Introduction
As the malignant tumor with highest morbidity and
mortality, lung cancer remains a global major public health problem
(1). In contrast to a steady
increase in the survival of the majority of tumors, advances in
lung cancer have been slow, and its 5-year relative survival rate
is still ~19% (1). This may be
attributed to the unsatisfactory efficacy of traditional treatments
such as chemotherapy, radiotherapy and tumorectomy, particularly at
advanced stages of the disease (2).
Therefore, the introduction of immunotherapy targeting immune
checkpoint molecules, such as programmed cell death ligand 1
(PD-L1), into clinical practice has recently gained increasing
attention (3). Since PD-L1
expression on tumor cells (TC) is the mechanism by which TC could
escape immune system surveillance, its expression is not only
linked to the response of immune checkpoint therapy, but is also
correlated with the prognosis of non-small cell lung cancer (NSCLC)
(4,5). PD-L1, the predominant ligand for PD-1,
can be expressed in multiple tissues, including tumor-infiltrating
T and B lymphocytes, dendritic cells and other immune cells
(5,6). To date, all previous meta-analyses
have identified PD-L1 expression on TC as a putative prognostic
biomarker in NSCLC (7-9).
However, meta-analyses of the prognostic significance of PD-L1 in
tumor-infiltrating immune cells (TIICs) have not yet been
conducted. Recent clinical trials have revealed that higher
response rate to PD-1/PD-L1 targeted therapy was associated with
positive expression of PDL-1 in TIICs (10,11).
This suggests that, in addition to using tumor cell-based PD-L1
expression, immune cell-based PD-L1 expression may be clinically
relevant. Although a number of studies have reported the prognostic
significance of PD-L1 expression on TIICs in lung cancer, the
findings remain controversial.
The aim of the present study was to conduct a
meta-analysis and to evaluate the prognostic value of
PD-L1-positive expression in TIICs, which was expressed as the
hazard ratio (HR) for overall survival (OS) and disease-free
survival (DFS) in patients with lung cancer.
Materials and methods
Search strategy
For study search and selection, the recommendation
(12) of the Preferred Reporting
Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines
were followed. Relevant articles were retrieved from the electronic
databases of PubMed, PMC, Embase and Web of Science. Studies
published before October 30, 2020 were screened by their title and
abstract. The key words used in the search strategy were ‘PD-L1 OR
PD-L1 OR B7-H1 OR C274’ AND ‘tumor-infiltrating lymphocytes OR TIL
OR tumor infiltrating immune cells OR TIIC’ AND ‘lung’ AND ‘cancer
OR carcinoma OR neoplasm OR tumor’ AND ‘survival OR prognosis. A
manual search for references cited in the retrieved articles on the
topic was also conducted.
Inclusion and exclusion criteria
Studies were considered eligible if they met the
following criteria: i) Studies on patients with lung cancer in whom
PD-L1 expression on TIICs was determined by immunohistochemistry
(IHC); ii) studies reporting the prognostic value of PD-L1 on TIICs
alongside OS and/or DFS, and HR with 95% confidence interval (CI);
iii) and studies reporting sufficient data to calculate or extract
HR values, such as Kaplan-Meier (KM) survival curves, were included
in the meta-analysis.
Articles were excluded from the meta-analysis if
they met the following criteria: i) The articles were conference
abstracts, reviews or case studies; or ii) contained insufficient
data to calculate or extract HR, or if KM information was not
available; or) were not written in English.
Data extraction and quality
assessment
Data extraction was performed by two authors using a
predesigned data extraction form. The following data were extracted
from eligible studies: Name of first author, publication year,
country, subtype of lung cancer, number of patients, format of
pathological section, tumor stage, IHC evaluation methods, antibody
clones used for PD-L1 detection and positive cutoff value for
expression of PD-L1 on TIICs.
Quality assessment of the included studies was
performed by Newcastle-Ottawa scale. Studies that scored ≥6 points
were considered to have a high quality.
Statistical analysis
Survival data were primarily extracted as HR and 95%
CI. When studies reported HR of Cox univariate and multivariate
analyses, the HR of Cox multivariate analysis was used, since it is
good for situation with missing outcomes and usually leads to more
precise conclusion. If HR was not directly available in the
article, it was calculated from available data or extracted from
the KM survival curve using WebPlotDigitizer (13). Statistical heterogeneity was
assessed by χ2 (I2) and visual inspection of
the forest plot. I2>50% was considered to indicate
the presence of heterogeneity. The random-effects model was used
due to the presence of heterogeneous studies. To identify the
source of heterogeneity and evaluate the influence of different
adjustment factors or confounders, subgroup analysis was performed.
Funnel plotswere used to estimate publication bias and small-study
effects. HR>1 and HR<1 indicated poor and improved prognosis,
respectively, while HR=1 considered no effect. For all analyses,
Stata software, version 16.0 was used. P<0.05 was considered to
indicate a statistically significant difference.
Results
Search results and study
characteristics
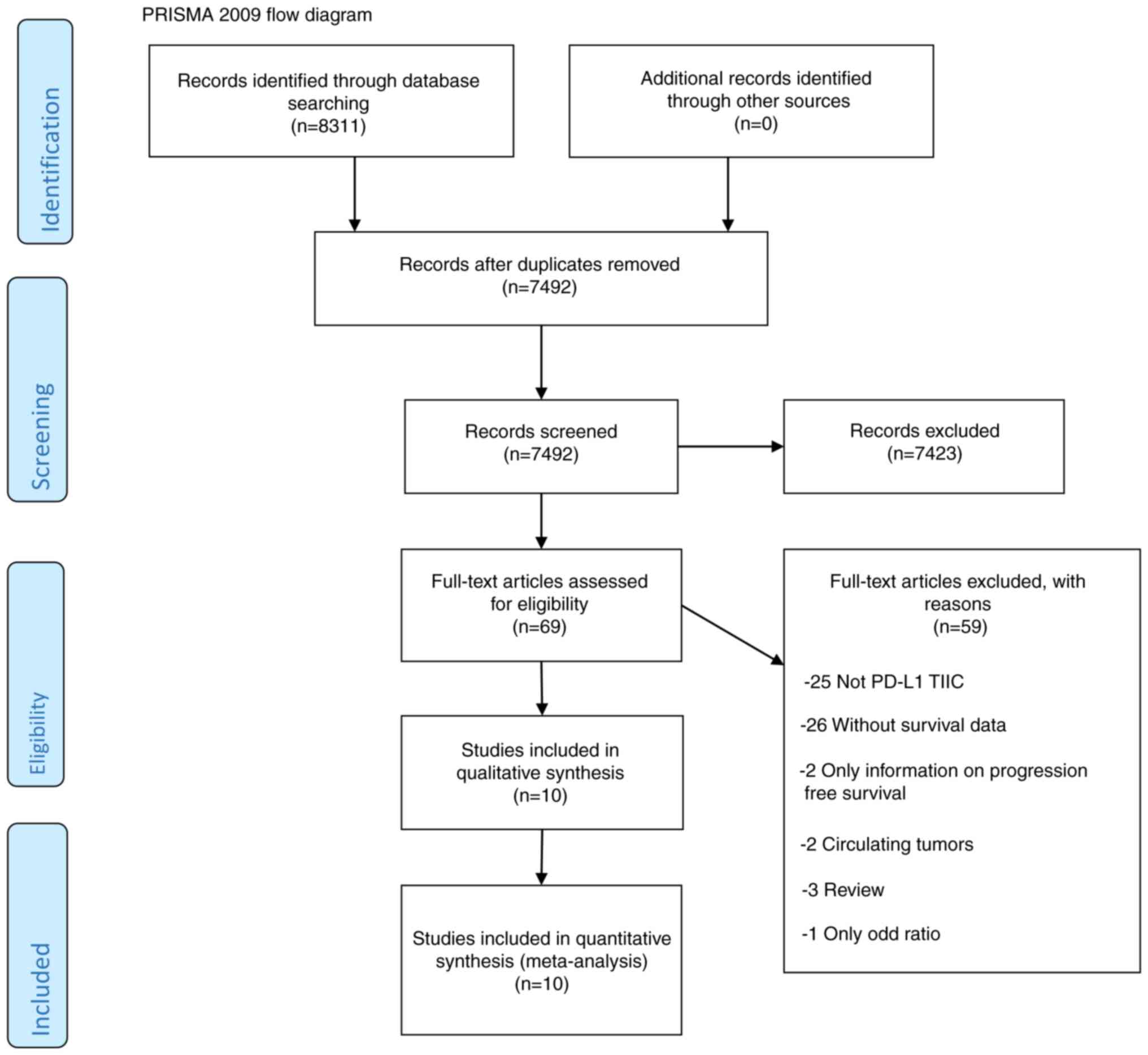
The search was completed in October 2020. The
detailed flow diagram for screening studies that identified a total
of 8,311 search records is shown in Fig. 1. EndNote software, version X7 was
used to identify duplicates and to manage citations. According to
the established inclusion and exclusion criteria, and by thoroughly
reviewing articles, 11 studies (2,685 patients) were included in
the final analysis using the Preferred Reporting Items for
Systematic Reviews and Meta-Analyses flow diagram (12) for identified articles. The
characteristics of the included studies are summarized in Tables I and II.
 | Table ICharacteristics of the studies
included in the present meta-analysis. |
Table I
Characteristics of the studies
included in the present meta-analysis.
| Author (year) | Country | Subtype of lung
cancer | Stage | Number of
patients | Age, median years
(range) | (Refs.) |
|---|
| Fehrenbacher et
al (2016) | USA | NSCLC | NR | 86 | NR | (11) |
| Yang et al
(2016) | China | NSCLC (Squamous
cell carcinoma) | I | 105 | 40-85 | (14) |
| Theelen et
al (2019) | Netherlands | NSCLC | I-IV | 286 | NR | (15) |
| Mignon et al
(2020) | Belgium | Adenocarcinoma | I-III | 237 | NR | (16) |
| Vallonthaiel et
al (2017) | India | NSCLC | 1-IV | 62 | 58 (29-78) | (17) |
| Chen et al
(2109) | China | NSCLC | I-IV | 234 | NR | (18) |
| Paulsen et
al (2017) | Norway | NSCLC | I-IIIA | 505 | 67 (28-85) | (19) |
| Sumitomo et
al (2019) | Japan | NSCLC | I-III | 160 | NR | (20) |
| Wang et al
(2018) | China | Pulmonary
neuroendocrine tumors | I-III | 159 | 59.5 (30-83) | (21) |
| Kim et al
(2018) | Korea | High-grade
neuroendocrine carcinoma | I-IV | 192 | 66 (36-89) | (22) |
| Bonanno et
al (2018) | Italy | Small cell lung
cancer | I-IV | 104 | 69 (47-86) | (23) |
 | Table IIAssessment methods of the included
studies. |
Table II
Assessment methods of the included
studies.
| Study | Tissue slides | Antibody | Staining
location | Median follow up
(months) | Outcome | PD-L1 TIIC cutoff
value (%) | NOS quality
assessment | (Refs.) |
|---|
| Fehrenbacher et
al (2016) | Whole slide | SP142 | NR | 15 | OS | 1 | 7 | (11) |
| Yang et al
(2016) | Whole slide | NR | Membrane | 79 | OS and DFS | 5 | 6 | (14) |
| Theelen et
al (2019) | Whole slide | SP142 | Membrane | 96 | OS | 1 | 6 | (15) |
| Mignon et al
(2020) | Whole slide | NR | NR | NR | OS | 1 | 6 | (16) |
| Vallonthaiel et
al (2017) | Whole slide | SP142 | Membrane/
cytoplasm | 16 | DFS | 5 | 7 | (17) |
| Chen et al
(2109) | Whole slide | E1L3N | Membrane | NR | DFS | 1 | 6 | (18) |
| Paulsen et
al (2017) | Tissue
microarray | E1L3N | Membrane/
cytoplasm | NR | OS and DFS | 1 | 7 | (19) |
| Sumitomo et
al (2019) | Whole slide | SP263 | Membrane | 42.8 | OS and DFS | 1 | 6 | (20) |
| Wang et al
(2018) | Whole slide | SP142 | Membrane/
cytoplasm | NR | OS | 1 | 6 | (21) |
| Kim et al
(2018) | Whole slide | NR | Membrane/
cytoplasm | 80.7 | OS | 1 | 7 | (22) |
| Bonanno et
al (2018) | Whole slide | 22C3 | Membrane | 13.4 | OS | 1 | 6 | (23) |
Among the included studies, 8 (11,14-20)
were focused on non-small cell lung cancer (NSCLC), of which, 1
study was on pulmonary squamous cell carcinoma (14) and 1 on lung adenocarcinoma (16). The remaining studies were on
pulmonary neuroendocrine carcinoma (21,22)
and SCLC (23). All studies
assessed PD-L1 expression on TIICs using IHC. In total, 7 different
types of monoclonal antibody were used, among which, SP142 clone
was used in 4 studies (11,15,17,21)
while the other antibodies were used once. A total of 4 studies
(17,19,21,22)
defined membranous and/or cytoplasmic staining of PDL-1 in TIICs as
positive, whereas 5 studies (14,15,20,23,24)
considered positive expression only the cases with membranous
staining of PD-L1 in TIICs. The cutoff values for evaluating the
positive expression of PD-L1 in TIICs were divided into two types:
i) Proportion of stained cells ≥5% and ii) proportion of stained
cells ≥1%. While 9 studies (11,15,16,19-23)
used 1% cutoff value for evaluation, only 2 studies (14,17)
used 5% cutoff value for evaluation. In 10 of 11 studies, whole
slides (11,14-17,20-23)
were used for evaluating cancer samples, while only 1 study used
tissue microarray assay (19).
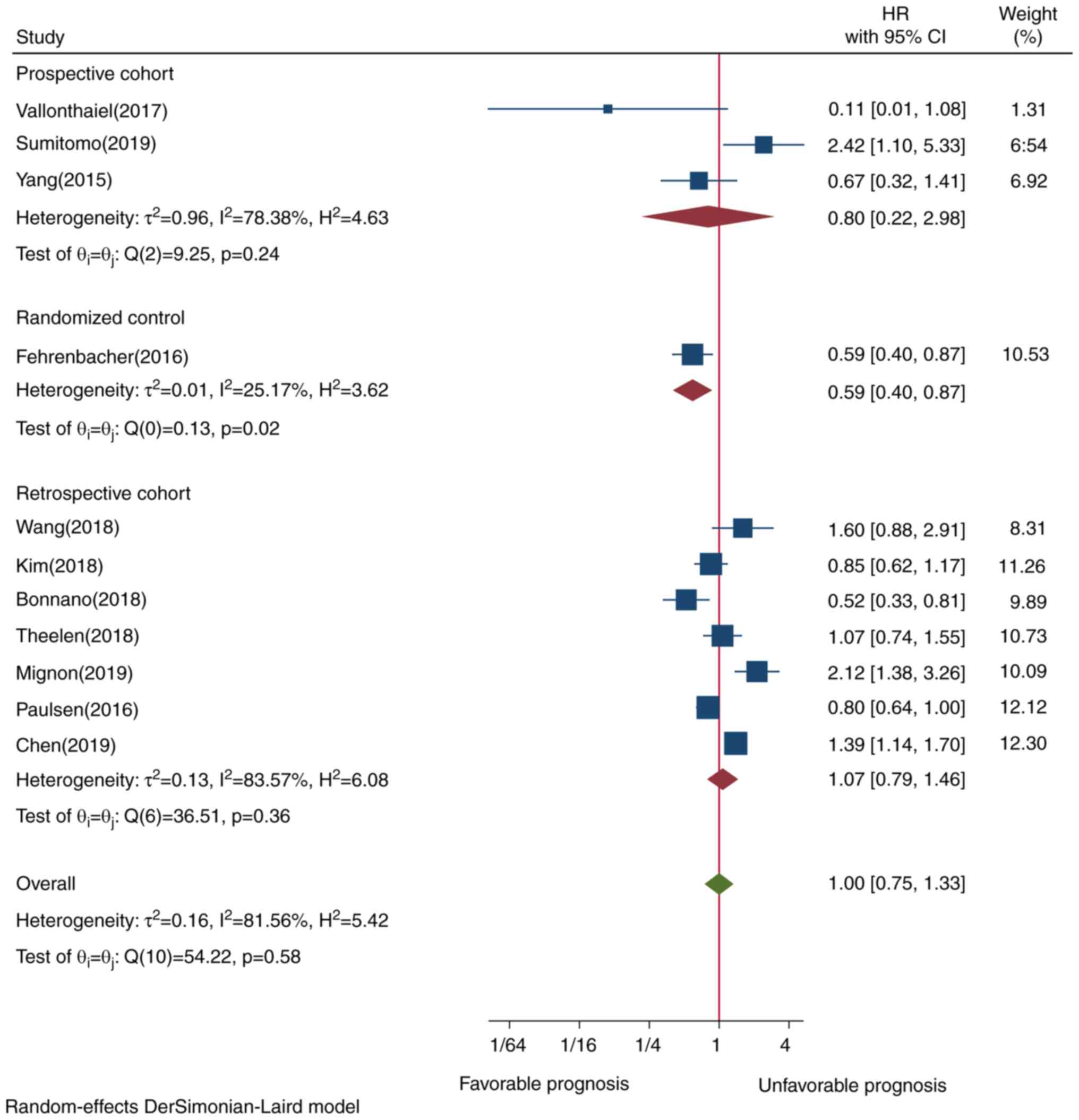
Among the included studies, 3 were prospective cohort studies, 7
were retrospective cohort studies and 1 was a randomized control
trial study (RCT).
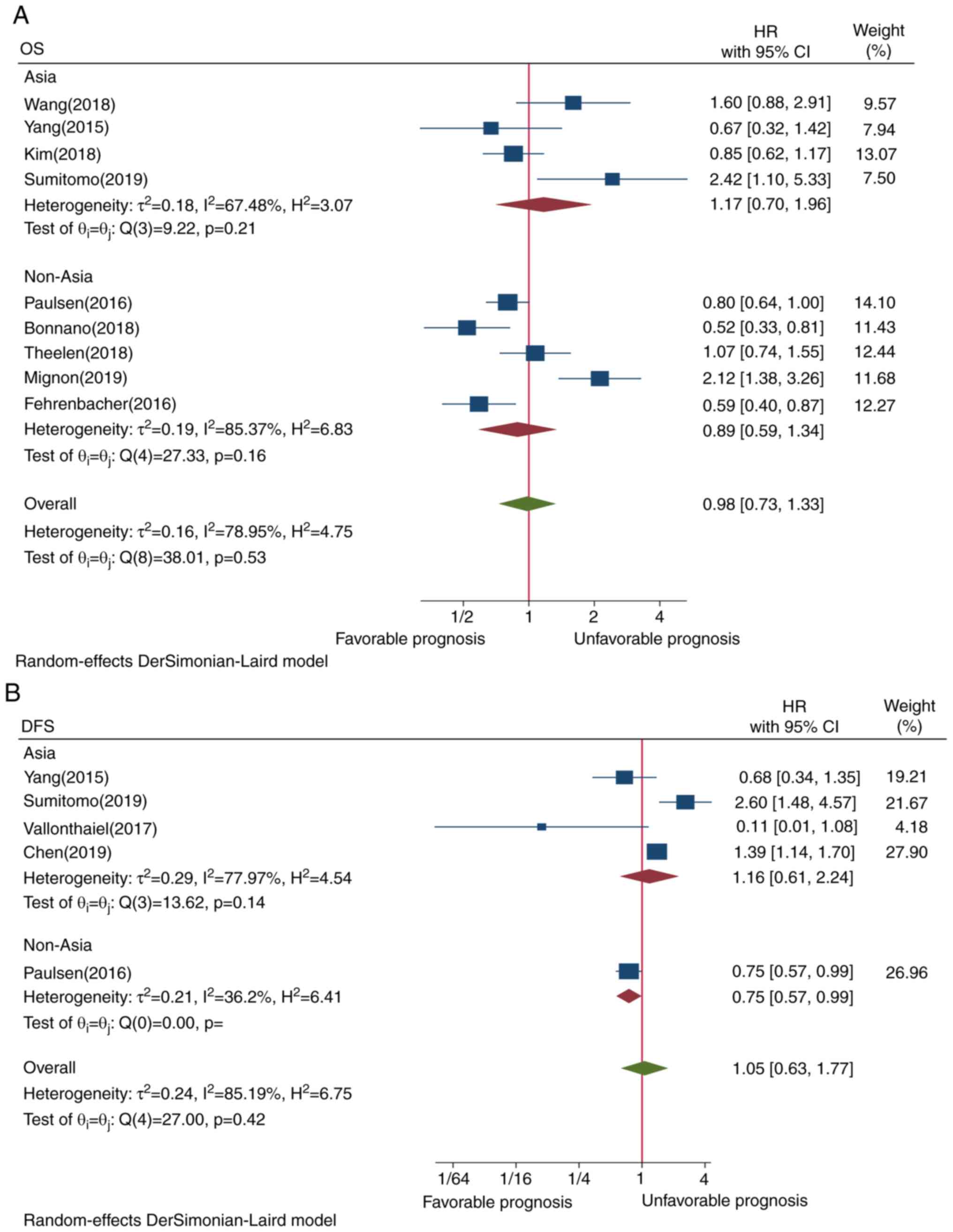
PD-L1 expression and OS
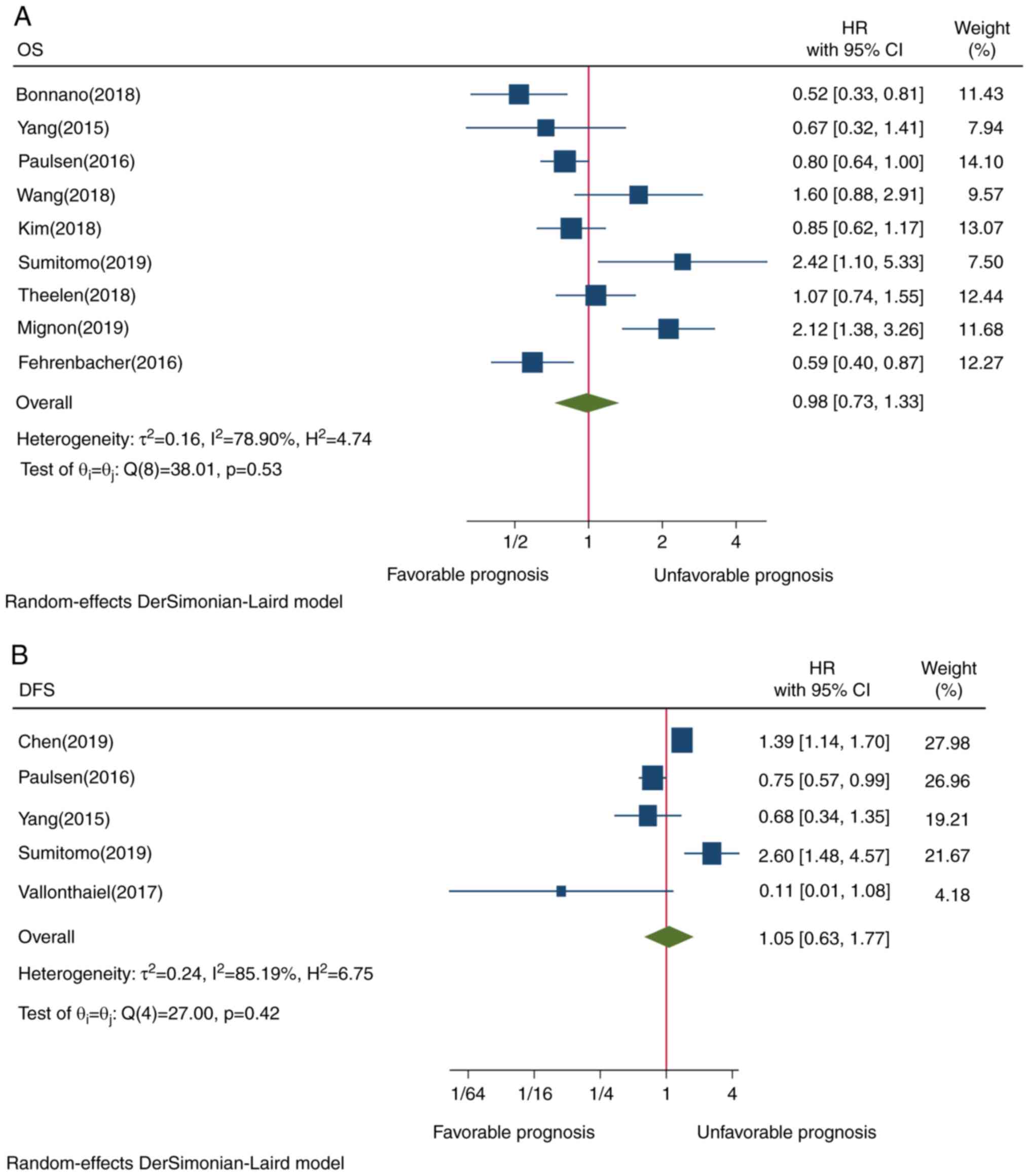
The pooled result of 9 studies did not reveal a
statistically significant association between PD-L1 expression in
TIICs and OS (HR=0.98, CI=0.73-1.33, P=0.53; Fig. 2A). This may be attributed to the
presence of heterogeneity among all the included studies.
Subgroup analysis between PD-L1 TIIC
expression and OS
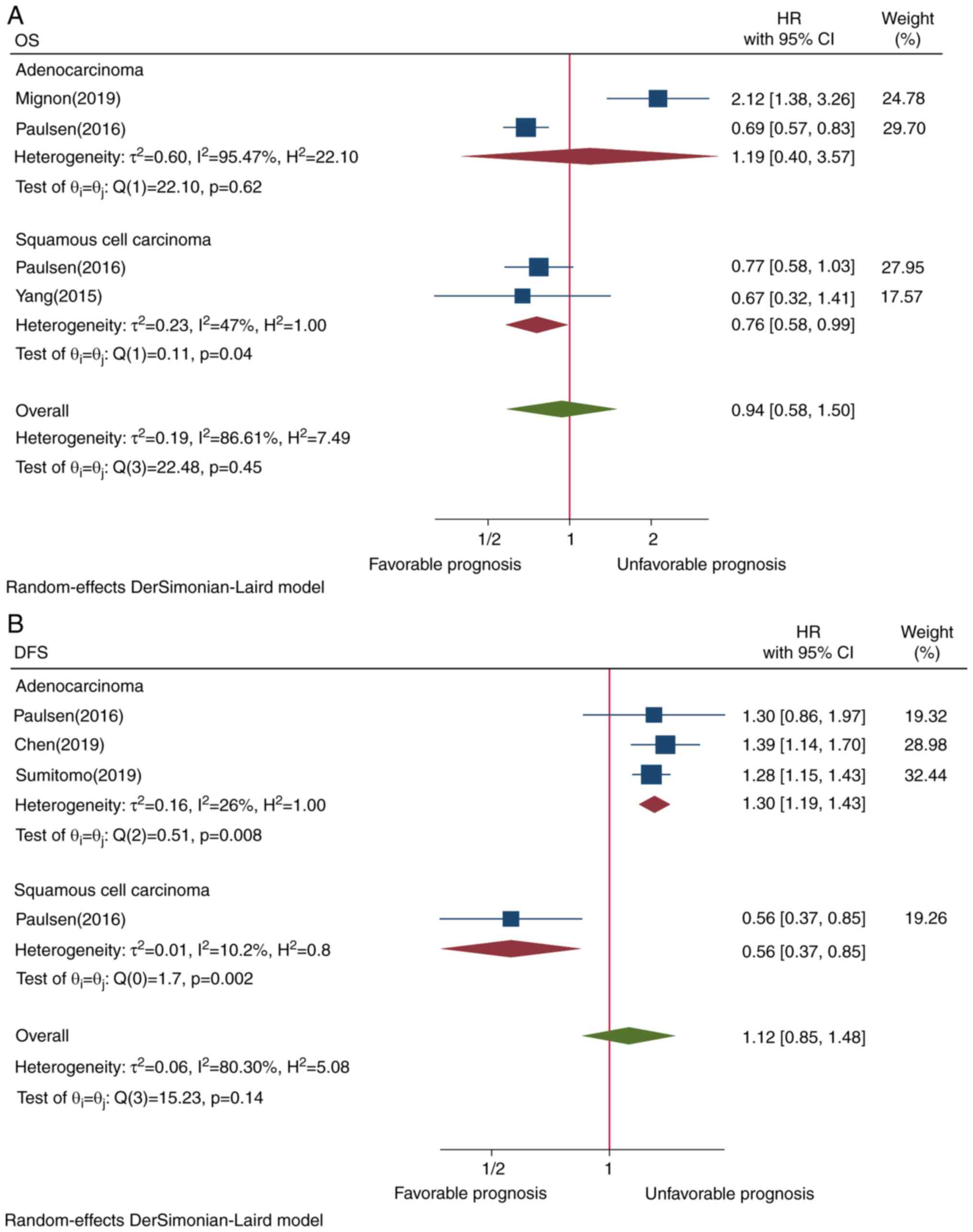
To identify the source of heterogeneity, subgroup
analysis in subtypes of lung cancer, cutoff values, ethnicity,
study design and staining localization was conducted. Lung squamous
cell carcinoma was associated with improved prognosis (HR=0.76,
CI=0.58-0.99, P=0.04; Fig. 3A).
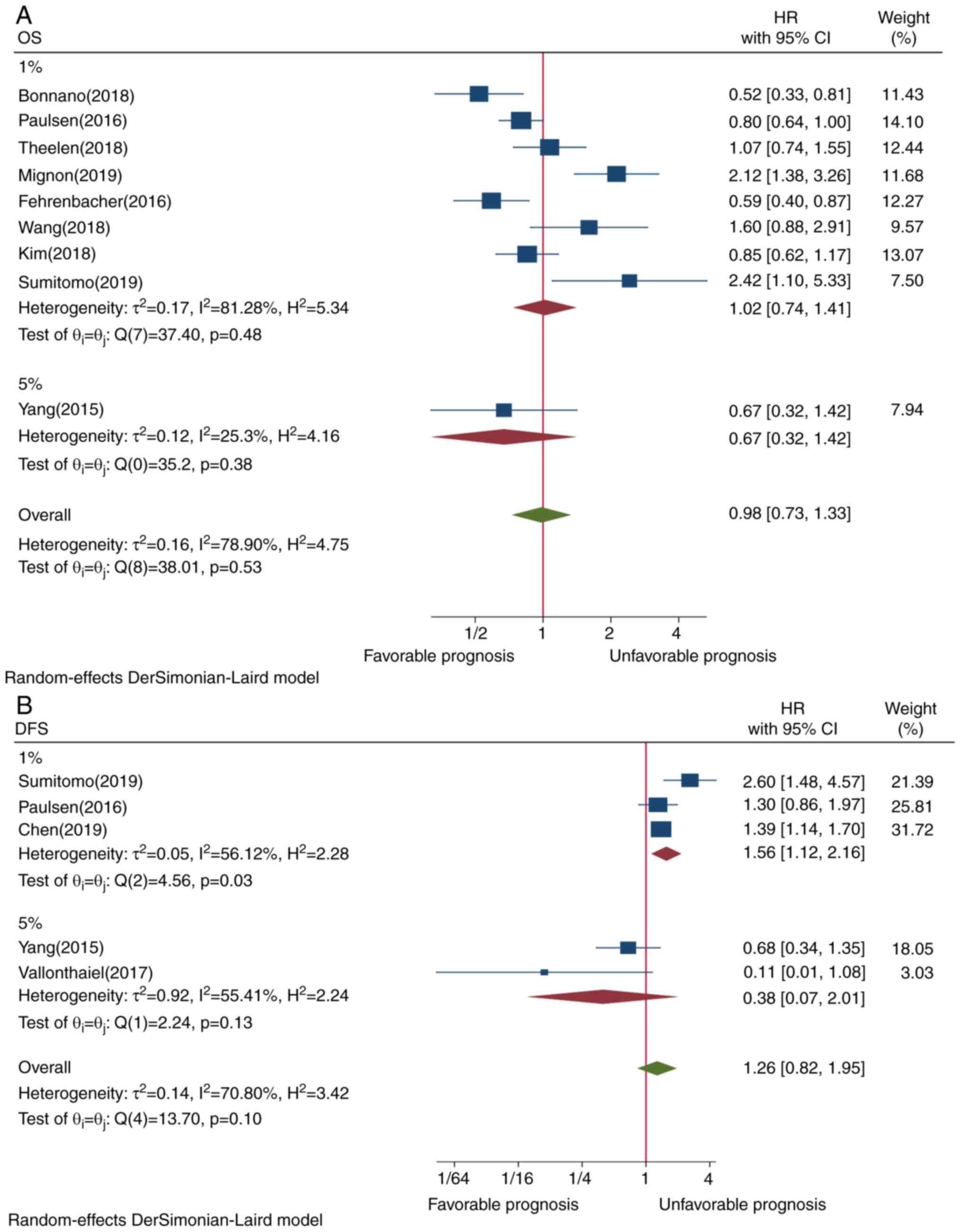
However, PD-L1 expression in TIICs assessed by ≥1% cutoff value
(P=0.48) and ≥5% cutoff value (P=0.38) was not significantly
associated with OS (P=0.53) (Fig.
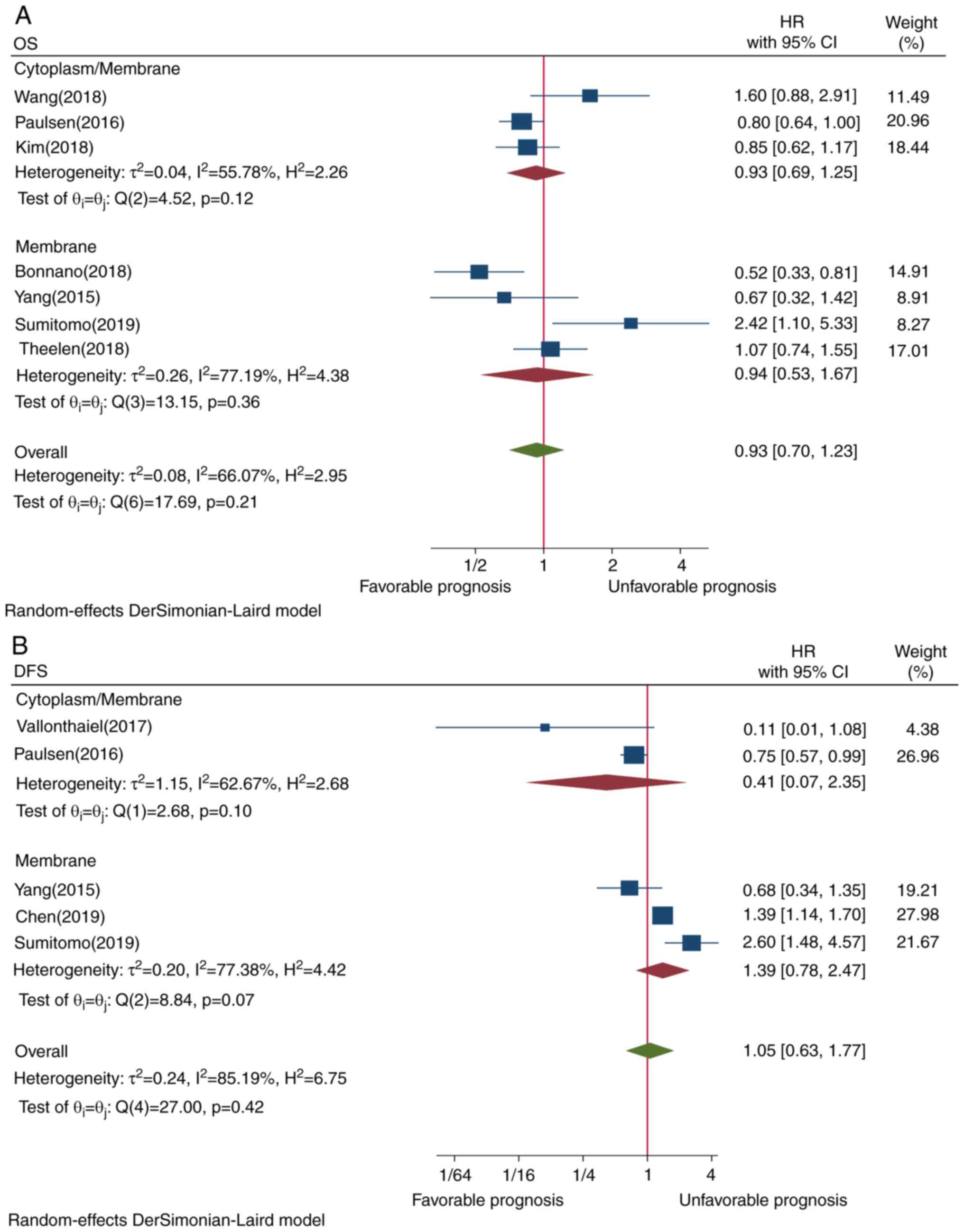
4A). Although Asian ethnicity (P=0.21) and membrane-only
staining (P=0.21) tended tobe associated with worse OS, the
association was not statistically significant (Figs. 5A and 6A, respectively). Subgroup analysis by
research design revealed that prospective cohort studies tended to
be associated with better prognosis (HR=0.79, CI=0.21-2.96,
P=0.24), while retrospective cohort studies had a borderline effect
(HR=1.07, CI=0.79-1.45, P=0.36), although neither design exhibited
a statistically significant difference (Fig. 7). Only a randomized control study
that demonstrated improved survival was reported. Excluding this
RCT study design from all other study designs did not alter the
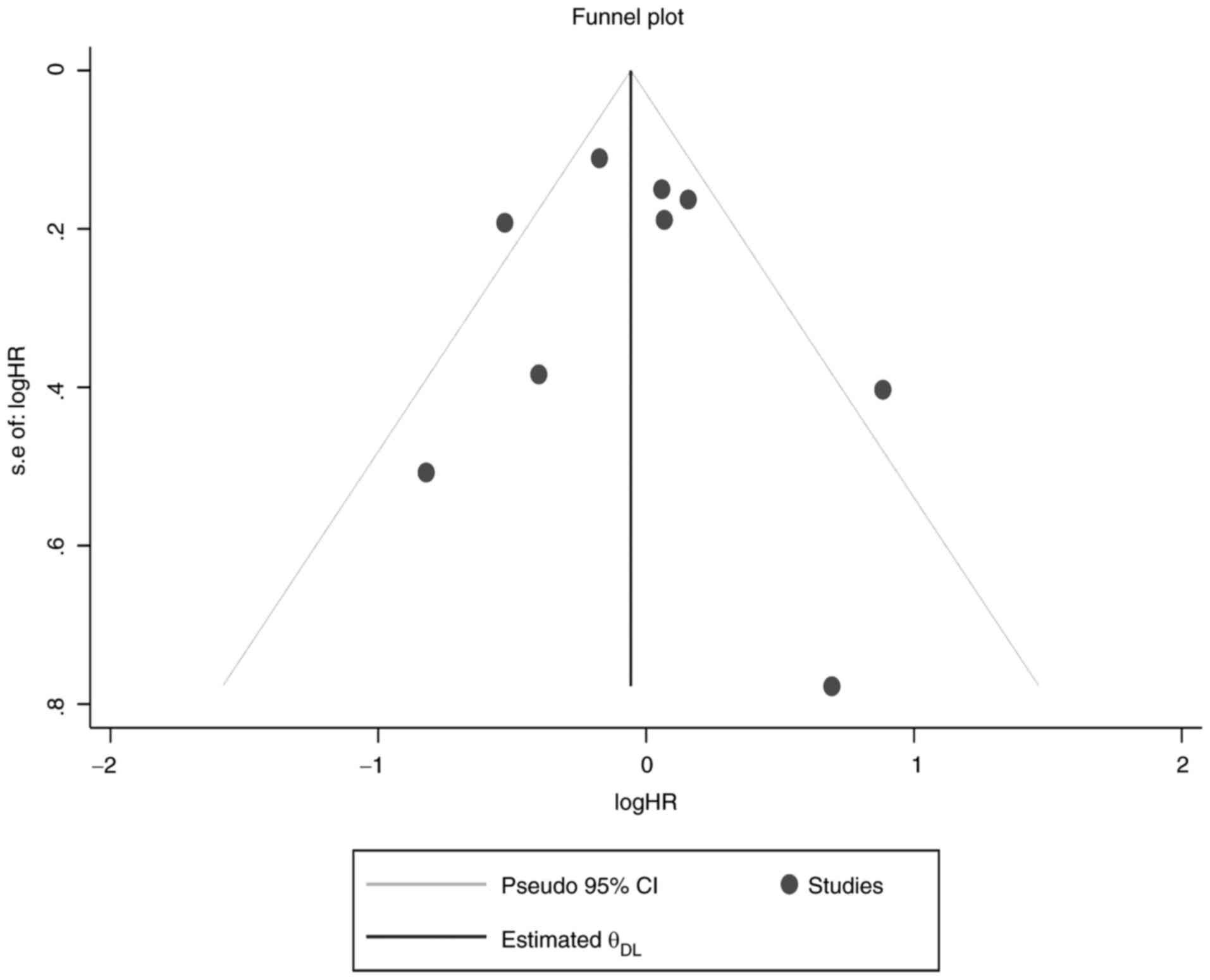
meta-analysis pooled result. Furthermore, funnel plot testing for
publication bias showed that there were no small-study effects for
OS (Fig. 8).
PD-L1 expression in TIICs and DFS
Only 5 studies were investigated the pooled results
of which did not show a statistically significant association
between PD-L1 TIIC and DFS (HR=1.05, CI=0.63-1.77, P=0.42; Fig. 2B). This result may be due to the
presence of heterogeneity.
Subgroup analysis between PD-L1 TIIC
expression and DFS
Subgroup analysis showed that PD-L1 TIIC expression
in pulmonary adenocarcinoma (HR=1.30, CI=1.19-1.43, P=0.008) and
assessment by ≥1% staining cutoff value (HR=1.56, CI=1.12-2.16,
P=0.03) exhibited an opposite survival trend (Figs. 3B and 4B, respectively). Although Asian ethnicity
(P=0.14) and membrane-only staining appeared to be poor predictors
of prognosis (P=0.07), the correlation was not statistically
significant (Figs. 5B and 6B, respectively).
Discussion
A number of meta-analyses (25-29)
demonstrated that PD-L1 expression on TC is becoming a powerful
prognostic tool in guiding patient selection for PD-1/PD-L1
inhibitor therapy in various cancer types, including lung cancer.
To the best of our knowledge, the present study is the first
meta-analysis to assess the prognostic value of PD-L1 expression in
TIICs in patients with lung cancer. Here, 10 of 11 included studies
in the meta-analysis were observational studies. As univariate
analysis data are difficult for clinical interpretation in
observational studies, multivariate model was used as this model
usually leads to more precise conclusion. There were three studies
that reported both univariate and multivariate results.
Interestingly, in these studies, the difference between univariate
and multivariate result was small and statistical/clinical
conclusions didn't change.
The overall pooled result showed that PD-L1
expression in TIICs was not associated with OS or DFS in any of the
included populations with lung cancer. This finding is consistent
with a meta-analysis of the included cancer types (30), and with the results reported in
tumors of the digestive system (6),
as well as cohort studies (31,32).
However, the present result contradicts previous studies that
revealed that PD-L1 TIIC in primary breast cancer (26) was associated with improved OS, while
in SCLC (33) and in head and neck
cancer (34) it was associated with
longer DFS. The differences in the definition of PD-L1 positivity,
cancer type, IHC methods used, therapeutic regimen and other
clinicopathological variables may account for the contradictory
results. Additionally, the differences in primary endpoint outcome
may also be another source of variation: While the present study
used OS and DFS, other studies (33,34)
reported relapse-free survival as the primary endpoint outcome.
In the present meta-analysis, due to the presence of
heterogeneity, subgroup analysis was performed by subtype of lung
cancer, cutoff value, ethnicity and staining localization in order
to identify the source of heterogeneity.
As regards tumor subtype, the present results showed
that PD-L1 TIIC overexpression in adenocarcinoma was associated
with poor DFS. This finding is consistent with a meta-analysis
(35) and a cohort study (36) on PD-L1 in TC in pulmonary
adenocarcinoma. However, the present study demonstrated that PD-L1
TIIC expression was associated with improved OS for patients with
pulmonary squamous cell carcinoma. This indicates that the presence
of a heterogeneous tumor microenvironment in the subset of cells
along with their unique molecular mechanism may greatly affect
PD-L1 induction (37) and, in turn,
the clinical outcome of the patients. Thus, PD-L1 expression
generate differently could respond to therapeutic strategies
distinctly (38). On this basis,
classifying patients with lung cancer is important for the safety
and efficacy of treatment, as well as for improving prognosis. The
results of the present meta-analysis suggest that patients with
lung adenocarcinoma with PD-L1-positive expression in TIICs may
benefit from anti-PD-L1 treatment. This hypothesis was supported by
previous cohort studies demonstrating that PD-L1 overexpression in
TIICs predicted response to anti-PD-1/PD-L1 therapy with
atezolizumuab (11,36,39).
This, in turn, suggests that adding PD-L1 TIIC to the existing
PD-L1 tumor cell (TC) in the diagnostic algorithm could help to
select patients.
As regards staining localization, there are certain
concerns with regard to the challenges in distinguishing membranous
from cytoplasmic staining (19,40)
and controversies regarding the need to classify the localization
to predict prognosis. The results of the present meta-analysis
revealed that membrane-only staining tended to predict worse DFS,
although the association was not significant. This may be partly
due to the fact that, although there may exist variations in
staining expression status, the characteristics of PD-L1 IHC assay
and staining priority pattern of assays mainly determine prognosis.
Previous studies indicate that both the SP142 and E1L3N clones are
more specific against intracellular PD-L1 (40,41),
while SP142 is less sensitive compared with other assays (42). In other words, antibodies that are
directed against the extracellular compartment could have limited
influence on other intracellular PD-L1 forms (cytoplasmic and
nuclear-PD-L1), which could, to certain extent, affect the efficacy
of PD-L1-based immunotherapy (43).
By contrast, it was previously reported that specific monoclonal
antibodies that recognize PD-L1 variants in the extracellular
compartment could also detect PD-L1 determinants retained in the
cytoplasm (40). Taken together,
these findings suggest that having a detailed knowledge of the
functional and structural integrity of membranous and cytoplasmic
(mPD-L1/cPD-L1) is important for selecting patients who may benefit
from this classification, and to reduce anti-PD-1/PD-L1
therapy-associated toxicity. Large, comprehensive and well-designed
studies are required in this field, investigating cytoplasm-and/or
membrane-infiltrating immune cells.
Previous cohorts demonstrated that the largest
survival benefit was obtained for the highest level of PD-L1
(TC≥50% or IC≥10%) (11,39). However, ≥50% PD-L1 was not included
in the present meta-analysis, as the included primary studies were
assessed either by 1 or 5% cutoff value. For cutoff value subgroup
analysis, it was reported that 1%, but not 5%, cutoff value for
PD-L1 TIIC was associated with poor DFS. This is partially in
agreement with previous meta-analyses (6,7,44).
However, there was a contradictory trend when ≥1 or ≥5% staining
cutoff value was used for assessing the association of PD-L1 TIIC
with the prognosis of patients with cancer. Thus,
multi-classification of cutoff values for evaluating PD-L1-positive
expression in TIICs may be feasible and reasonable.
The present meta-analysis has certain limitations.
First, different research designs, such as observational studies
and randomized control studies, were combined. Second, there were
differences in the antibody clone used and the scoring criteria.
Third, the present study was limited to studies published in
English. Fourth, due to insufficient data, clinicopathological
factors on PD-L1 TIIC could not be analyzed. However, the present
results may serve as a useful guide for future studies.
In conclusion, based on the present pooled
meta-analysis results, positive PD-L1 expression in TIICs was not
associated with OS or DFS in the total population of the included
studies on patients with lung cancer. However, it was correlated
with subtype of lung cancer, since PD-L1 expression in TIICs was
associated with worse DFS in patients with lung adenocarcinoma, but
with improved OS in patients with lung squamous cell carcinoma.
This suggests that adding PD-L1 TIIC to existing PD-L1 TC in the
diagnostic algorithm may help with the selection of patients who
may benefit from this classification, particularly those with lung
adenocarcinoma. However, future large-scale studies are required to
verify these findings.
Acknowledgements
Not applicable.
Funding
The research was supported by the Joint fund of Hubei Health and
Family Planning Commission (grant. no. WJ2018H0018).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BAB and GY conceived and designed the current study,
acquired and analyzed the data, and revised the manuscript. TW
conceived and designed the current study, analyzed the data and
revised the manuscript. BAB and GY confirm the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Luo D, Carter KA, Miranda D and Lovell JF:
Chemophototherapy: An emerging treatment option for solid tumors.
Adv Sci (Weinh). 4(1600106)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Shukuya T and Carbone DP: Predictive
markers for the efficacy of Anti-PD-1/PD-L1 antibodies in lung
cancer. J Thorac Oncol. 11:976–988. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Aguiar PN Jr, Santoro IL, Tadokoro H, de
Lima Lopes G, Filardi BA, Oliveira P, Mountzios G and de Mello RA:
The role of PD-L1 expression as a predictive biomarker in advanced
non-small-cell lung cancer: A network meta-analysis. Immunotherapy.
8:479–488. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chen DS, Irving BA and Hodi FS: Molecular
pathways: Next-generation immunotherapy-inhibiting programmed
death-ligand 1 and programmed death-1. Clin Cancer Res.
18:6580–6587. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhao T, Li C, Wu Y and Li B: Prognostic
value of PD-L1 expression in tumor infiltrating immune cells in
cancers: A meta-analysis. PLoS One. 12(e0176822)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li H, Xu Y, Wan B, Song Y, Zhan P, Hu Y,
Zhang Q, Zhang F, Liu H, Li T, et al: The clinicopathological and
prognostic significance of PD-L1 expression assessed by
immunohistochemistry in lung cancer: A meta-analysis of 50 studies
with 11,383 patients. Transl Lung Cancer Res. 8:429–449.
2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang A, Wang HY, Liu Y, Zhao MC, Zhang HJ,
Lu ZY, Fang YC, Chen XF and Liu GT: The prognostic value of PD-L1
expression for non-small cell lung cancer patients: A
meta-analysis. Eur J Surg Oncol. 41:450–456. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhang M, Li G and Wang Y and Wang Y, Zhao
S, Haihong P, Zhao H and Wang Y: PD-L1 expression in lung cancer
and its correlation with driver mutations: A meta-analysis. Sci
Rep. 7(10255)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Herbst RS, Soria JC, Kowanetz M, Fine GD,
Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger
SN, et al: Predictive correlates of response to the anti-PD-L1
antibody MPDL3280A in cancer patients. Nature. 515:563–567.
2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Fehrenbacher L, Spira A, Ballinger M,
Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D,
Artal-Cortes A, Lewanski C, et al: Atezolizumab versus docetaxel
for patients with previously treated non-small-cell lung cancer
(POPLAR): A multicentre, open-label, phase 2 randomised controlled
trial. Lancet. 387:1837–1846. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Liberati A, Altman DG, Tetzlaff J, Mulrow
C, Gotzsche PC, Loannidis JPA, Clarke M, Devereaux PJ, Kleijnen J
and Moher D: The PRISMA statement for reporting systematic reviews
and meta-analyses of studies that evaluate healthcare
interventions: Explanation and elaboration. BMJ.
339(b2700)2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Rohatgi A: WebPlotDigitizer, https://automeris.io/WebPlotDigitizer.
Accessed November, 2020.
|
|
14
|
Yang CY, Lin MW, Chang YL, Wu CT and Yang
PC: Programmed cell death-ligand 1 expression is associated with a
favourable immune microenvironment and better overall survival in
stage I pulmonary squamous cell carcinoma. Eur J Cancer. 57:91–103.
2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Theelen WSME, Kuilman T, Schulze K, Zou W,
Krijgsman O, Peters DDGC, Cornelissen S, Monkhorst K, Sarma P,
Sumiyoshi T, et al: Absence of PD-L1 expression on tumor cells in
the context of an activated immune infiltrate may indicate impaired
IFNγ signaling in non-small cell lung cancer. PLoS One.
14(e0216864)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mignon S, Willard-Gallo K, Van den Eynden
G, Salgado R, Decoster L, Marien KM, Vansteenkiste JF, Teugels E
and De Grève J: The relationship between tumor-infiltrating
lymphocytes, PD-L1 expression, driver mutations and clinical
outcome parameters in non-small cell lung cancer adenocarcinoma in
patients with a limited to no smoking history. Pathol OncolRes.
26:1221–1228. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Vallonthaiel AG, Malik PS, Singh V, Kumar
V, Kumar S, Sharma MC, Mathur S, Arava S, Guleria R and Jain D:
Clinicopathologic correlation of programmed death ligand-1
expression in non-small cell lung carcinomas: A report from India.
Ann Diagn Pathol. 31:56–61. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chen L, Cao MF, Zhang X, Dang WQ, Xiao JF,
Liu Q, Tan YH, Tan YY, Xu YY, Xu SL, et al: The landscape of immune
microenvironment in lung adenocarcinoma and squamous cell carcinoma
based on PD-L1 expression and tumor-infiltrating lymphocytes.
Cancer Med. 8:7207–7218. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Paulsen EE, Kilvaer TK, Khanehkenari MR,
Al-Saad S, Hald SM, Andersen S, Richardsen E, Ness N, Busund LT,
Bremnes RM and Donnem T: Assessing PDL-1 and PD-1 in non-small cell
lung cancer: A novel immunoscore approach. Clin Lung Cancer.
18:220–233.e8. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sumitomo R, Hirai T, Fujita M, Murakami H,
Otake Y and Huang CL: PD-L1 expression on tumor-infiltrating immune
cells is highly associated with M2 TAM and aggressive malignant
potential in patients with resected non-small cell lung cancer.
Lung Cancer. 136:136–144. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang H, Li Z, Dong B, Sun W, Yang X, Liu
R, Zhou L, Huang X, Jia L and Lin D: Prognostic significance of
PD-L1 expression and CD8+ T cell infiltration in
pulmonary neuroendocrine tumors. Diagn Pathol.
13(30)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kim HS, Lee JH, Nam SJ, Ock CY, Moon JW,
Yoo CW, Lee GK and Han JY: Association of PD-L1 expression with
tumor-infiltrating immune cells and mutation burden in high-grade
neuroendocrine carcinoma of the lung. J Thorac Oncol. 13:636–648.
2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bonanno L, Pavan A, Dieci MV, Di Liso E,
Schiavon M, Comacchio G, Attili I, Pasello AG, Calabrese F, Rea F,
et al: The role of immune microenvironment in small-cell lung
cancer: Distribution of PD-L1 expression and prognostic role of
FOXP3-positive tumour infiltrating lymphocytes. Eur J Cancer.
101:191–200. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Song P, Guo L, Li W, Zhang F, Ying J and
Gao S: Clinicopathologic correlation with expression of PD-L1 on
both tumor cells and tumor-infiltrating immune cells in patients
with non-small cell lung cancer. J Immunother. 42:23–28.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Huang W, Ran R, Shao B and Li H:
Prognostic and clinicopathological value of PD-L1 expression in
primary breast cancer: A meta-analysis. Breast Cancer Res Treat.
178:17–33. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang BC, Zhang ZJ, Fu C and Wang C:
Efficacy and safety of anti-PD-1/PD-L1 agents vs. chemotherapy in
patients with gastric or gastroesophageal junction cancer: A
systematic review and meta-analysis. Medicine (Baltimore).
98(e18054)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Huang Y and Shen A: The prediction
potential of neutrophil-to-lymphocyte ratio for the therapeutic
outcomes of programmed death receptor-1/programmed death ligand 1
inhibitors in non-small cell lung cancer patients: A meta-analysis.
Medicine (Baltimore). 99(e21718)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang C, Yu X and Wang W: A meta-analysis
of efficacy and safety of antibodies targeting PD-1/PD-L1 in
treatment of advanced nonsmall cell lung cancer. Medicine
(Baltimore). 95(e5539)2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Li JH, Ma WJ, Wang GG, Jiang X, Chen X, Wu
L, Liu ZS, Zeng XT, Zhou FL and Yuan YF: Clinicopathologic
significance and prognostic value of programmed cell death ligand 1
(PD-L1) in patients with hepatocellular carcinoma: A meta-analysis.
Front Immunol. 9(2077)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kim Y, Wen X, Cho NY and Kang GH:
Intratumoral immune cells expressing PD-1/PD-L1 and their
prognostic implications in cancer: A meta-analysis. Int J Biol
Markers: May 1, 2018 (Epub ahead of print). doi:
10.1177/1724600818770941.
|
|
31
|
Dix Junqueira Pinto G, de Souza Viana L,
Scapulatempo Neto C and Vicente Serrano S: Evaluation of PD-L1
expression in tumor tissue of patients with lung carcinoma and
correlation with clinical and demographic data. J Immunol Res.
2016(9839685)2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Pezzuto A, Cappuzzo F, D'Arcangelo M,
Ciccozzi M, Navarini L, Guerrini S, Ricci A, D'Ascanio M and Carico
E: Prognostic value of p16 protein in patients with surgically
treated non-small cell lung cancer; Relationship with Ki-67 and
PD-L1. Anticancer Res. 40:983–990. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Sun C, Zhang L, Zhang W, Liu Y, Chen B,
Zhao S, Li W, Wang L, Ye L, Jia K, et al: Expression of PD-1 and
PD-L1 on tumor-infiltrating lymphocytes predicts prognosis in
patients with small-cell lung cancer. Onco Targets Ther.
13:6475–6483. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kim HR, Ha SJ, Hong MH, Heo SJ, Koh WY,
Choi EC, Kim EK, Pyo KH, Jung I, Seo D, et al: PD-L1 expression on
immune cells, but not on tumor cells, is a favorable prognostic
factor for head and neck cancer patients. Sci Rep.
6(36956)2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ma J, Chi D, Wang Y, Yan Y, Zhao S, Liu H,
Jing J, Pu H and Zhang M: Prognostic value of PD-L1 expression in
resected lung adenocarcinoma and potential molecular mechanisms. J
Cancer. 9:3489–3499. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Cha YJ, Kim HR, Lee CY, Cho BC and Shim
HS: Clinicopathological and prognostic significance of programmed
cell death ligand-1 expression in lung adenocarcinoma and its
relationship with p53 status. Lung cancer. 97:73–80.
2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chen S, Crabill GA, Pritchard TS, McMiller
TL, Wei P, Pardoll DM, Pan F and Topalian SL: Mechanisms regulating
PD-L1 expression on tumor and immune cells. J Immunother Cancer.
7(305)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wei Y, Zhao Q, Gao Z, Lao XM, Lin WM, Chen
DP, Mu M, Huang CX, Liu ZY, Li B, et al: The local immune landscape
determines tumor PD-L1 heterogeneity and sensitivity to therapy. J
Clin Invest. 129:3347–3360. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Rittmeyer A, Barlesi F, Waterkamp D, Park
K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols
MC, et al: Atezolizumab versus docetaxel in patients with
previously treated non-small-cell lung cancer (OAK): A phase 3,
open-label, multicentre randomised controlled trial. Lancet.
389:255–265. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Mahoney KM, Sun H, Liao X, Hua P, Callea
M, Greenfield EA, Hodi FS, Sharpe AH, Signoretti S, Rodig SJ and
Freeman GJ: PD-L1 Antibodies to its cytoplasmic domain most clearly
delineate cell membranes in immunohistochemical staining of tumor
cells. Cancer Immunol Res. 3:1308–1315. 2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Mino-Kenudson M: Programmed cell death
ligand-1 (PD-L1) expression by immunohistochemistry: Could it be
predictive and/or prognostic in non-small cell lung cancer? Cancer
Biol Med. 13:157–170. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kintsler S, Cassataro MA, Drosch M,
Holenya P, Knuechel R and Braunschweig T: Expression of programmed
death ligand (PD-L1) in different tumors. Comparison of several
current available antibody clones and antibody profiling. Ann Diagn
Pathol. 41:24–37. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Wu Y, Chen W, Xu ZP and Gu W: PD-L1
distribution and perspective for cancer immunotherapy-blockade,
knockdown, or inhibition. Front Immunol. 10(2022)2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Abdel-Rahman O: Correlation between PD-L1
expression and outcome of NSCLC patients treated with
anti-PD-1/PD-L1 agents: A meta-analysis. Crit Rev Oncol Hematol.
101:75–85. 2016.PubMed/NCBI View Article : Google Scholar
|