Introduction
Pituitary apoplexy (PA) is a relatively rare
syndrome with characteristic features; early diagnosis and
treatment can be crucial in saving a patient's vision and life.
This condition is caused by sudden and extensive bleeding into a
tumor of the anterior lobe of the pituitary gland, which is the
cause or consequence of acute necrosis (1). PA is rare, with an estimated
prevalence of ~ 6.2 cases per 100,000 inhabitants (2).
The clinical syndrome of PA is well known. The
pathological-anatomical finding of pituitary adenoma infarction was
described in sudden and unexpected deaths by Brougham et al
in 1950 and designated PA. It was believed that the cause of
necrosis and bleeding is the rapid growth of the tumor, which
exceeds the capacity of the blood supply. Swollen tumor masses
further compress blood vessels and worsen ischemia (3).
The complete clinical findings are characterized by
the following: i) Sudden-onset severe headache, mostly at the front
of the head and behind the eyes, progressing to coma and often
meningeal symptoms. ii) Rapid development of visual impairment,
often bilateral amaurosis. iii) Extraocular muscle palsies-often
bilateral total ophthalmoplegia and innervation disorders in the
trigeminal area. iv) Cerebrospinal fluid findings such as
xanthochromia, pleocytosis, erythrocytes, and increased protein
levels.
However, the above list may be incomplete, and
symptoms may be laterally asymmetric. Acute expansion of the
necrotic and hemorrhagic adenoma is directed to the sides and
compresses the structures of the cavernous sinus, as well as
upwards through the diaphragm sellae, and affects the optic chiasm
and hypothalamic centers on the floor of the third ventricle
(1).
The cause of PA is not always adenoma. Cases of
nonadenomatous lesions, including hypophysitis (4,5),
metastasis to the pituitary gland [especially renal cell carcinoma
(6)], craniopharyngioma, Rathke's
cleft cyst (7,8) and sellar hemangioblastoma, have been
described (9).
The precise pathophysiology is not completely
understood. A proposed hypothesis involves tumor vascular occlusion
due to tumor growth, tumor blood flux reduction, and abnormal tumor
(immature) vascularization. VEGF mRNA levels may be increased in
pituitary tumors, especially in nonfunctioning pituitary adenomas,
which could be related to abnormal vascularization (10).
As the usual trigger for angiogenesis is not
present, vasculopathy may occur in apoplectic tumors. Four
categories of triggering factors have been suggested: i) Vascular
flux reduction: From surgery, especially cardiac surgery,
radiotherapy, and postspinal anesthesia. ii) Acute increases in
blood flow: From physical activity and systemic hypertension. iii)
Pituitary stimulation: Provocative pituitary tests, especially from
TRH and GnRH analog use. iv) Coagulation disturbances: From
thrombocytopenia and anticoagulation.
A more detailed analysis of 42 studies on possible
inducing factors in PA is given in the summary study of Capatina
et al (11).
The same authors report ophthalmologic abnormalities
in 85% of cases (rapid decrease in visual acuity in 39-56% of
cases, unilateral or bilateral blindness exceeding 39% of cases,
visual field changes in 36-71% of cases, and oculomotor disorders
in 40-78% of cases).
Similar results were reported by other authors who
analyzed eight studies in patients with PA. They reported changes
in visual fields in 33.3-82% of cases and ophthalmoplegia in
36.8-83% of cases (12).
In all of the abovementioned studies, the chiasm was
compressed with a hemorrhagic tumor. Although our patient did not
show a similar compression of the visual pathway, there were still
visual impairments, which we would like to present in this case
report.
Case report
In March 2019, a patient (born in 1975) with a
history of renal colic experienced headache and pain in the right
half of the face, including teeth predominantly on the right side.
He went to sleep and, after waking up, was disoriented. He
experienced blurred vision of the right eye and restriction of the
visual field in the upper periphery. He sought out an
ophthalmologist who removed a foreign body from his cornea. Any
other ocular abnormalities were not detected. In July 2019, he was
examined at an eye clinic for continued decreased vision in his
right eye. The visual acuity of the right eye was 0.3 naturally,
0.7 with astigmatism correction -1.5 D cyl. ax. 80˚, and the visual
acuity of the left eye was 0.7 naturally, 1.0 with astigmatism
correction +0.75 D cyl. ax. 90˚. Ocular findings, including
perimetric examination, were normal. There was a small nubecula in
the paracentral area of the right cornea.
In August 2019, the visual acuity with the same
correction was 0.9 in the right eye and 1.0 in the left eye during
follow-up examination. Ocular findings, including visual field
tests, were normal. Optical coherence tomography (OCT) also showed
normal RNFL thickness.
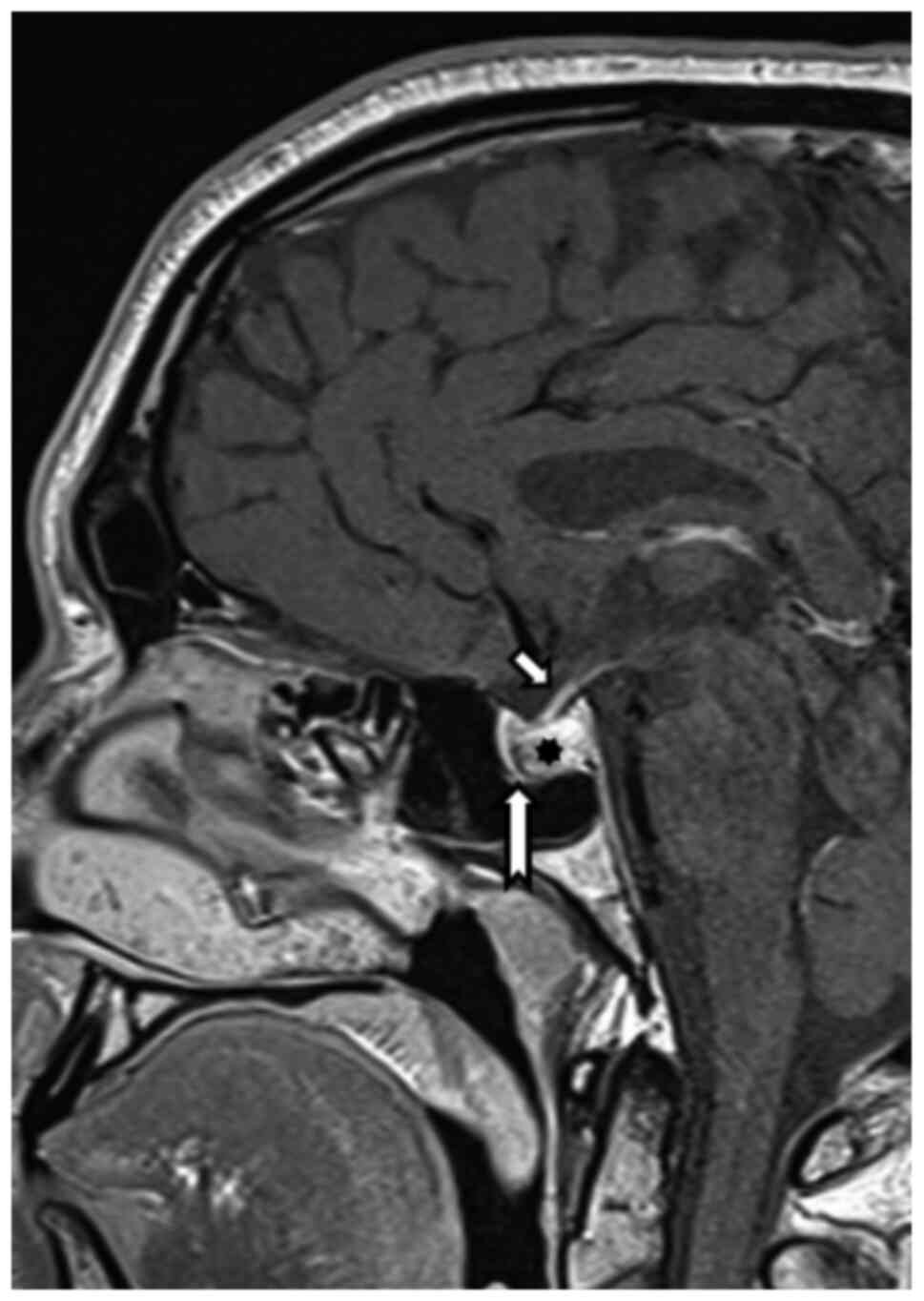
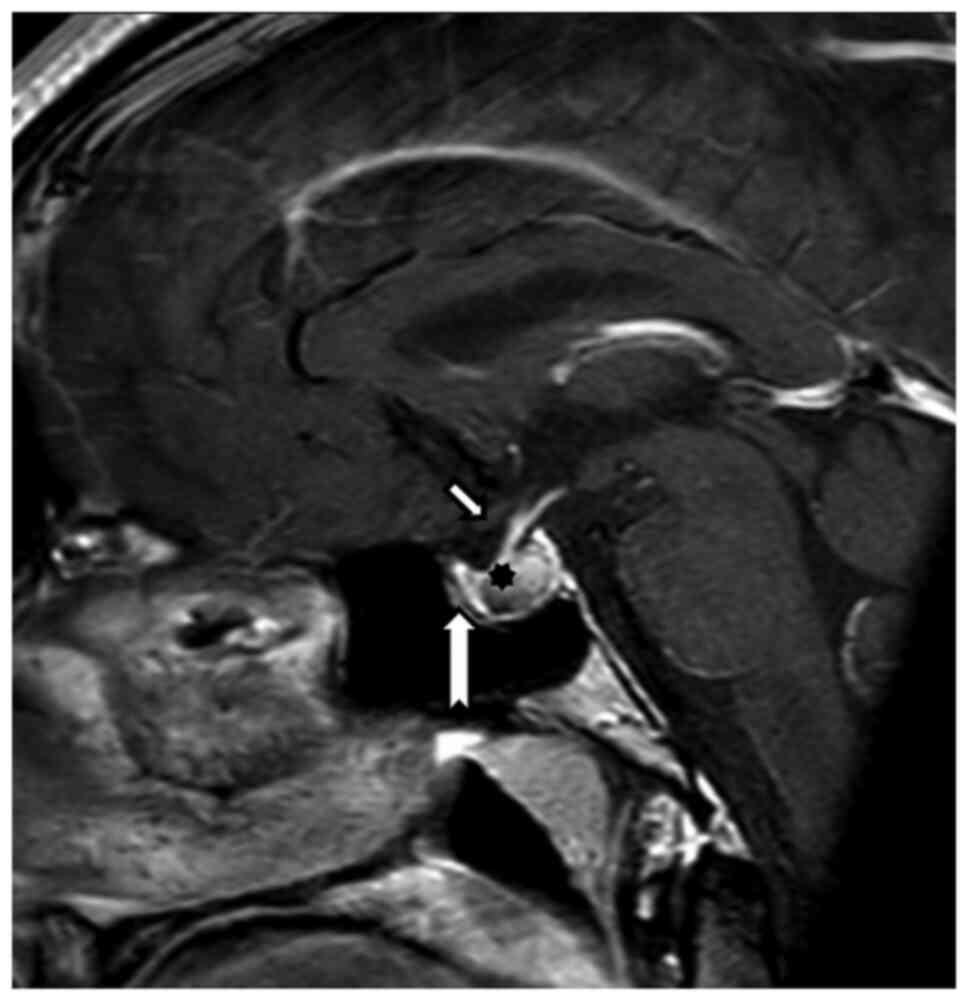
Because of the reduced visual acuity, MRI
examination was recommended, which revealed (August 2019) expansion
of the Turkish saddle with hemorrhage, with probable benign
etiology. The pituitary gland itself was pushed away ventrally
(Fig. 1).
Endocrinological examination was recommended for
abnormalities in Turkish saddles. In September 2019, the patient
was examined endocrinologically for the first time. A pituitary
adenoma measuring 12x14x16 mm was found during MRI examination.
From the patient's own account, he had experienced occasional
headaches and erectile dysfunction that had slowly worsened over
the prior 12 years, without further symptomatology. Physical
findings were normal, and gynecomastia was not present. The
laboratory results showed a moderate elevation of prolactin (4983
mIU/l) and low testosterone (5.6 nmol/l) with low LH (0.9 mIU/l),
indicative of central hypogonadism; the other pituitary axes were
normal in function. A secondary laboratory finding in the patient
was prolonged aPTT, and further investigation showed mild
coagulation factor XII deficiency (35%). This deficiency does not
cause bleeding; in contrast, more severe deficits may increase the
risk of thrombosis. Only preventive anticoagulant treatment at the
time of reduced mobility after possible surgery was recommended by
the hematologist.
Treatment with cabergoline was initiated at a dose
of 0.25 mg once a week with slow, gradual titration (to a maximum
dose of 0.5 mg per day). During the next follow-up visit in
November 2019, the prolactin level had decreased to 416 mg/l, and
adenoma size was reduced according to MRI examination. The patient
was also free of headaches. At another follow-up in January 2020,
the patient reported a complete remission of erectile dysfunction;
the patient's prolactin level was normal (158 mIU/l), correction of
central hypogonadism was evident in the laboratory results
(testosterone 10.8 nmol/l), and the other pituitary axes remained
normal.
During follow-up visits in March 2020 and May 2020,
normal laboratory and clinical findings were present, except for
the reported visual impairment. Cabergoline treatment was reduced
to 0.5 mg 5 times a week, and in the follow-up visit in September
2020, the dosage was further reduced to 0.5 mg 3 times a week. The
physiological hormonal profile demonstrated suppression of
prolactin levels.
Thus, from an endocrinological point of view, the
finding can be concluded as a macroprolactinoma without compression
symptoms of other pituitary axes, with a good clinical, laboratory
and graphical response to the usual cabergoline treatment, which
does not correspond to the progression of visual field defects.
The control MRI examination in 11/2019 showed a
reduction in cystic expansion in the Turkish saddle; the other
findings did not show significant changes.
Another ophthalmologic examination occurred in
12/2019. Subjectively, the patient complained of decreased visual
acuity of the right eye, occasional periocular pain, and headaches
but denied other problems.
Right eye VA: 0.4 s=1.5 cyl. ax. 82 st. Left eye VA:
1.0 s -0.75 cyl. ax. 90. Intraocular pressure (IOP): 13/15
mmHg.
The ocular findings were normal except inactive
nubecula on the cornea of the right eye after foreign body removal.
Perimetric examination showed complete temporal hemianopsia on the
right, while examination of the left eye was normal. RNFL remained,
without signs of progression.
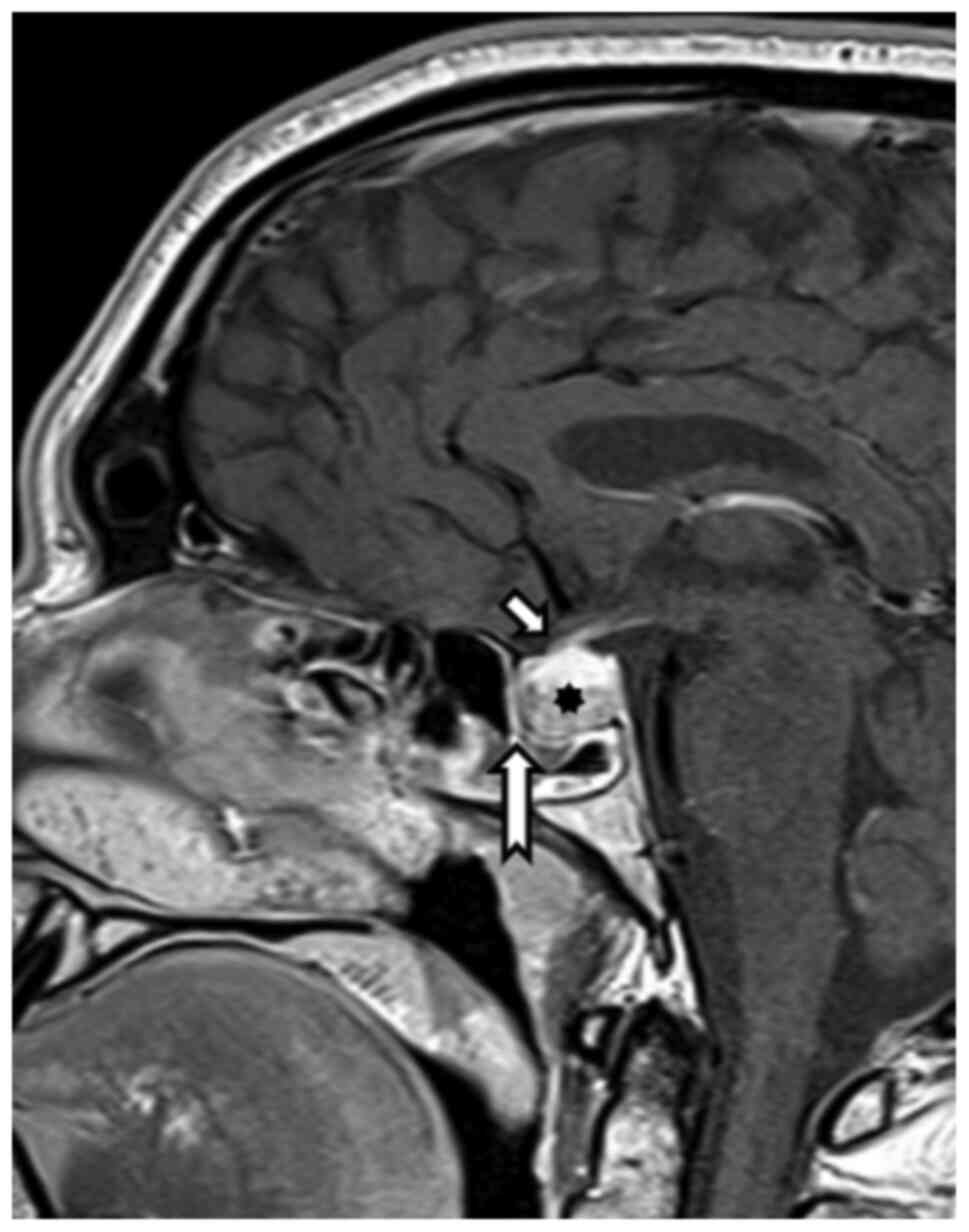
Magnetic resonance imaging from 12/2020 showed a
regression of pituitary expansion. There was a clearly defined
distance of the chiasm and cranial contour of the pituitary gland
and a common location of the infundibulum (Fig. 2).
The eye examination in 1/2020 showed no changes
compared with the examination in 12/2019. When examined in 2/2020,
the VA of the right eye had decreased to 0.3 with correction. Other
findings showed no evidence of progression. One month after this
examination, there was a further decrease in VA of the right eye to
0.15, and correction did not improve the patient's visual acuity.
Perimetric examination showed changes in the left visual field,
while there were no signs of progression on the right. For
unexplained changes in the visual fields, the examination was
supplemented by brain computed tomography angiography. Even this
examination did not show vascular abnormalities. The ocular
examination in 6/2020 remained unchanged from the previous
examination.
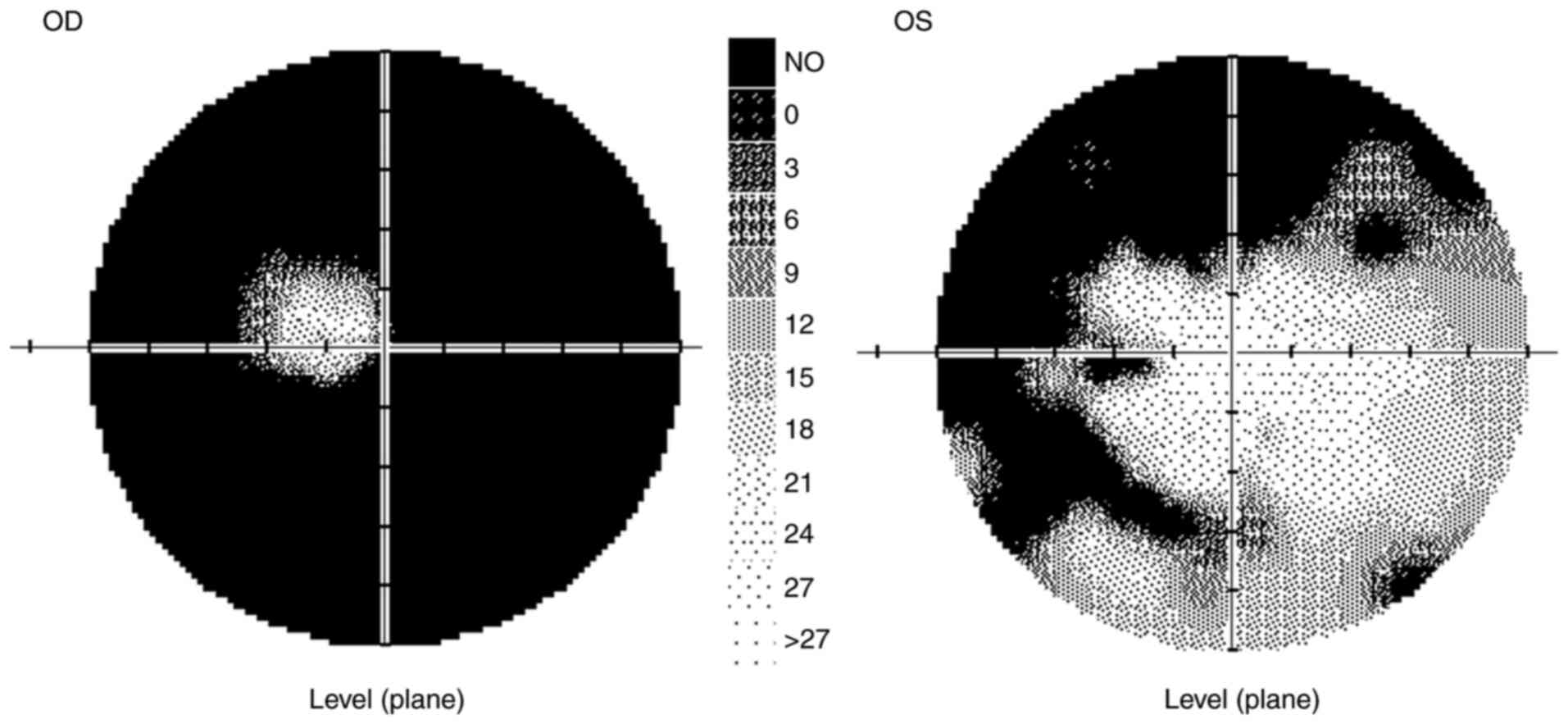
In 9/2020, the VA of the right eye was partially
0.12, with no improvement in visual acuity with correction, and the
VA of the left eye was naturally 1.0. The ocular findings,
including of the ocular fundus, were normal. The IOP was 10/11
mmHg. The patient's color vision was normal. The peripapillary
vessel density (Avanti RTVue XR from Optovue), as in the entire
image, was normal in both eyes, as was the RNFL. Visual field
testing revealed abnormalities in both eyes (Fig. 3).
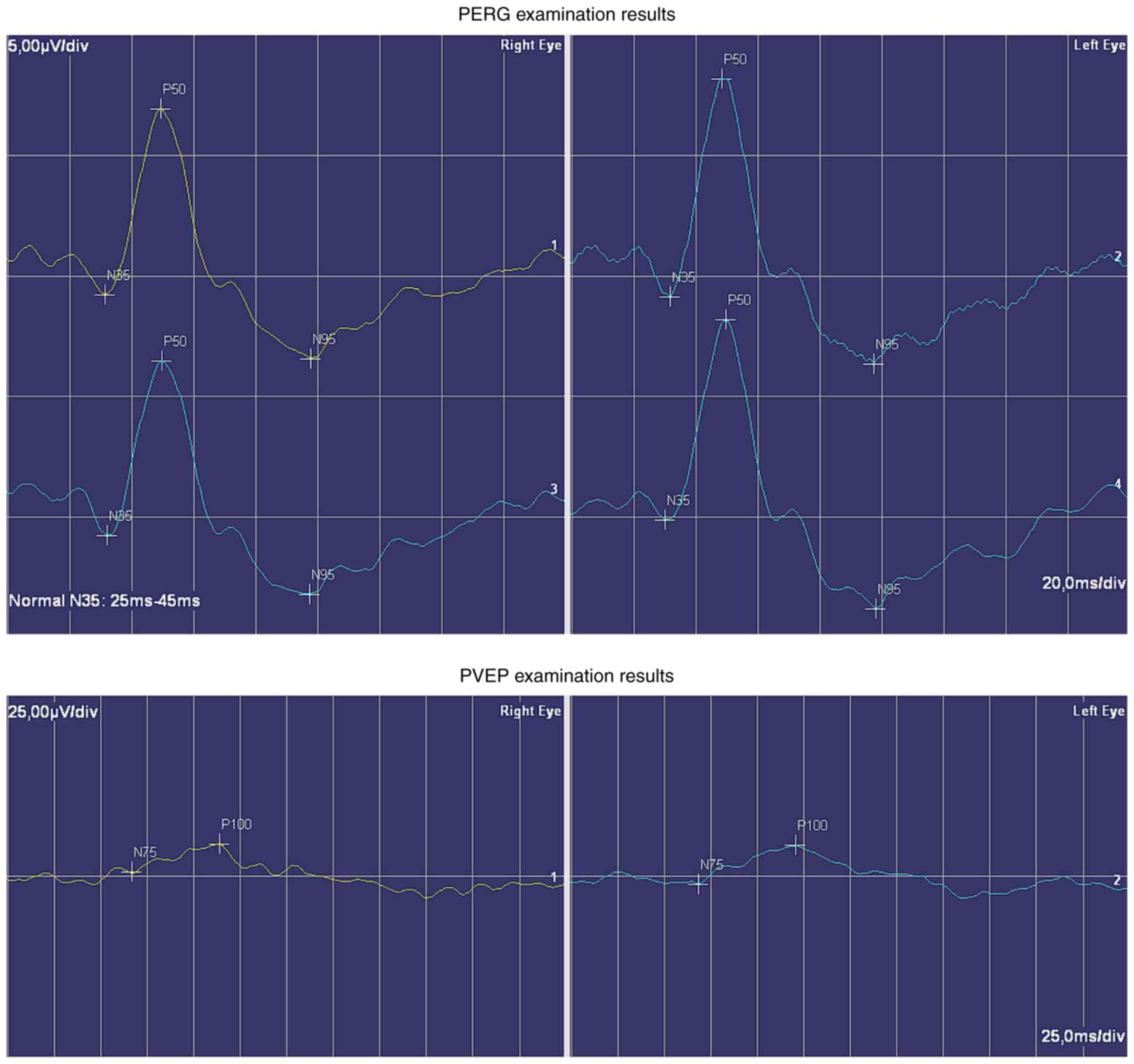
The Retiscan electrophysiological examination
(Roland Consult), performed according to the ISCEV methodology,
revealed a bilateral normal response on the bottom regulation
border in the pattern electroretinogram (P50-N95: 10.4 µV, resp. 12
µV). The latency of N95 was not prolonged. Larger square visual
evoked responses proved a distinctive amplitude decrease. Smaller
square stimulation resulted in a similar decision (4.5 vs. 4.3 µV).
The latency of P100 was not prolonged (Fig. 4).
Structural MRI of the hypophysis and orbits was
carried out on a 3T Achieva dStream TX SERIES (Philips HealthCare,
Best) with a 32-channel SENSE RF head coil. The hypophysis was
examined according to the protocol, with intravenous application of
10 ml of gadolinium contrast substance. MRI of the hypophysis
employed a T1 TSE sagittal sequence, with 2-mm slice thickness, gap
0, TR 500, TE 10 native and after intravenous contrast substance
application (Fig. 5). The targeted
orbital examination employed an sT1 3D TFE sequence, with
1-mm-slice thickness, gap 0, TR 6.7, TE 3.1, primarily in the
sagittal plane, T2 TSE mDIXON, 2.5-mm-slice thickness, gap 0.3, TR
3000, TE 80, in the coronal plane and targeted orbital sequences on
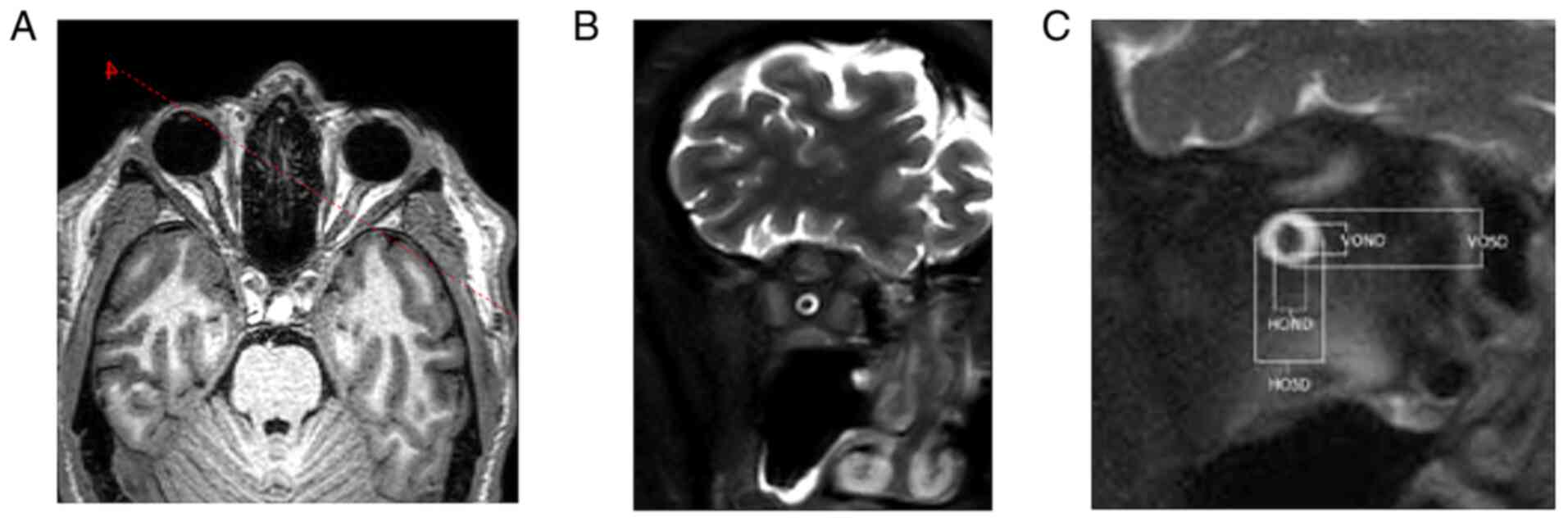
both sides, T2 SPIR SSh, 3-mm-slice thickness, gap 0.3, TR 9520.2,
TE 120.0. Optic nerve measurements were analyzed on IntelliSpace
Portal working station version 10-1 (Philips Medical Systems).
Coronal T2 SPIR SSh sequences were planned in the axial plane
upright to the optical nerve in 4-8-16-20-mm intervals behind the
dorsal eye contour for both sides. The measurement included the
optic nerve's largest outer diameters in two perpendicular axes
horizontally (ONDH) and vertically (ONDV), and optic nerve sheath
diameters in two perpendicular axes horizontally (OSDH) and
vertically (OSDV) in both previously mentioned intervals separately
(Fig. 6 and Table I). The optic nerve chiasma was
detected in the coronal plane in the T1 TFE 3D sequence at the
point of its narrowest range, and the outer diameter in the
horizontal plane was measured. The Parks et al algorithm was
used to distinguish PA from craniopharyngioma (13).
 | Table IOND and OSD values at distances of 4,
8, 16 and 20 mm from the eyeball in the V and H plane. |
Table I
OND and OSD values at distances of 4,
8, 16 and 20 mm from the eyeball in the V and H plane.
| | Right eye, mm | Left eye, mm |
|---|
| Distance from
eyeball | OND | OSD | OND | OSD |
|---|
| V4 | 3.0 | 6.8 | 2.8 | 6.7 |
| H4 | 2.8 | 7.0 | 3.1 | 7.0 |
| V8 | 2.9 | 6.3 | 3.1 | 6.3 |
| H8 | 3.0 | 7.4 | 3.4 | 7.1 |
| V16 | 2.5 | 5.0 | 2.2 | 4.4 |
| H16 | 2.9 | 5.8 | 2.4 | 5.1 |
| V20 | 2.8 | 5.4 | 2.4 | 5.6 |
| H20 | 2.7 | 5.4 | 2.3 | 5.3 |
Functional magnetic resonance imaging (fMRI) was
carried out on a Philips Achieva TX SERIES system with a magnetic
field strength of 3 Tesla. A 6-channel and later 32-channel SENSE
RF head coil were used for scanning. Optical stimulation during
fMRI measurements was performed by inverse alternation of a black
and white checkerboard with a frequency of 2 Hz. Both measurements
(with separate stimulation of the left and right eyes) consisted of
five periods of stimulation (duration 30 sec) that alternated with
five resting periods of the same length when the crosshair was
projected into the visual field. Every measurement included 100
dynamic scans of EPI gradient echo sequences with the following
basic parameters: TR=3 sec, TE=30 msec, and spatial resolution
2x2x2 mm3.
Evaluation of fMRI was performed with SPM 12
software using general linear model statistics and standard data
preprocessing (motion correction, spatial normalization and
smoothing with a 4x4x4-mm core). The resulting statistical maps
were thresholded at a significance level of P=0.05 with family-wide
error (FWE) correction for multiple observations. The extent of
activation was then assessed by the number of statistically
significant voxels using a statistical threshold, i.e., voxels
where there are a high probability of activation of brain tissue at
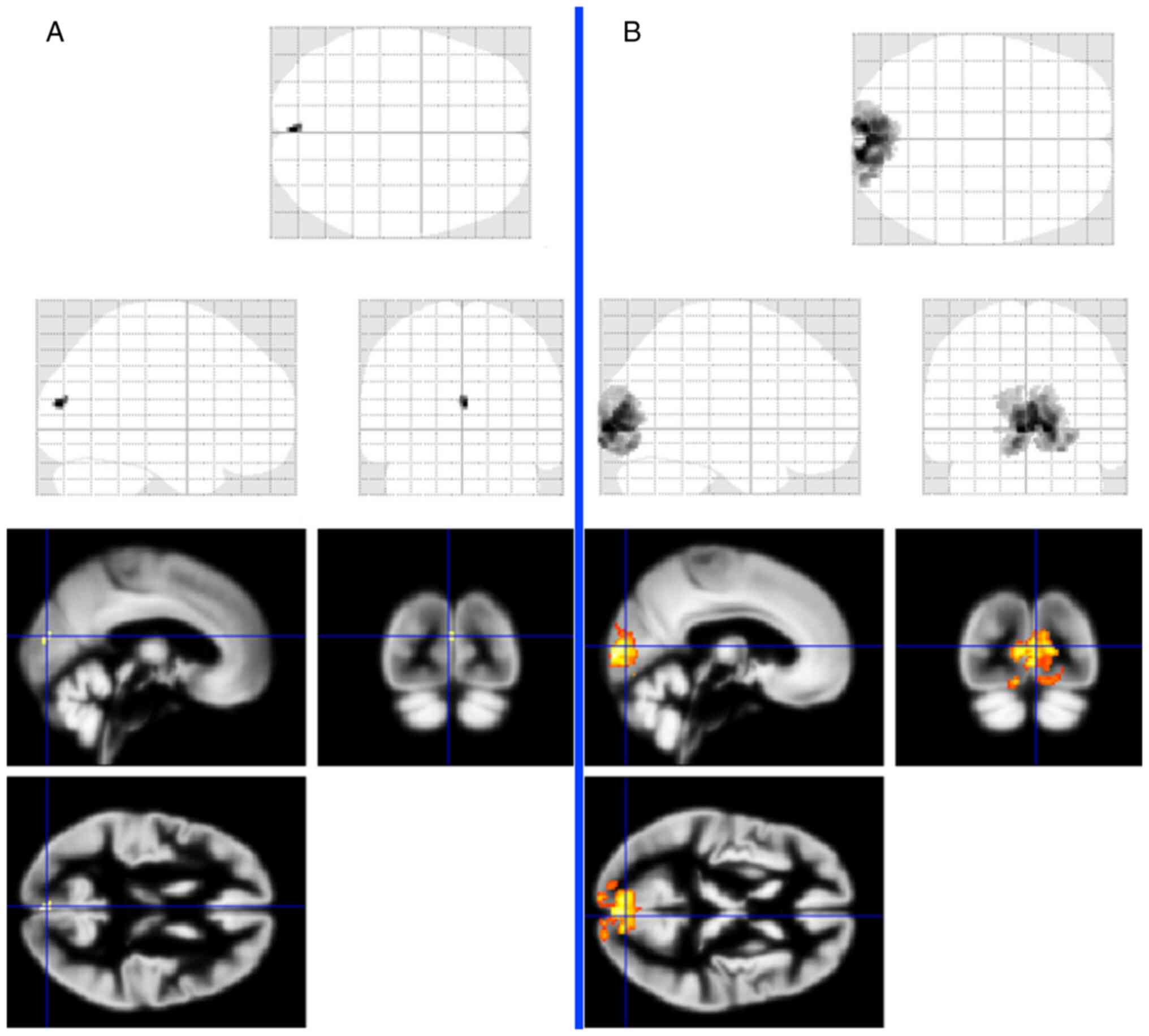
a given stimulation (Fig. 7).
After right eye stimulation, voxel activity was
significantly reduced (20 voxels), and the left eye was normal
(2358 voxels) (14).
Discussion
Our PA observation is noticeable, especially from an
ophthalmological point of view. Chiasmatic syndrome visual field
changes arise from growing hypophyseal compression in an upward
direction. The lower chiasmatic face lies approximately 10-15 mm
above the sellar diaphragm, and this diameter grows in the
ventrodorsal direction (15).
Pituitary upward-growing tumors must distend at
least 10 mm above the diaphragm in order to come into contact with
the chiasm (16). That is,
hypophyseal expansion should reach a volume such that it reaches
the chiasma. In non-MRI scans, it was obvious that the tumor
formation neither exceeded the sellar diaphragm nor contacted the
chiasm. The hypophysis was not in contact with the chiasm,
according to the first perimetric change recognition times. If
chiasm compression was present, we would observe consecutive visual
field change regression. Further explanation of the visual field
changes can be explained by inadequate perfusion in the chiasmatic
region. The chiasmatic area is nourished by the Willis arterial
circle, which extends from the arteria carotis interna. Even though
its arterial hypophyseal branches are superior, anterior and
posterior, arteria chiasmatica lateralis and arteria comunicans
posterior are important for nourishment (15). If there was compression of
hypophyseal arteries, which could have caused PA, it might also
have caused spasms in an area nourishing the touched visual
pathway. There was no alteration in the visual field until nine
months after the first PA attack. It is assumed that if there is
compression and vascular closure caused by growing tumors, this
would cause neighboring artery spasms. Both the hypophysis and
chiasm share nearly the same arterial source. It is still not
logical why the visual field changes appeared nine months after PA.
This could be explained by long-term hypophyseal vascular
compression, first by a prolactinoma and consequential hypophyseal
bleeding. Visual field changes came contemporaneously with the drop
in right eye corrected central visual acquisition from 0.7 (7/2017)
to 0.4 (12/2019). In a case of chronic ischemia, similar to
normotensive glaucoma cases, changes should not occur to either an
affected optic nerve or chiasm.
Our patient had all of the listed parameters of both
optic nerves symmetrically, and there was no chiasmatic extent
alteration of -13.7 mm (17,18).
In a prior case in which the PA attack persisted
during chiasmatic compression, we also detected optic nerve papilla
changes or nerve fiber layer changes more precisely (19-21).
We did not note anything similar in our case report.
There are still standing questions regarding
abnormal functional MRI results by visual paradigm. We detected a
distinctive voxel activity decrease after right eye stimulation,
with regular activity in the right eye (22).
Similar functional MRI changes were found by
Chouinard et al in a 68-year-old woman with hypophyseal
macroadenoma accompanied by visual field changes (23).
It is possible that there was an afferent visual
cortex impairment or that the results are from a lower action
potential quantity coming to the brain. The evidence of this
hypothesis is indicated by the lower right eye retinal ganglion
cell reply (10.4 vs. 12 µV) and the right eye lower visual evoked
potential's larger square amplitude (3.9 vs. 5.4 µV).
Bleeding of a prolactinoma with quickly escalated
dopaminergic agonist therapy is more frequent, and can be the first
tumor manifestation. The general macroprolactinoma bleeding
prevalence is estimated to be approximately 20%, greater than that
of microprolactinomas (3%). There were no signs of chiasmatic
compression in our case report; even with frequent MRI studies,
there was no evidence of bleeding. It is possible that bleeding
occurred prior to the first MRI, which would have caused transient
macroprolactinoma enhancement, visual pathway compression and
consequential spontaneous bleeding resolution. However, as evidence
against this hypothesis, the patient reported no blistering
headaches, which commonly accompany larger bleeding events, and
analysis of the endocrine studies indicated gradual progression.
The presence of the first MRI adenoma bleeding sign would test this
hypothesis (24).
There are some literature case reports mentioning
visual disturbances with delay after prolactinoma therapy, which
were caused by quick tumor reduction and chiasmatic prolapse into
the sella. A previous study reported twelve months of visual
improvement after dopaminergic agonist dose reduction without
resolution of chiasmatic prolapse. Despite the lack of evidence for
optic chiasm prolapse in our case report, we attempted to maximally
reduce the cabergoline dose (25,26).
In conclusion, in this rare case report, chiasm
compression was not demonstrated. The authors hypothesize that the
visual field changes resulted from the chiasmatic area and optic
nerve ischemia, similar to PA.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MKy contributed to study design and management. ZK,
MF and JL contributed to electrophysiology (PVEP and PERG) and
visual field examinations, writing of related ophthalmology parts
of the manuscript and final review of the manuscript. MKy and JT
contributed to the examinations and interpretation of MRI and FMRI
results. MKr contributed to the endocrinology examination results
and writing related parts of the manuscript with an
endocrinological perspective. MS and SR contributed to the
neuropathology examinations, analysis and interpretation. JL and MF
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was performed according to the
Declaration of Helsinki and was approved by the internal ethics
committee of the Ophthalmology Clinic Jana Leštáka (Prague, Czech
Republic).
Patient consent for publication
All details, medical records, figures, medical
history or test results were used with the written consent for
publication from the patient. All data used were anonymized.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Otradovec J and Jindrová M: Pituitary
apoplexy. Cesk Oftalmol. 28:329–333. 1972.PubMed/NCBI(In Czech).
|
|
2
|
Fernandez A, Karavitaki N and Wass JA:
Prevalence of pituitary adenomas: A community-based,
cross-sectional study in Banbury (Oxfordshire, UK). Clin
Endocrinol. 72:377–382. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Brougham M, Heusner AP and Adams RD: Acute
degenerative changes in adenomas of the pituitary body with special
reference to pituitary apoplexy. J Neurosurg. 7:421–439.
1950.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Dan NG, Feiner RI, Houang MT and Turner
JJ: Pituitary apoplexy in association with lymphocytic
hypophysitis. J Clin Neurosci. 9:577–580. 2002.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Husain Q, Zouzias A, Kanumuri VV, Eloy JA
and Liu JK: Idiopathic granulomatous hypophysitis presenting as
pituitary apoplexy. J Clin Neurosc. 21:510–512. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chhiber SS, Bhat AR, Khan SH, Wani MA,
Ramzan AU, Kirmani AR, Malik NK, Wani AA and Rather T: Apoplexy in
sellar metastasis: A case report and review of literature. Turk
Neurosurg. 21:230–234. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chaiban JT, Abdelmannan D, Cohen M, Selman
WR and Arafah BM: Rathke cleft cyst apoplexy: A newly characterized
distinct clinical entity. J Neurosurgery. 114:318–324.
2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Trifanescu R, Ansorge O, Wass JA, Grossman
AB and Karavitaki N: Rathke's cleft cysts. Clin Endocrinol (Oxf).
76:151–160. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Schar RT, Vajtai I, Sahli R and Seiler RW:
Manifestation of a sellar hemangioblastoma due to pituitary
apoplexy: A case report. J Med Case Rep. 5(496)2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Möller-Goede DL, Brändle M, Landau K,
Bernays RL and Schmid C: Pituitary apoplexy: Re-evaluation of risk
factors for bleeding into pituitary adenomas and impact on outcome.
Eur J Endocrinol. 164:37–43. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Capatina C, Inder W, Karavitaki N and Wass
JA: Management of endocrine disease: Pituitary tumour apoplexy. Eur
J Endocrinol. 172:R179–R190. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Glezer A and Bronstein MD: Pituitary
apoplexy: Pathophysiology, diagnosis and management. Arch
Endocrinol Metab. 59:259–264. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Park M, Lee SK, Choi J, Kim SH, Kim SH,
Shin NY, Kim J and Ahn SS: Differentiation between cystic pituitary
adenomas and rathke cleft cysts: A diagnostic model using MRI. Am J
Neuroradiol. 36:1866–1873. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lešták J and Tintěra J: Functional
magnetic resonance imaging in selected eye diseases. Cesk Slov
Oftalmol. 71:127–133. 2015.PubMed/NCBI(In Czech).
|
|
15
|
Otradovec J: Klinická Neurooftalmologie.
GRADA Publishing, Prague, 2003. (In Czech).
|
|
16
|
Slamovits TL and Burde R:
Neuro-ophthalmology. Textbook of Ophthalmology. Vol. 6. Mosby,
1994.
|
|
17
|
Kyncl M, Lestak J, Sverepa M, Ettler L and
Rozsival P: The anterior visual pathway in normal-tension glaucoma.
Papirex-Indian J Res. 4:10–13. 2015.
|
|
18
|
Lešták J, Kyncl M, Fůs M and Marešová K:
Optic chiasm width in normotensive and hypertensive glaucomas. Cesk
Slov Oftalmol. 76:126–128. 2020.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Danesh-Meyer HV, Wong A, Papchenko T,
Matheos K, Stylli S, Nichols A, Frampton C, Daniell M, Savino PJ
and Kaye AH: Optical coherence tomography predicts visual outcome
for pituitary tumours. J Clin Neurosci. 22:1098–1104.
2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yoneoka Y, Hatase T, Watanabe N, Jinguji
S, Okada M, Takagi M and Fujii Y: Early morphological recovery of
the optic chiasm is associated with excellent visual outcome in
patients with compressive chiasmal syndromecaused by pituitary
tumours. Neurol Res. 37:1–8. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Póczoš P, Kremláček J, Česák T, Macháčková
M and Jirásková N: The use of optical coherence tomography in
chiasmal compression. Cesk Slov Oftalmol. 75:120–127.
2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lestak J, Kalvodova B, Karel I and Tintera
J: Functional magnetic resonance imaging following epimacular and
internal limiting membrane peeling-ipsilateral and contralateral
findings. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub.
164:273–276. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chouinard PA, Striemer CL, Ryu WH,
Sperandio I, Goodale MA, Nicolle DA, Rotenberg B and Duggal N:
Retinotopic organization of the visual cortex before and after
decompression of the optic chiasm in a patient with pituitary
macroadenoma. J Neurosurg. 117:218–224. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sarwar KN, Huda MS, Van de Velde V,
Hopkins L, Luck S, Preston R, McGowan BM, Carroll PV and Powrie JK:
The prevalence and natural history of pituitary haemorrhage in
prolactinoma. J Clin Endocrinol Metab. 986:2362–2367.
2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chuman H, Cornblath WT, Trobe JD and
Gebarski SS: Delayed visual loss following pergolide treatment of a
prolactinoma. J Neuroophthalmol. 22:102–106. 2002.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Raverot G, Jacob M, Jouanneau E, Delemer
B, Vighetto A, Pugeat M and Borson-Chazot F: Secondary
deterioration of visual field during cabergoline treatment for
macroprolactinoma. Clin Endocrinol (Oxf). 70:588–592.
2009.PubMed/NCBI View Article : Google Scholar
|