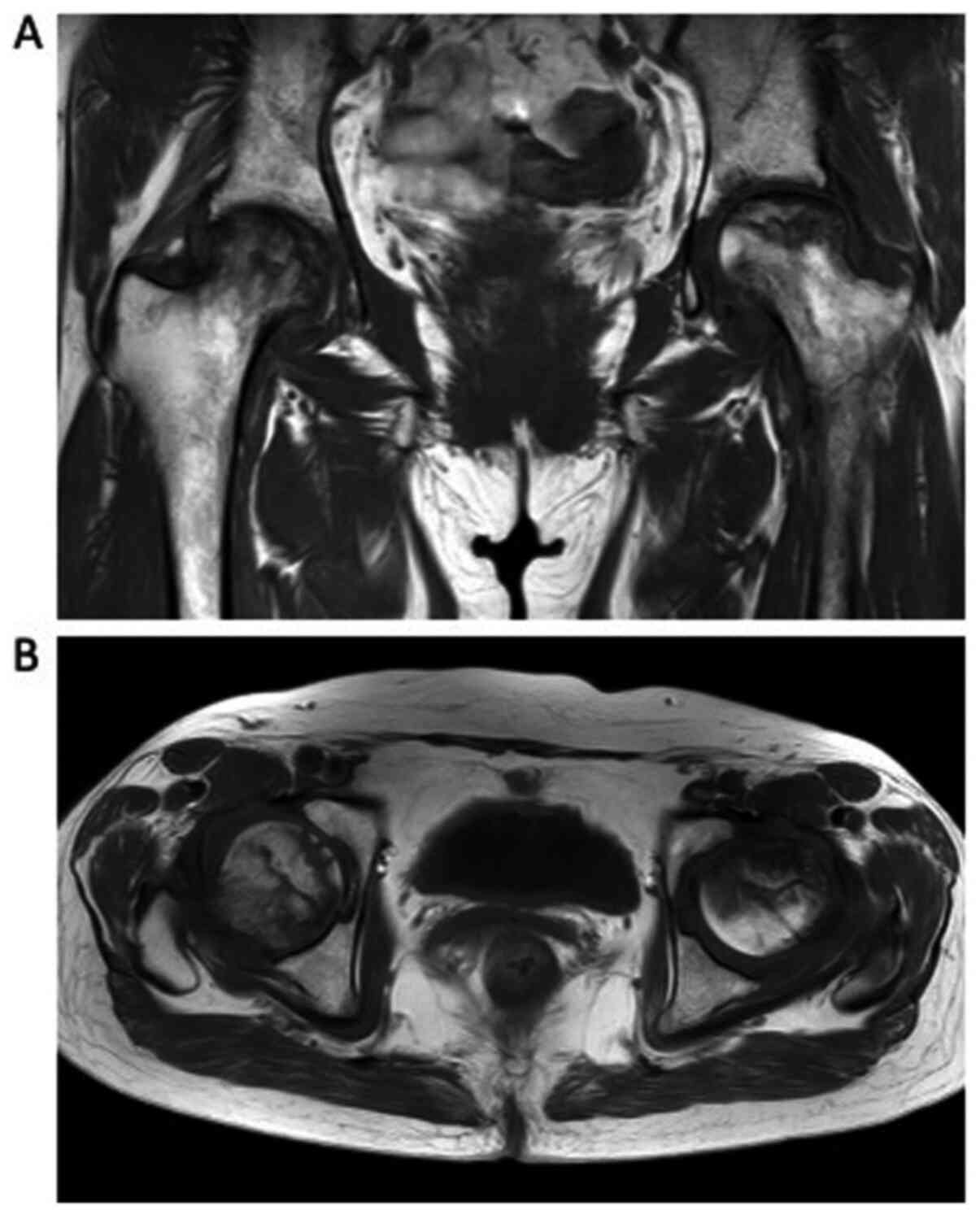
In October 2015, the patient complained of bilateral
hip pain, and PET-CT suggested hip arthritis rather than relapse of
bone metastases. In July 2016, she underwent magnetic resonance
imaging (MRI), and was diagnosed with ONFH with no bone metastases
(Fig. 5). Following this, she
received bilateral artificial hip joint replacements. After the
orthopedic surgery, she received zoledronic acid every 4 weeks for
3 years. Her multiple distant metastases, including brain
metastasis, remain in complete remission based on PET-CT and brain
MRI scans.
Discussion
Triple-negative breast cancer is difficult to treat,
although chemotherapy can sometimes be effective. In this case, the
patient experienced complete remission after chemotherapy, BMA
treatment, and irradiation. The patient was administered zoledronic
acid and has survived longer than 7 years after her initial
diagnosis. This case suggests that long-term use of BMAs may result
in improved control of bone metastases and support long-term
survival. However, they may also induce femoral head necrosis.
Although it is difficult to determine the underlying mechanisms,
this case suggests to be aware regarding the occurrence of femoral
necrosis in patients who receive long-term treatment with
zoledronic acid. Our patient also received prophylactic
corticosteroid treatment concurrent with irradiation. The total
accumulated dose of betamethasone was 44 mg. Avascular necrosis of
the femoral head is a rare complication related to glucocorticoid
administration that has long been associated with high-dosage
and/or prolonged treatment (12). A
meta-analysis by Mont et al previously reported on the
relationship between corticosteroid dose and hip osteonecrosis
(13). They reported that
osteonecrosis incidence associated with corticosteroid treatment of
>2 g was 6.7% (prednisone-equivalent). Moreover, Dharmshaktu
et al reported the case of a 38-year-old man who experienced
ONFH after receiving 5 mg zoledronic acid and the lowest oral dose
of prednisolone. He also received 60 mg dexamethasone in oral doses
of 2 mg for less than a month (14). In our case, the patient received 44
mg of betamethasone with zoledronic acid, suggesting that steroids
might not be the major factor in inducing ONFH. However, concurrent
use of steroids and BMAs may enhance the incidence of ONFH. Lee
et al reported the results of a prospective randomized trial
for the prevention of femoral head osteonecrosis using zoledronic
acid (9). They demonstrated that
zoledronic acid was not effective in preventing femoral head
necrosis. Based on basic and clinical evidence for the efficacy of
bisphosphonates in improving bone strength, bisphosphonates could
be a candidate for ONFH treatment. Nonetheless, we believe that
long-term use of BMAs could induce ONFH because of the relationship
between bisphosphonates and osteonecrosis of the jaw. Therefore, it
is important in clinical practice to pay attention to chronic
adverse events of BMAs. A systematic review by Bal et al
revealed that bisphosphonate treatment may be prolonged for over 2
years in bone metastatic breast cancer patients with an acceptable
toxicity profile (15). But the
incidence of femoral head necrosis was low in that study, and not
specified as one of the adverse events. Rossi et al reported
a case of osteonecrosis of the distal femur associated with the use
of bisphosphonates in a 74-year-old woman with metastatic breast
cancer (16).
Bisphosphonates inhibit bone desorption and have
therefore been used to treat osteoporosis (17). Additionally, bisphosphonates can
modulate the activity of both osteoblasts and osteoclasts in
metastatic bone tumors. While they can improve bone strength, they
sometimes lead to bone necrosis, a severe adverse event (11). Hence, the mechanisms of
bisphosphonate action on the bone remain controversial. It is also
important to point out that appropriate BMA treatment regimens
after complete remission need to be discussed in the development of
treatment guidelines.
In conclusion, BMAs must not be discontinued until
serious adverse events are recognized; however, the difference
between relapse of bone metastasis and osteonecrosis caused by BMAs
should be taken into consideration.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TS and KW provided the clinical data included in the
text. KM wrote the manuscript draft. All authors contributed to the
conception and design of the study, and interpreted and revised the
PET-CT, MRI imaging and the laboratory test results. TTo and TH
critically revised the manuscript and modified the text. TTa, AO
and TTo assessed all the raw data and confirmed the authenticity of
the data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
All procedures performed in the present case report
were in accordance with the ethical standards of Saitama Medical
University and/or national research committee and with the 1964
Declaration of Helsinki and its later amendments or comparable
ethical standards. Written informed consent was provided by the
patient included in the current case report.
Patient consent for publication
Written consent for publication was provided by the
patient.
Competing interests
TS received research grants from Eisai Co., Ltd.,
Taiho Pharmaceutical Co., Ltd., and Chugai Pharmaceutical Co.,
Ltd., and received personal fees from Taiho Pharmaceutical Co. and
Chugai Pharmaceutical Co. AO received research grants from
AstraZeneca K.K., Eisai Co., Ltd., MSD K.K., Ono Pharmaceutical
Co., Ltd., Kyowa Hakko Kirin Co., Ltd., Daiichi Sankyo Co., Ltd.,
Taiho Pharmaceutical Co., Ltd., Sawai Pharmaceutical Co., Ltd.,
Chugai Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Novartis
Pharma K.K., Hamamatsu Photonics K.K., Parexel International Inc.,
and Fuji Pharma Co. Additionally, AO received personal fees from
AstraZeneca K.K., Kyowa Hakko Kirin Co., Ltd, Daiichi Sankyo Co.,
Ltd, Chugai Pharmaceutical Co., Ltd., and Novartis Pharma K.K. All
other authors declare that they have no competing interests.
References
|
1
|
Yang M and Yu X: Management of bone
metastasis with intravenous bisphosphonates in breast cancer: A
systematic review and meta-analysis of dosing frequency. Support
Care Cancer. 28:2533–2540. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Jeon HL, Oh IS, Baek YH, Yang H, Park J,
Hong S and Shin JY: Zoledronic acid and skeletal-related events in
patients with bone metastatic cancer or multiple myeloma. J Bone
Miner Metab. 38:254–263. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Van Poznak C, Somerfield MR, Barlow WE,
Biermann JS, Bosserman LD, Clemons MJ, Dhesy-Thind SK, Dillmon MS,
Eisen A, Frank ES, et al: Role of bone-modifying agents in
metastatic breast cancer: An American Society of Clinical
Oncology-cancer care Ontario focused guideline update. J Clin
Oncol. 35:3978–3986. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cardoso F, Senkus E, Costa A, Papadopoulos
E, Aapro M, André F, Harbeck N, Aguilar Lopez B, Barrios CH, Bergh
J, et al: 4th ESO-ESMO International Consensus Guidelines for
Advanced Breast Cancer (ABC 4). Ann Oncol. 29:1634–1657.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Migliorati CA, Siegel MA and Elting LS:
Bisphosphonate-associated osteonecrosis: A long-term complication
of bisphosphonate treatment. Lancet Oncol. 7:508–514.
2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mont MA, Zywiel MG, Marker DR, McGrath MS
and Delanois RE: The natural history of untreated asymptomatic
osteonecrosis of the femoral head: A systematic literature review.
J Bone Joint Surg Am Volume. 92:2165–2170. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gangji V, Soyfoo MS, Heuschling A, Afzali
V, Moreno-Reyes R, Rasschaert J, Gillet C, Fils JF and Hauzeur JP:
Non traumatic osteonecrosis of the femoral head is associated with
low bone mass. Bone. 107:88–92. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Takahashi M, Ozaki Y, Kizawa R, Masuda J,
Sakamaki K, Kinowaki K, Umezu T, Kondoh C, Tanabe Y, Tamura N, et
al: Atypical femoral fracture in patients with bone metastasis
receiving denosumab therapy: A retrospective study and systematic
review. BMC Cancer. 19(980)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lee YK, Ha YC, Cho YJ, Suh KT, Kim SY, Won
YY, Min BW, Yoon TR, Kim HJ and Koo KH: Does zoledronate prevent
femoral head collapse from osteonecrosis? A prospective,
randomized, open-label, multicenter study. J Bone Joint Surg Am.
97:1142–1148. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Russell RG, Watts NB, Ebetino FH and
Rogers MJ: Mechanisms of action of bisphosphonates: Similarities
and differences and their potential influence on clinical efficacy.
Osteoporos Int. 19:733–759. 2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ristow O, Gerngross C, Schwaiger M,
Hohlweg-Majert B, Kehl V, Jansen H, Hahnefeld L, Otto S and Pautke
C: Is bone turnover of jawbone and its possible over suppression by
bisphosphonates of etiologic importance in pathogenesis of
bisphosphonate-related osteonecrosis? J Oral Maxillofac Surg.
72:903–910. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Weinstein RS: Glucocorticoid-induced
osteonecrosis. Endocrine. 41:183–190. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Mont MA, Pivec R, Banerjee S, Issa K,
Elmallah RK and Jones LC: High-dose corticosteroid use and risk of
hip osteonecrosis: Meta-analysis and systematic literature review.
J Arthroplast. 30:1506–1512.e5. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Dharmshaktu P, Aggarwal A, Dutta D and
Kulshreshtha B: Bilateral femoral head avascular necrosis with a
very low dose of oral corticosteroid used for panhypopituitarism.
BMJ Case Rep. 2016:2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bal O, Oksuzoglu B, Dogan M, Durnali A,
Uyeturk U, Demirci A, Arslan UY, Ekinci AS, Yildirim N, Alkis N and
Kilic S: Long-term outcomes of prolonged bisphosphonates more than
2 years in bone metastatic breast cancer: Risk vs benefit. Ir J Med
Sci. 189:805–810. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rossi L, Lascio SD, Kouros M and Pagani O:
A rare avascular osteonecrosis of the knee related to
bisphosphonate treatment in a patient with metastatic breast
cancer. Breast Dis. 35:203–206. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lyles KW, Colón-Emeric CS, Magaziner JS,
Adachi JD, Pieper CF, Mautalen C, Hyldstrup L, Recknor C,
Nordsletten L, Moore KA, et al: Zoledronic acid and clinical
fractures and mortality after hip fracture. N Engl J Med.
357:1799–1809. 2007.PubMed/NCBI View Article : Google Scholar
|