Introduction
Childhood cancer is a major public health challenge
in Mexico, with >5,000 new cases diagnosed annually, and is the
second leading cause of mortality among children aged 5-14 years
(1,2). In the Mexican state of Guerrero, acute
lymphoblastic leukemia (ALL) is the most common malignancy in
children (1,3). The prognosis of patients with ALL may
be partially predicted based on certain clinical characteristics,
such as age, karyotype, cytogenetic alterations, immunophenotype,
sex and initial leukocyte count (4,5).
Hence, it is important to identify novel and more reliable
prognostic biomarkers for leukemia. YKL-40, also known as
chitinase-3-like protein 1 (CHI3L1), is a secreted inflammatory
glycoprotein produced by different types of cells, such as
inflammatory cells (neutrophils and macrophages), stem cells,
vascular smooth muscle cells and chondrocytes (6). YKL-40 is involved in different
cellular processes, such as cancer cell proliferation, survival and
invasiveness, peritumoral inflammation, angiogenesis and remodeling
of the extracellular matrix (7).
High serum or plasma levels of YKL-40 have been detected in
patients with various types of cancer. This upregulation has been
linked to shorter progression-free and overall survival times in
patients with glioma, melanoma, pancreatic, lung and breast cancer
(8-12).
YKL-40 has also been found to be elevated in the
serum or plasma of patients with non-cancerous diseases that are
characterized by inflammation, tissue remodeling and fibrosis
(13), including rheumatic diseases
(14) and lung diseases, such as
asthma (15) and chronic
obstructive pulmonary disease (16). In cardiovascular diseases, YKL-40
has been shown to be expressed by macrophages in atherosclerotic
plaques (17). In patients with
diabetes, elevated levels of YKL-40 have been associated with
insulin resistance (18).
A previous study revealed that high serum levels of
YKL-40 in adult patients with acute myeloblastic leukemia (AML) led
to shorter survival times, and that the serum level of this protein
was an independent predictor of survival in patients with AML
(19). In another study in adult
patients with leukemia, the YKL-40 plasma levels were found to
differ significantly between leukemia patients and healthy
individuals (20).
Therefore, YKL-40 protein has been attracting a
notable amount of attention as a biomarker of poor prognosis in
patients with cancer (7,13). However, the role of YKL-40 as
prognostic biomarker in childhood ALL has not been explored to
date. Therefore, the present study was undertaken to analyze the
plasma levels of YKL-40 in Mexican children with ALL and
investigate its role as a prognostic factor.
Materials and methods
Study population
A case-control study in a population of children
aged 1-18 years was performed. The patients were treated at the
Pediatric Oncology Clinic of the State Cancer Institute ‘Dr. Arturo
Beltran Ortega’ in Acapulco, México, between July 2016 and
September 2018. The study involved 45 children who had not received
treatment and who were recently diagnosed with ALL according to the
morphological findings of bone marrow aspirate and the criteria
reported by Organista et al in 2015(21).
At the time of admission of each patient, a physical
examination was performed to determine their general health status.
In addition, genetic and biochemical tests were performed, as well
as imaging tests, such as ultrasound, nuclear magnetic resonance or
computed axial tomography, in order to assess the involvement of
other organs. Furthermore, all patients included in the study
underwent a procalcitonin test, as well as blood and urine cultures
to rule out secondary infections. This was taken into account, in
order to rule out the possible effects of inflammation on YKL-40
levels.
Overall survival was determined as the time between
the date of study registration and the date of death from any cause
or the date of the last contact (21,22).
Survival data were obtained by reviewing the clinical records. Risk
classification in children with ALL was as follows: i) Low-risk,
children aged 1-10 years with a leukocyte count
<50,000/mm3; and ii) high-risk, children aged <1
and >10 years with a leukocyte count
>50,000/mm3.
Patients with ALL were divided into groups with low
and high expression of YKL-40. The median expression of YKL-40
(36.34 ng/ml) was used as the cut-off point to divide the 45
patients with ALL into these two groups. Those who expressed YKL-40
at levels <36.34 ng/ml were assigned to the downregulation group
(n=20), and those with expression levels >36.34 ng/ml were
assigned to the upregulation group (n=25).
Patients were treated with VDCPM (vincristine,
daunorubicin, cyclophosphamide, prednisone and intrathecal
methotrexate) or VDLPM (vincristine, daunorubicin, asparaginase,
prednisone and intrathecal methotrexate) regimens according to the
protocol reported by Organista et al (21). Following the aforementioned
treatment schemes and a follow-up of ~36 months, 64.5% of children
with ALL survived and 35.5% succumbed to the disease.
The control group comprised 45 hematologically
healthy children aged 1-18 years, who visited the Health Center
‘Dr. Ramón Carreto Leyva’ in Chilpancingo, México, for reasons such
as vaccination, weight and height monitoring, and medical
consultation. Blood count measurements confirmed normal leukocyte
count (4.5-11.0x103/mm3).
Peripheral blood samples (5 ml) were collected from
both study groups. The parents or guardians of all the subjects
signed informed consent forms; the original study protocol was
approved by the Ethics Committee of the State Cancer Institute of
the State of Guerrero. All procedures followed were in accordance
with the ethical standards of the responsible committees on human
experimentation (institutional and national) and with the
principles outlined in the Helsinki Declaration.
The blood samples used in both study groups were
obtained through venous puncture and were collected in tubes with
EDTA as the anticoagulant (BD Vacutainer™;
Becton-Dickinson and Company). Plasma was separated from each
sample by centrifugation at 1,200 x g for 10 min at 4˚C and stored
at -70˚C until use.
Determination of YKL-40 plasma levels
by ELISA
YKL-40 plasma levels were measured using ELISA
(Human CHI3L1 ELISA Kit, cat. no. DC3L10; R&D Systems, Inc.),
according to the manufacturer's instructions. Plasma samples were
diluted 1:10 [90 µl assay diluent RD5P (1:5) + 10 µl of plasma].
The optical density of each well was determined using a microplate
reader (MultiSkan Go; Thermo Fisher Scientific, Inc.) at a
wavelength of 450 nm. The sensitivity of the ELISA technique was
3.55 pg/ml. All samples were measured in duplicate.
Statistical analysis
Statistical analysis was performed using SPSS
version 25.0 (IBM Corp.) and GraphPad Prism version 5.0 (GraphPad
Software, Inc.). The Mann-Whitney U-test was used to identify
significant differences between study populations. Kaplan-Meier
curves were constructed to analyze the effect of YKL-40 plasma
levels on the survival of patients with ALL, and the differences
were compared using log-rank tests. A univariate logistic
regression model was also used to identify factors associated with
survival in children with ALL, adjusted for sex, presence of
cytogenetic alterations such as translocations, risk by age and
initial leukocyte count, invasion of the central nervous system
(CNS) and YKL-40 plasma levels. Those associated factors were
included in a second multivariate logistic regression model.
P<0.05 was considered to indicate statistically significant
differences.
Results
General characteristics of the study
population
Overall, 45 plasma samples from children with ALL as
well as 45 control samples from healthy children were collected.
The characteristics of the included subjects are summarized in
Table I.
 | Table IGeneral characteristics and clinical
data of children with and without ALL. |
Table I
General characteristics and clinical
data of children with and without ALL.
| Variables | Children with ALL
(n=45) | Children without
ALL (n=45) |
|---|
| Age, years (mean ±
SD) | 8.7±4.9 | 9.5±4.0 |
| Leukocyte count,
/mm3 | 24,400
(11,950-63,925)a | 6,650
(5,700-8,325)a |
| Sex, n (%) | | |
|
Male | 24 (53.3) | 21 (46.7) |
|
Female | 21 (46.7) | 24 (53.3) |
| Patient status, n
(%) | | |
|
Alive | 29 (64.4) | 45 (100.0) |
|
Deceased | 16 (35.6) | - |
| ALL type, n
(%) | | |
|
Type B | 42 (93.0) | - |
|
Type T | 3 (7.0) | - |
| Translocation, n
(%) | 10 (24.0) | |
|
t(1;19) | 2 (4.0) | - |
|
t(9;22) | 5 (11.0) | - |
|
TAL1 | 1 (3.0.) | - |
|
t(10;11) | 1 (3.0) | - |
|
t(12;21) | 1 (3.0) | |
|
None | 35 (76.0) | |
| Risk according to
age, n (%) | | |
|
Low (1-10
years) | 24 (53.3) | - |
|
High (<1
and >10 years) | 21 (46.7) | - |
| Risk according to
leukocyte count at diagnosis, n (%) | | |
|
Low
(<50,000/mm3) | 30 (66.7) | - |
|
High
(≥50,000/mm3) | 15 (33.3) | - |
| CNS invasion, n
(%) | | |
|
Yes | 14 (31.1) | |
|
No | 31 (68.9) | |
YKL-40 plasma levels in children with
ALL
The median YKL-40 plasma level in children with ALL
was 59.7 ng/ml, which was significantly higher compared with that
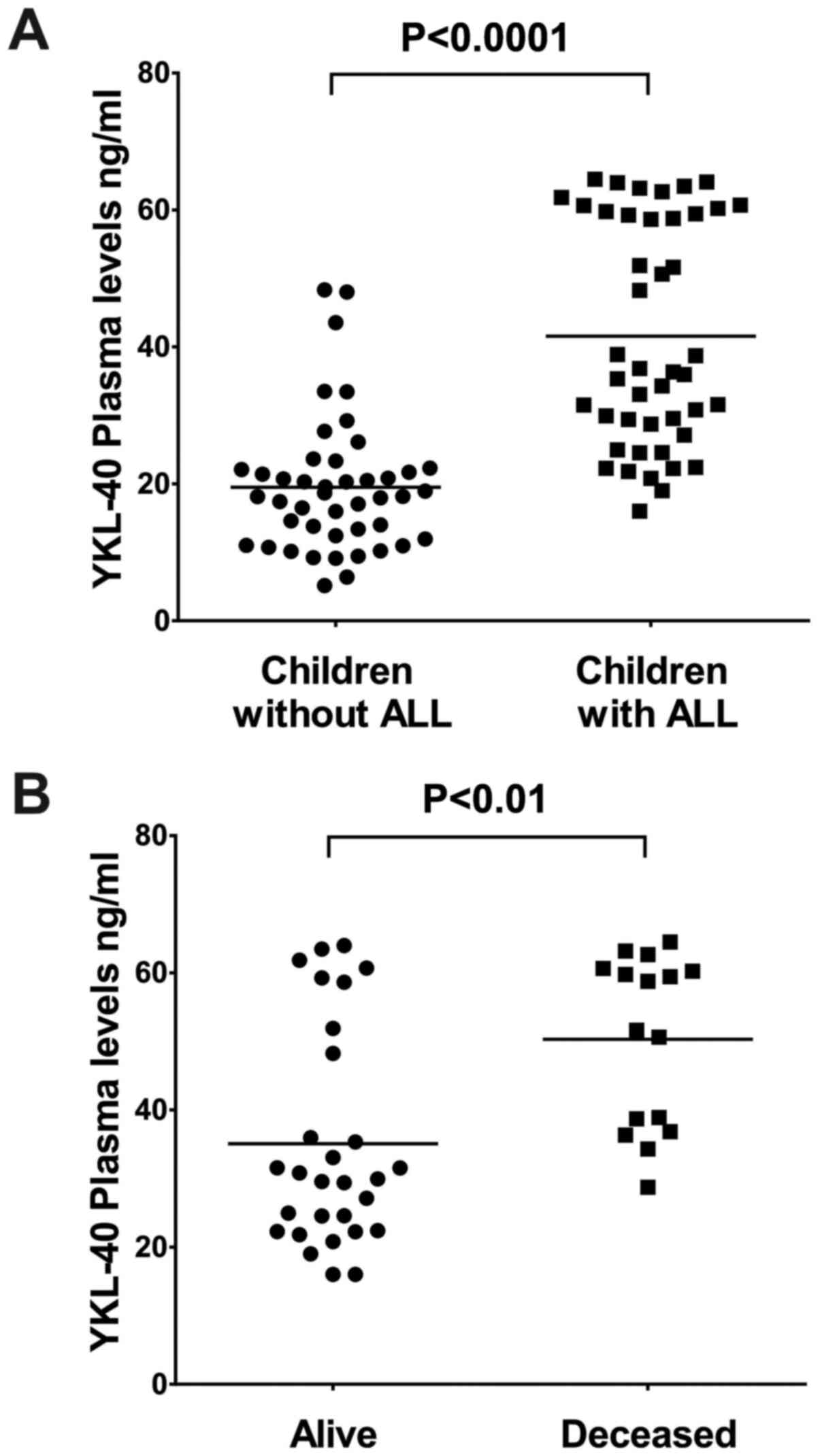
in children without ALL (22.5 ng/ml; P<0.0001; Fig. 1A).
Clinical characteristics of children
with ALL and identification of prognostic factors
The pathological parameters associated with survival
in children with ALL in the present study were as follows: Age,
sex, initial leukocyte count, cytogenetic abnormalities, such as
translocations, and invasion of the CNS by leukemic cells.
Univariate and multivariate logistic regression
analyses were performed to determine which of the parameters, such
as age, sex, presence of translocations, initial leukocyte count
and CNS invasion, in addition to YKL-40 plasma levels, were
associated with the survival of children with ALL (Table II). Univariate and multivariate
regression analyses in this study revealed that sex and the
presence of translocations were not associated with survival in
children with ALL. By contrast, age, initial leukocyte count, CNS
invasion and YKL-40 plasma levels were found to be associated with
survival in children with ALL. High-risk children with ALL were
8.53 times more likely to succumb to the disease compared with
their low-risk counterparts [95% confidence interval (CI):
1.2-58.2; P=0.03]. Children with ALL with CNS invasion had a
6.45-fold higher risk of death compared with those without CNS
invasion (95% CI: 1.01-41.2; P=0.04). Children with ALL who had
high levels of YKL-40 (≥36.34 ng/ml) had a 6.06-fold higher risk of
death compared with those with low levels (<36.34 ng/ml; 95% CI:
1.1-31.6; P=0.03). Low-risk children with ALL with high plasma
levels of YKL-40 (>36.34 ng/ml) may develop an infection with
associated inflammation, which is a characteristic sign of ALL,
leading to an increase in the plasma levels of YKL-40 (Table II).
 | Table IIMultivariate analysis of prognostic
factors in children with acute lymphoblastic leukemia. |
Table II
Multivariate analysis of prognostic
factors in children with acute lymphoblastic leukemia.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Factors | Alive, n (%)
[total, 29 (64.4)] | Deceased, n (%)
[total, 16 (35.6)] | OR | 95% CI |
P-valuea | OR | 95% CI |
P-valueb |
|---|
| Sex | | | | | | | | |
|
Male | 15 (33.3) | 9 (20.0) | 1.09 | 0.59-2.02 | 0.76 | | | |
|
Female | 14 (31.1) | 7 (15.6) | | | | | | |
| Translocation | | | | | | | | |
|
Absent | 24 (53.3) | 11 (24.5) | 2.18 | 0.52-9.12 | 0.28 | | | |
|
Present | 5 (11.1) | 5 (11.1) | | | | | | |
| Risk by age and
initial leukocyte count | | | | | | | | |
|
Lowc | 16 (35.5) | 3 (6.6) | 5.33 | 1.24-22.8 | 0.02 | 8.53 | 1.2-58.2 | 0.03 |
|
Highd | 13 (28.9) | 13(29) | | | | | | |
| CNS invasion | | | | | | | | |
|
Yes | 6 (13.3) | 8 (17.8) | 3.83 | 1.01-14.4 | 0.04 | 6.45 | 1.01-41.2 | 0.04 |
|
No | 23 (51.1) | 8 (17.8) | | | | | | |
| YKL-40 plasma
levels | | | | | | | | |
|
Low | 19 (42.2) | 3 (6.6) | 8.2 | 1.89-35.82 | 0.005 | 6.06 | 1.1-31.6 | 0.03 |
|
High | 10 (26.7) | 13 (24.5) | | | | | | |
Association of YKL-40 plasma levels
with survival in children with ALL
Measurement of YKL-40 plasma levels revealed that
the median YKL-40 level was significantly higher in children who
had succumbed to the disease during the study (55.1 ng/ml) compared
with that in children who remained alive (31.53 ng/ml; P<0.05).
YKL-40 is a protein the levels of which increase in the presence of
inflammation, and it is considered as an independent marker of
inflammation (8,18). Patients who remained alive and
exhibited high levels of YKL-40 likely had a prominent inflammatory
response, which led to the increase of the YKL-40 levels(Fig. 1B).
Children with ALL were followed up for ~36 months;
during this period, 16 (35.5%) succumbed to the disease and 29
(64.5%) remained alive. The follow-up was complete for all the
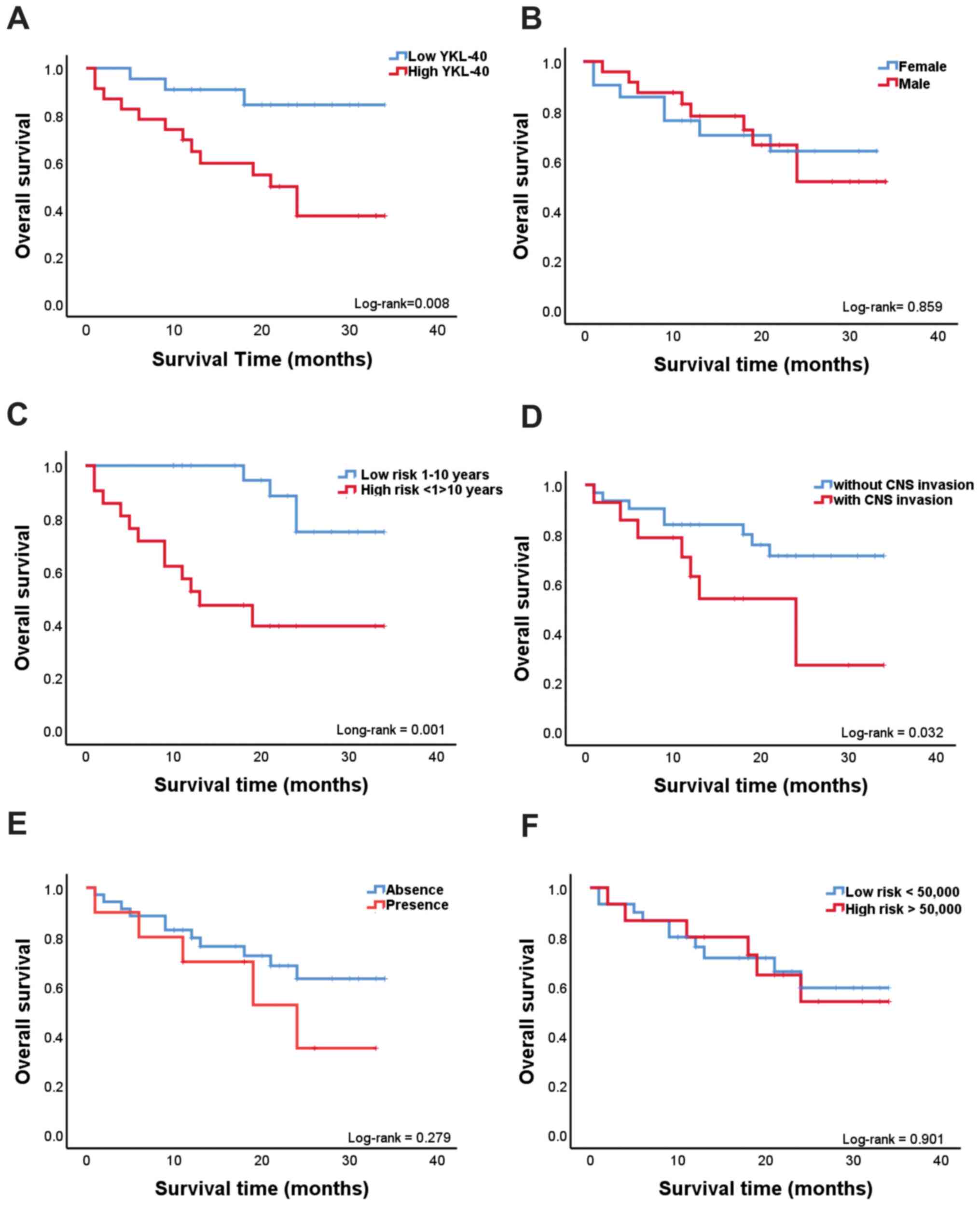
patients. Kaplan-Meier analysis revealed a positive association
between overall survival and the plasma levels of YKL-40. As shown
in Fig. 2A, children with ALL who
had high YKL-40 plasma levels (≥36.34 ng/ml) had shorter survival
times compared with those with low YKL-40 plasma levels (<36.34
ng/ml; log-rank P=0.008). The cut-off value of 36.34 ng/ml was
based on the median value obtained from the YKL-40 plasma levels in
children with ALL in the present study. Kaplan-Meier analysis was
performed with variables of poor prognosis in ALL, such as age,
sex, presence of translocations, initial leukocyte count and CNS
invasion. Kaplan-Meier analysis revealed a positive association of
overall survival with age and CNS invasion. As shown in Fig. 2C, Kaplan-Meier analysis was
performed in children with ALL grouped by age (low-risk, children
aged 1-10 years; and high-risk, children aged <1 and >10
years), and it was observed that children with ALL aged <1 and
>10 years exhibited a shorter survival compared with those aged
1-10 years (log-rank P=0.001). As shown in Fig. 2D, children with ALL with CNS
invasion had a shorter survival compared with those without CNS
invasion (log-rank P=0.032). Sex, presence or absence of
translocations and initial leukocyte count were not associated with
the overall survival of children with ALL in the present study
(Fig. 2B, E and F).
Discussion
Ongoing research is aimed at identifying novel
biomarkers that may help determine the prognosis of patients with
ALL. Several studies to date have demonstrated that YKL-40 may
serve as a prognostic biomarker in patients with different types of
malignancies, including lung cancer, breast cancer,
cholangiosarcoma and glioma (23-27).
YKL-40 levels were previously evaluated in tissue samples from
healthy individuals using immunohistochemistry, where the YKL-40
staining intensity was higher in tissues that exhibited high
metabolic activity compared with those with low activity (28).
In the present study, the plasma levels of YKL-40
were analyzed in 45 children with ALL who were aged 1-18 years. The
pathological parameters associated with survival in children with
ALL are as follows: i) Age: Patients aged <1 and >10 years
are considered as high-risk and may have an unfavorable prognosis;
ii) sex: Female patients have a better prognosis compared with male
patients, primarily due to the appearance of testicular infiltrates
that lead to a higher risk of relapse (4,5); iii)
initial leukocyte count: An initial leukocyte count of
50,000/mm3 is the cut-off value that determines
favorable or dismal prognosis; patients with a count greater than
this cut-off value are considered as high-risk (4); iv) cytogenetic abnormalities, such as
translocations: The presence of t(12;21) is associated with a good
prognosis, while t(9;22) and t(1;19) are considered to be
associated with poor prognosis, since patients who harbor these
cytogenic abnormalities have a lower rate of resolution of the
disease (4,29); and v) invasion of the CNS by
leukemic cells, which is also considered as a poor prognostic
factor (30).
The statistical analyses suggested that high plasma
levels of YKL-40 are associated with shorter survival, as well as
with poor prognostic factors, such as age, leukocyte count at the
time of diagnosis and CNS invasion, whereas they were not
associated with other prognostic factors, such as the presence of
translocations and sex. Our statistical analysis also suggested
that, in addition to the association of high plasma YKL-40 levels
with survival, other variables, such as age and CNS invasion, were
also associated with the survival of children with ALL in this
study.
The present study demonstrated that YKL-40 plasma
levels were elevated in children with ALL, which was also
consistent with other studies that found high serum or plasma
levels of YKL-40 in different types of solid cancers, and were
associated with shorter survival durations in patients with
cholangiosarcoma, breast, lung, bladder and endometrial cancer
(23-27).
It has also been reported that high levels of YKL-40 may be
associated with poor prognostic factors; for example, high levels
of YKL-40 in patients with breast cancer were associated with
higher tumor-node-metastasis stage (23). High serum levels of YKL-40 were
associated with more advanced disease stage in small cell lung
cancer (25) and International
Federation of Gynecology and Obstetrics stage of endometrial cancer
(26).
YKL-40 has been shown to increase tumor growth,
metastatic potential and angiogenesis (31,32).
At the cellular level, YKL-40 protein expression is high in fetal
and embryonic tissues, which are characterized by morphogenetic
changes and marked cell proliferation and differentiation (6). No specific cell surface or soluble
receptor for this protein has been identified to date. The growth
of fibroblasts derived from osteoarthritic synovial fluid, fetal
lungs and adult skin after stimulation with YKL-40 is similar to
that when stimulated by insulin-like growth factor 1(32). The expression of YKL-40 was found in
a variety of tumors and cell lines derived from different types of
tumors, such as those of the bone, brain, breast and lung (33). YKL-40 may play a role in the
regulation of the Ras/mitogen-activated protein kinase (MAPK)
pathway, which is one of the most extensively investigated signal
transduction pathways related to mitogenesis and the conduction of
anti-apoptotic and mitogenic signals. YKL-40 has also been linked
to MAPK and phosphoinositide 3-kinase (PI-3K) signaling cascade in
fibroblasts, which leads to the phosphorylation of extracellular
signal-regulated kinase-1/2 and Akt-mediated signaling cascades
that are associated with mitogenesis and cell survival.
Dysregulated activation of these signaling pathways leads to
proliferative and antiapoptotic responses that are implicated in
the development of different types of cancer (34). YKL-40 can therefore play a direct or
indirect role in the development of leukemia, as it has been
reported that activation of the PI-3K/Akt pathway is associated
with poor prognosis and drug resistance in pediatric patients with
ALL. Inhibition of the PI-3K/Akt pathway leads to a decrease in
cell proliferation in chronic lymphoblastic leukemia and AML
(35,36). High expression of YKL-40 in patients
with leukemia may promote disease progression (20). It has been suggested that serum or
plasma levels of YKL-40 may reflect certain aspects of tumor growth
and cell proliferation and may be used as a biomarker for
monitoring cancer patients during and after treatment (13).
The results of the present study are consistent with
those published in 2013 by Hurmale et al (20), where they analyzed the plasma levels
of YKL-40 in adult patients with leukemia, and observed that the
plasma levels of YKL-40 were elevated in patients with leukemia
compared with those in healthy individuals. However, in that study,
the mean YKL-40 plasma levels in patients with leukemia was 168
ng/ml, which was quite different from that obtained in the present
study, which was 59.7 ng/ml. This may be due to the age of the
patients included in our study (mean age, 8.7±4.9 years). YKL-40
levels increase with age and may be associated with lifestyle
changes (37-39).
YKL-40 plasma levels in both sexes are highly correlated with age,
which is therefore an important factor to consider when conducting
clinical studies on the role of YKL-40 in the prognosis of patients
with cancer (40).
Several limitations are important to consider in the
present study. This study had a small sample size, which was not
sufficient for validation of the plasma levels of YKL-40 as a
prognostic biomarker. It is necessary to increase the number of
samples and investigate the specific role of YKL-40 in
hematological malignancies. It was herein demonstrated that high
plasma levels of YKL-40 are associated with poor prognosis in
children with ALL. Therefore, YKL-40 may be a new useful biomarker
for actual risk classification in the prognosis of patients with
ALL, in Mexico as well as globally.
The findings of the present study provide evidence
that high plasma YKL-40 levels, age and CNS invasion are associated
with shortened survival in children with ALL. High plasma YKL-40
levels were also found to be correlated with poor prognostic
factors, such as age, CNS invasion and high initial leukocyte
count. To the best of our knowledge, this is the first study to
elucidate the role of YKL-40 plasma levels in the prognosis of
children with ALL; however, additional studies with larger cohorts
are required to verify the role of YKL-40 in this type of cancer.
Elevated YKL-40 protein levels may serve as a marker of poor
prognosis and shorter survival in children with this hematological
malignancy. However, our data may generate novel hypotheses
regarding the effect of YKL-40 levels on the survival of patients
with ALL, which will have to be confirmed in independent studies.
In addition, in vitro studies are necessary to further
elucidate the role of YKL-40 in ALL.
Acknowledgements
AARA was the recipient of a PhD scholarship from
CONACYT (CVU/Becario: 2366757/211722). This manuscript is part of
the doctoral dissertation project of AARA a student of the Programa
de Doctorado en Ciencias Biomédicas, Facultad de Ciencia Químico
Bilógicas, Universidad Autónoma de Guerrero (UAGro), Chilpancingo,
Guerrero, México. The authors wish to thank the State Cancer
Institute ‘Dr. Arturo Beltran Ortega’ for the support provided.
Funding
The present study was supported by a grant from CONACYT, México
(Fondo Sectorial de Investigación en Salud y Seguridad Social,
FSSS01-C-2018-1; grant no. A3-S-47392). This study was also
supported by Universidad Autónoma de Guerrero.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AARA conducted the experiments, analyzed the data
and wrote the paper. YGG, JON and MALV analyzed the data and wrote
the paper. MALV and BIA contributed the reagents and materials.
EIS, MVSH, MAJL and ABRR provided the biological samples and
clinical data of the patients. AARA, MALV, YGG and JON are
responsible for and can confirm the authenticity of the raw data.
All the authors have read and approved the final manuscript.
Ethics approval and consent to
participate
This study was performed with the approval of the
Ethics Committee of the State Cancer Institute of the State of
Guerrero. The parents or guardians of all the subjects signed
informed consent forms. All procedures followed were in accordance
with the ethical standards of the responsible committees on human
experimentation (institutional and national) and with the
principles outlined in the Helsinki Declaration.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Centro Nacional para la Salud de la
Infancia y la Adolescencia. (CENSIA): Comportamiento epidemiológico
del cáncer en menores de 18 años. México 2008-2014. Secretaria de
Salud, 2014. http://censia.salud.gob.mx/contenidos/descargas/cancer/20160601_Boletin2014_SEDP12sep16_4.pdf.
|
|
2
|
Smith MA, Seibel NL, Altekruse SF, Ries
LA, Melbert DL, O'Leary M, Smith FO and Reamen GH: Outcomes for
children and adolescents with cancer: Challenges for the
twenty-first century. Int J Clin Oncol. 28:2625–2634.
2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rendón-Macías ME, Reyes-Zepeda NC,
Villasís-Keever MÁ, Meneses SJ and Núñez AE: Tendencia mundial de
la supervivencia en pacientes pediátricos con leucemia
linfoblástica aguda. Revisión de las últimas cuatro décadas. Bol
Med Hosp Infant Mex. 69:153–163. 2012.
|
|
4
|
Layton-Tovar C: Factores de pronóstico en
leucemia linfoblástica aguda pediátrica: Posibles marcadores
moleculares. Rev Med Investig. 3:85–91. 2015.
|
|
5
|
Terwilliger T and Abdul-Hay M: Acute
lymphoblastic leukemia: A comprehensive review and update. Blood
Cancer J. 7(e577)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Johansen JS, Hoyer PE, Larsen LA, Price PA
and Møllgård K: YKL-40 protein expression in the early developing
human musculoskeletal system. J Histochem Cytochem. 55:1213–1228.
2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Johansen JS, Jensen BV, Roslind A, Nielsen
D and Price P: Serum YKL-40, a new prognostic biomarker in cancer
patients? Cancer Epidemiol Biomarkers Prev. 15:194–202.
2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Prakash M, Bodas M, Prakash D, Nawani N,
Khetmalas M, Mandal A and Eriksson C: Diverse pathological
implications of YKL-40: Answers may lie in ‘outside-in’ signaling.
Cell Signal. 25:1567–1573. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chen HT, Zheng JM, Zhang YZ, Yang M, Wang
YL, Man XH, Chen Y, Cai QC and Li ZS: Overexpression of YKL-40
predicts poor prognosis in patients undergoing curative resection
of pancreatic cancer. J Pancreas. 46:323–334. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kang EJ, Jung H, Woo OH, Park KH, Woo SU,
Yang DS, Kim AR, Lee JB, Kim YH, Kim JS and Seo JH: YKL-40
expression could be a poor prognostic marker in the breast cancer
tissue. Tumor Biol. 35:277–286. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Steponaitis G, Skiriutė D, Kazlauskas A,
Golubickaitė I, Stakaitis R, Tamašauskas A and Vaitkienė P: High
CHI3L1 expression is associated with glioma patient survival. Diagn
Pathol. 11(42)2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Erturk K, Tas F, Serilmez M, Bilgin E and
Yasasever V: Clinical significance of serum Ykl-40
(chitinase-3-like-1 protein) as a biomarker in melanoma: An
analysis of 112 Turkish patients. Asian Pac J Cancer Prev.
18:1383–1387. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Schultz NA and Johansen JS: YKL-40-A
protein in the field of translational medicine: A role as a
biomarker in cancer patients? Cancers (Basel). 2:1453–1491.
2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Volck B, Johansen JS, Stoltenberg M,
Garbarsch C, Price PA, Ostergaard M, Ostergaard K, Løvgreen-Nielsen
P, Sonne-Holm S and Lorenzen I: Studies on YKL-40 in knee joints of
patients with rheumatoid arthritis and osteoarthritis. Involvement
of YKL-40 in the joint pathology. Osteoarthritis Cartilage.
9:203–214. 2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chupp GL, Lee CG, Jarjour N, Shim YM, Holm
CT, He S, Dziura JD, Reed J, Coyle AJ, Kiener P, et al: A
chitinase-like protein in the lung and circulation of patients with
severe asthma. N Engl J Med. 357:2016–2027. 2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Letuve S, Kozhich A, Arouche N,
Grandsaigne M, Reed J, Dombret MC, Kiener PA, Aubier M, Coyle AJ
and Pretolani M: YKL-40 is elevated in patients with chronic
obstructive pulmonary disease and activates alveolar macrophages. J
Immunol. 181:5167–5173. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Boot RG, Van Achterberg TA, Van Aken BE,
Renkema GH, Jacobs MJ, Aerts JM and de Vries CJ: Strong induction
of members of the chitinase family of proteins in atherosclerosis.
Chitotriosidase and human cartilage gp-39 expressed in lesion
macrophages. Arterioscler Thromb Vasc Biol. 19:687–694.
1999.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rathcke CN, Johansen JS and Vestergaard H:
YKL-40, a biomarker of inflammation, is elevated in patients with
type 2 diabetes and is related to insulin resistance. Inflamm Re.
55:53–59. 2006.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bergmann OJ, Johansen JS, Klausen TW,
Mylin AK, Kristensen JS, Kjeldsen E and Johnsen HE: High serum
concentration of YKL-40 is associated with short survival in
patients with acute myeloid leukemia. Clin Can Res. 11:8644–8652.
2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hurmale AK, Choudhary SK, Bhaskar SK,
Jatawa SK, Yadav M and Tiwari A: Overexpression of chitinase like
protein YKL-40 in leukemia patients. J BioSci Biotech. 2:215–220.
2013.
|
|
21
|
Organista-Nava J, Gómez-Gómez Y,
Illades-Aguiar B, Del Carmen Alarcón-Romero L, Saavedra-Herrera MV,
Rivera-Ramírez AB, Garzón-Barrientos VH and Leyva-Vázquez MA: High
miR-24 expression is associated with risk of relapse and poor
survival in acute leukemia. Oncol Rep. 33:1639–1649.
2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
US Food, Drug Administration: Clinical
trial endpoints for the approval of cancer drugs and biologics:
Guidance for industry. https://www.fda.gov/media/71195/download. Published
December 2018. Accessed August 14, 2019.
|
|
23
|
Wang D, Zhai B, Hu F, Liu C, Zhao J and Xu
J: High YKL-40 serum concentration is correlated with prognosis of
Chinese patients with breast cancer. PLoS One.
7(e51127)2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Thongsom S, Chaocharoen W, Silsirivanit A,
Wongkham S, Sripa B, Choe H, Suginta W and Talabnin C:
YKL-40/chitinase-3-like protein 1 is associated with poor prognosis
and promotes cell growth and migration of cholangiocarcinoma. Tumor
Biol. 37:9451–9463. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Xu CH, Yu LK and Hao KK: Serum YKL-40
level is associated with the chemotherapy response and prognosis of
patients with small cell lung cancer. PLoS One.
9(e96384)2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kotowicz B, Fuksiewicz M, Jonska-Gmyrek J,
Wagrodzki M and Kowalska M: Preoperative serum levels of YKL 40 and
CA125 as a prognostic indicators in patients with endometrial
cancer. Eur J Obstet Gynecol Reprod Biol. 215:141–147.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhang JP, Yuan HX, Kong WT, Liu Y, Lin ZM,
Wangs WP and Guo JM: Increased expression of Chitinase 3-like 1 and
microvessel density predicts metastasis and poor prognosis in clear
cell renal cell carcinoma. Tumour Biol. 35:12131–12137.
2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ringsholt M, Hogdall EV, Johansen JS,
Price PA and Christensen LH: YKL-40 protein expression in normal
adult human tissues-an immunohistochemical study. J Mol Histol.
38:33–43. 2007.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Borowitz MJ, Devidas M, Hunger SP, Bowman
WP, Carroll AJ, Carroll WL, Linda S, Martin PL, Pullen DJ,
Viswanatha D, et al: Clinical significance of minimal residual
disease in childhood acute lymphoblastic leukemia and its
relationship to other prognostic factors: A children's oncology
groups study. Blood. 111:5477–5485. 2008.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lenk L, Alsadeq A and Schewe DM:
Involvement of the central nervous system in acute lymphoblastic
leukemia: Opinions on molecular mechanisms and clinical
implications based on recent data. Cancer Metastasis Rev.
39:173–187. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Shao R, Hamel K, Petersen L, Cao QJ,
Arenas RB, Bigelow C, Bentley B and Yan W: YKL-40, a secreted
glycoprotein, promotes tumor angiogenesis. Oncogene. 28:4456–4468.
2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Recklies AD, White C and Ling H: The
chitinase 3-like protein human cartilage glycoprotein 39 (HC-gp39)
stimulates proliferation of human connective-tissue cells and
activates both extracellular signal regulated kinase- and protein
kinase B-mediated signalling pathways. Biochem J. 365:119–126.
2002.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Johansen JS, Drivsholm L, Price PA and
Christensen IJ: High serum YKL-40 level in patients with small cell
lung cancer is related to early death. Lung Cancer. 46:333–340.
2004.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sanchez VE, Nichols C, Kim HN, Gang EJ and
Kim YM: Targeting PI3K signaling in acute lymphoblastic leukemia.
Int J Mol Sci. 20(412)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hoellenriegel J, Meadows SA, Sivina M,
Wierda WG, Kantarjian H, Keating MJ, Giese N, O'Brien S, Yu A,
Miller LL, et al: The phosphoinositide 3'-kinase delta inhibitor,
CAL-101, inhibits B-cell receptor signaling and chemokine networks
in chronic lymphocytic leukemia. Blood. 118:3603–3612.
2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Billottet C, Grandage VL, Gale RE,
Quattropani A, Rommel C, Vanhaesebroeck B and Khwaja A: A selective
inhibitor of the p110delta isoform of PI 3-kinase inhibits AML cell
proliferation and survival and increases the cytotoxic effects of
VP16. Oncogene. 25:6648–6659. 2006.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bojesen SE, Johansen JS and Nordestgaard
BG: Plasma YKL-40 levels in healthy subjects from the general
population. Clin Chim Acta. 412:709–712. 2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Johansen JS: Studies on serum YKL-40 as a
biomarker in diseases with inflammation, tissue remodelling,
fibroses and cancer. Dan Med Bull. 53:172–209. 2006.PubMed/NCBI
|
|
39
|
Recklies AD, Ling H, White C and Bernier
SM: Inflammatory cytokines induce production of CHI3L1 by articular
chondrocytes. J Biol Chem. 280:41213–41221. 2005.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Johansen JS, Lottenburger T, Nielsen HJ,
Jensen JE, Svendsen MN, Kollerup G and Christensen IJ: Diurnal,
weekly, and long-time variation in serum concentrations of YKL-40
in healthy subjects. Cancer Epidemiol Biomarkers Prev.
17:2603–2608. 2008.PubMed/NCBI View Article : Google Scholar
|