Introduction
Obesity is an emerging and increasingly prevalent
condition in the Western world. It is associated with well-known
metabolic disorders, leading to the development of different
diseases, including cancer (1).
Chronic inflammation, a typical feature of obesity, increases the
imbalance of the tissue microenvironment, promoting the appearance
of the preneoplastic status. In fact, the increase in white adipose
tissue (WAT) affects the development of the disease due to the
release of several adipokines, interleukins and other cytokines by
this type of tissue (2). These
factors cause greater hormonal signaling, increased proliferation
and survival of adjacent and distal cells which could induce
tumorigenesis (3). There is
convincing evidence that excess body weight is associated with an
increased risk for cancer of at least 13 anatomic sites, including
colorectal (4). Thence, the
relationship between body mass index (BMI) and risk of colorectal
cancer (CRC) has been the focus of several investigations (4,5), and
these studies have shown that an increase in BMI is related to a
higher incidence of CRC. However, only few of the publications have
shown poorer results for patients with obesity (6,7) and,
therefore, the relationship between obesity and the prognosis of
CRC has not been widely studied.
As is known, one of the hallmarks in the development
of tumorigenesis is the gain of replicative immortality through the
maintenance of telomere length (8).
Telomeres are a complex of nucleoproteins, which have a fundamental
role in the protection of genomic DNA, and they are shortened with
each cell cycle of replication (9).
Individuals with short telomeres should be at increased risk for
cancer, since short telomeres lead to genomic instability-a
hallmark of cancer. However, individuals with long telomeres also
display an increased risk for major cancers, thus creating a
cancer-telomere length paradox (10). A recent study investigating relative
telomere length in white blood cells provides evidence in support
of longer telomeres being associated with a higher risk of
colorectal cancer, particularly rectal cancer (11). In most cases, shortened telomeres
induce a cell-cycle arrest or trigger apoptosis, although for those
cells that bypass such signals during tumour progression, a
critical length threshold is reached at which telomere dysfunction
may ensue (12). Therefore,
telomere attrition resulting in replicative senescence,
simultaneously by passing cell cycle checkpoints, is a hallmark of
malignant transformation of the cell (13).
Telomerase enzyme is the main responsible for
telomere maintenance (14). The
majority of tumors (80-85%) sustain their capacity to grow
indefinitely through the ectopic expression of telomerase, as shown
in previous studies (15,16). Telomerase is almost ubiquitous in
advanced solid cancers, including CRC, and its expression is
essential for cell immortalization (13).
Many studies have shown that telomere dysfunction,
understood as a critical telomere shortening, has a dual role in
the development of the tumor, since it can act as a tumor
suppressor or an oncogenic factor, depending on the cellular
context (17). Attrition of
telomeres induces the loss of telomere function, which increases
genomic instability and activates regulatory molecules such as p53
that lead to senescence and cell death (18,19).
On the other hand, this genomic instability causes mutations which
could lead to tumorigenesis (20).
In addition, it has been described that shorter telomeres are found
in patients with higher BMI values (21), due to the impact of oxidative stress
and inflammatory processes, suggesting that obesity is related to
shortening of telomeres and potentially promotes colorectal
carcinogenesis (22-24).
Previous reports, in order to help clinicians
optimize their practice, considered crucial to introduce more
effective tools that will improve not only early diagnosis, but
also prediction of the most likely progression of the disease and
response to chemotherapy. In this context, telomere length and
telomerase activity have been included among the promising emerging
biomarkers in CRC monitoring (25).
The exposed data highlight the importance of
studying how obesity influences telomere function and its potential
role as a predictor of prognosis in CRC. We report a multivariate
predictor model for CRC prognosis. The main novelty of the present
work consists of jointly analyzing obesity and telomere status
regarding prognosis of CRC patients submitted to curative intention
surgery treatment.
Materials and methods
Patients and tissue samples
One hundred and sixty-two CRC samples and its paired
non-tumor tissue samples, used as controls, were obtained from
patients who had undergone potentially curative surgery at San
Carlos Hospital in Madrid, Spain, along the last 10 years. Paired
samples of non-tumor tissues located at least 10 cm from the margin
of the tumor were obtained and confirmed microscopically.
After surgical resection, all tissue samples were
instantly frozen in liquid nitrogen and stored at -80˚C until
processed. Cryostat-sectioned, hematoxylin and Eosin (H&E)
stained samples from each tumor block were examined microscopically
by two independent pathologists to confirm the presence of ≥80%
tumor cells. Tumors were pathologically staged according to the
modification of the original staging scheme of Dukes by Turnbull
et al (26). Location of the
tumor, grade of differentiation and other clinical-pathological
features were also recorded.
Cases were collected independently from gender, age
of the patient or tumor stage, and no patient had received previous
chemo- or radiotherapy before diagnosis and inclusion in the study.
Of all patients included in this study, 24 (14,4%) had diabetes, 7
(4,3%) had previously been diagnosed with an autoimmune disorder,
and 26 (16%) showed hyperlipidemia.
The mean follow-up period of the series was 5 years
(range, 1-147 months). Follow-up was defined by the elapsed time
between surgery and either last clinical evaluation of the patient
during the study period or death of the patient. Recurrence was
defined as the appearance, during follow-up, of any local or
distant lesion related to the tumoral process. Disease free
survival (DFS) was assessed from the day of surgery until confirmed
recurrence.
Written informed consent was obtained from patients
prior to investigation. This study was approved by the Ethical
Committee of the Hospital (C.I. 15/180-E FIS, 24/04/2015), assuring
the patients the confidentiality of their data.
Telomere length and telomerase
activity evaluation
The mean telomere length (MTL) values were obtained
by the Terminal Restriction Fragment (TRF) length procedure. TRF
measurement was performed using Telo TTAGGG Telomere Length Assay
kit, cat. no. 12 209 136 001 (Roche Applied Science) as previously
described (9). TRF lengths for
tumor and control tissues were determined by comparing the signals
relative to a standard molecular weight using Image Gauge software
version 3.46 (Fujifilm). The TRF length ratio was determined as the
ratio of the length of tumor tissue TRF and their paired normal
tissue TRF (T/N ratio). Shortening or lengthening of TRFs was
defined if the TRF length of tumor tissues was shorter or longer
than the corresponding non-tumor tissues, respectively.
In colorectal samples, telomerase was measured using
the Telomeric Repeat Amplification Protocol (TRAP)-based telomerase
polymerase chain reaction (PCR)-enzyme-linked immunosorbent assay
(ELISA) kit, cat. no. 11 854 666 910 (Roche Applied Science), which
allowed us to establish a semiquantitative assay (27). Thus, considering that the cut-off
for TRAP-ELISA negativity corresponds to an optical density
OD450 nm <0.2, all samples with OD450 nm
≥0.2 were considered telomerase positive (28).
Statistical analysis
Statistical analyses were performed using the SPSS
software package version 22 (SPSS Inc.). Differences between two or
more groups of study were calculated using parametric tests, in the
case of normality data (ANOVA following by the Bonferroni test for
comparing multiple groups) or non-parametric tests, in the case
that there were no normality data (Kruskal-Wallis and Mann-Whitney
U test). The normality of the data was investigated using the
Kolmogorov-Smirnov test and the homoscedasticity conditions of the
variables used in this study using the Levene's test for equality
of variances. To compare the means of two related variables,
Wilcoxon test was executed. Chi-square test was employed to compare
categorical, and correlations were assessed by Spearman test;
P-values <0.05 were considered statistically significant.
Disease-free survival (DFS) was calculated using the
Kaplan-Meier method and differences were evaluated by the Log-rank
test. Patients with Dukes' D-stage tumors or those who died in the
postoperative period were excluded from the survival analysis. The
mark of censored data indicated the end of an individual follow-up
period. The potential prognostic impact of the variables considered
in this work was evaluated jointly by Cox multivariate regression
analyses. Cutoff Finder Web Application (http://molpath.charite.de/cutoff/) (29) was used to determine the cut-off
points for prognosis analysis.
Results
Groups of patients
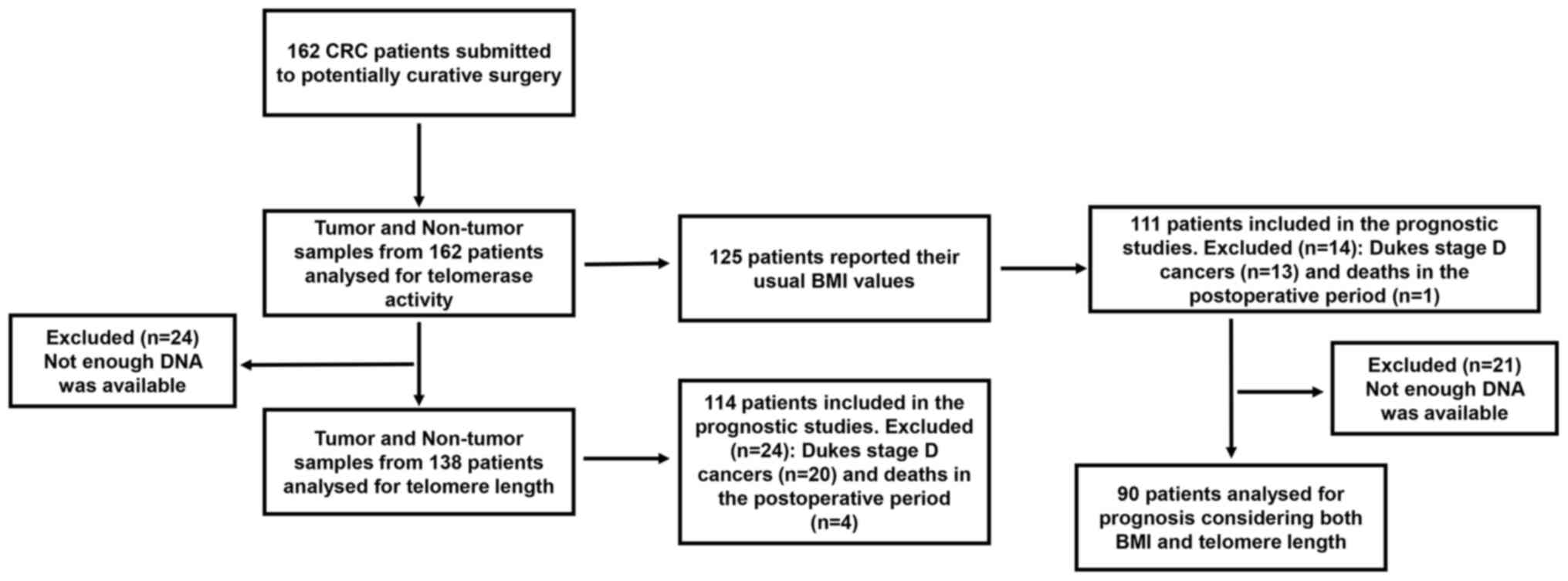
A flow diagram of the progress through the different
studies developed in the present work has been included in Fig. 1. Both the number of patients
involved in each one of the studies and the number of patients
excluded are indicated. Causes of exclusion are also specified.
Of all 162 CRC patients included in this study, 82
were females and 80 males, with a mean age of 70.6±0.9 years. Mean
age was comparable among both genders (female: 70.3±1.4 years vs.
male: 71.0±1.2; P=0.905).
Patients were classified according to their BMI
values following the criteria of the World Health Organization
(WHO). Thus, patients with BMI <25 kg/m2 were
considered to have normal weight; patients with BMI ≥25
kg/m2 and ≤ 29.9 kg/m2, as overweight; and
the ones with BMI ≥30 kg/m2 were defined as with
obesity. BMI data was available for 125 cases, 63 females and 62
males. Overall, 34 patients showed ‘normal weight’; 57 were
classified as ‘overweight’; and 34 were cataloged as ‘people with
obesity’, without significant gender differences (P=0.286).
Groups of patients established considering BMI
values did not show significant associations with Dukes' stage
(P=0.239), nor with tumor location (P=0.347) (Table I).
 | Table IBMI groups and clinicopathological
variables in patients with colorectal cancer. |
Table I
BMI groups and clinicopathological
variables in patients with colorectal cancer.
| Variable | Cases, n
(n=125) | Normoweight (BMI
<25), n (n=34) | Overweight
(BMI=25-29.9), n (n=57) | Obesity (BMI ≥30),
n (n=34) | P-value
(χ2 test) |
|---|
| Sex | | | | | 0.286 |
|
Female | 63 | 15 | 27 | 21 | |
|
Male | 62 | 19 | 30 | 13 | |
| Dukes' stage | | | | | 0.232 |
|
A | 19 | 7 | 6 | 6 | |
|
B | 60 | 13 | 34 | 13 | |
|
C | 33 | 11 | 10 | 12 | |
|
D | 13 | 3 | 7 | 3 | |
| Tumor location | | | | | 0.250 |
|
Right
colon | 46 | 11 | 22 | 13 | |
|
Left
colon | 33 | 9 | 19 | 5 | |
|
Rectum | 46 | 14 | 16 | 16 | |
Telomere function analysis
Telomere function was investigated through telomere
length determination, both in tumor (T) and non-tumor (NT) samples
from 138 patients, and by telomerase activity evaluation in paired
T and NT samples from 162 subjects. MTL in tumor tissues was
6.11±0.20 kb, whereas in non-tumor tissues MTL was 8.22±0.27 kb. A
positive correlation was detected between telomere length in tumor
and its paired non-tumor samples (r=0.501, P<0.001). Moreover,
an inverse correlation was found between non-tumor and tumor
telomere length and the age of patients (r=-0.170, P=0.028 for
non-tumor tissues, and r=-0.155; P=0.041 for tumor samples).
The mean value for telomere length in tumor and
non-tumor samples was correlated with Dukes' stage, as the lowest
mean values were observed in the earliest Dukes' stages (P=0.032
for tumor samples) (Table II).
Overall, telomere shortening was detected in CRC, as demonstrated
by the T/N ratio values (0.78±0.02). T/N ratio values were
significantly associated with BMI; in fact, patients with obesity
and CRC showed less shortening of tumor telomeres (0.85±0.05) than
non-obese patients affected by CRC (0.72±0.03) (P=0.047).
 | Table IITelomere status and
clinicopathological variables in patients with colorectal
cancer. |
Table II
Telomere status and
clinicopathological variables in patients with colorectal
cancer.
| | Telomere length
(kilobase pairs; mean ± standard error) | Telomerase
activity |
|---|
| Variable | Cases, n
(n=138) | Tumor samples | P-value (test) | Non-tumor
samples | P-value (test) | Cases, n
(n=162) | Positive, n
(n=121) | Negative, n
(n=41) | P-value
(χ2 test) |
|---|
| Sex | | | 0.423 | | 0.908 | | | | 0.526 |
|
Female | 73 | 5.93±0.29 | (Mann- Whitney U
test) | 8.14±0.36 | (Mann- Whitney U
test) | 82 | 63 | 19 | |
|
Male | 65 | 6.21±0.30 | | 8.43±0.48 | | 80 | 58 | 22 | |
| Dukes' stage | | | 0.032 | | 0.198 | | | | 0.784 |
|
A | 19 | 4.86±0.34 | (Kruskall- Wallis
test) | 7.08±0.56 | (Kruskall- Wallis
test) | 22 | 18 | 4 | |
|
B | 62 | 5.89±0.30 | | 8.09±0.39 | | 66 | 50 | 16 | |
|
C | 37 | 6.71±0.42 | | 9.23±0.74 | | 44 | 32 | 12 | |
|
D | 20 | 6.55±0.57 | | 8.22±0.68 | | 30 | 21 | 9 | |
| Tumor location | | | 0.075 | | 0.060 | | | | <0.001 |
|
Right
colon | 43 | 5.40±0.32 | (Kruskall- Wallis
test) | 8.36±0.70 | (Kruskall- Wallis
test) | 51 | 28 | 23 | |
|
Left
colon | 33 | 6.60±0.53 | | 9.34±0.58 | | 37 | 30 | 7 | |
|
Rectum | 62 | 6.24±0.28 | | 7.65±0.30 | | 74 | 63 | 11 | |
Telomerase activity was positive in 121 (75.9%) out
of the 162 cases, while 41 (24.1%) of the CRC were telomerase
negative. As shown in Table II, a
significantly higher proportion of tumors of the right colon
(45.1%) were negative for telomerase, compared to other locations
of cancers (18.9% of tumors from the left colon, or 14.7% of tumors
from the rectum), with significant differences (P<0.001).
Survival studies
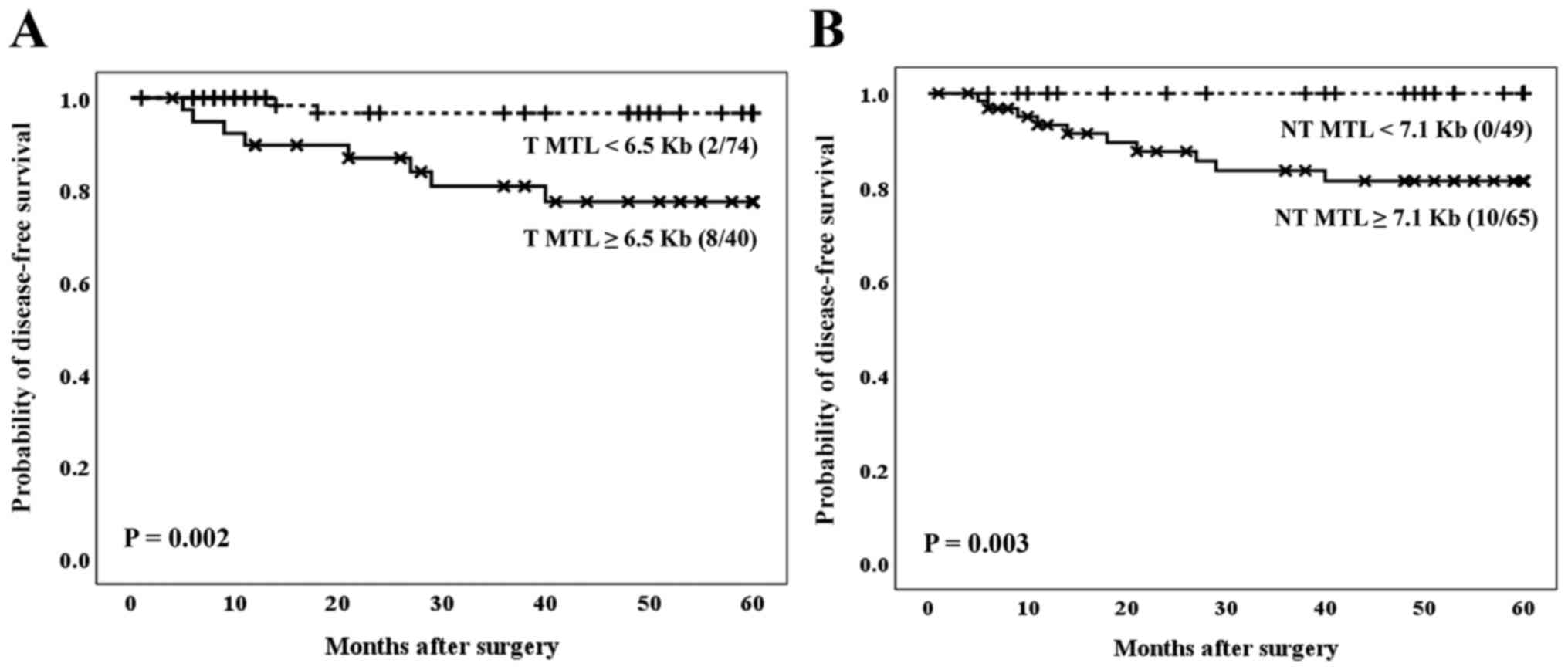
Regarding telomere status, the outcomes of the
patients were analyzed considering the optimal cut-off values for
the mean length of the tumor telomeres, as established in a
previous publication of our research team (12). With regard to the CRC population
included in the present manuscript, patients whose tumors had a MTL
<6.5 kb showed the best clinical evolution (Fig. 2A), regardless of Dukes' stage of the
tumor, as corroborated by Cox's multivariate analysis (Table III). Therefore, colorectal tumors
with an MTL <6.5 kb conferred a decreased relative risk of
recurrence, more than five times lower than the one of tumors
showing an MTL ≥6.5 kb (RR=0.169, 95% CI=0.036-0.700; P=0.025).
 | Table IIIMultivariate Cox regression analysis
in patients with CRC. |
Table III
Multivariate Cox regression analysis
in patients with CRC.
| | Multivariate
analysis |
|---|
| Variable | P-value | RR | CI |
|---|
| Dukes' stage (A + B
vs. C) | 0.004 | 4.690 | 1.660-13.240 |
| Tumor MTL in CRC
(<6.5 Kb vs. ≥6.5 Kb) | 0.025 | 0.169 | 0.036-0.800 |
Moreover, when survival was analyzed considering the
MTL data in non-tumor tissues, a better clinical evolution was
found in the group of patients who showed MTL values <7.1 kb
(P=0.003) (Fig. 2B). It was not
mathematically possible to establish the Cox multivariate study
considering MTL data in non-tumor tissues, because one subset of
events was empty (no cases of recurrence within the group of MTL
<7.1 kb), and we would have obtained an undefined value for
RR.
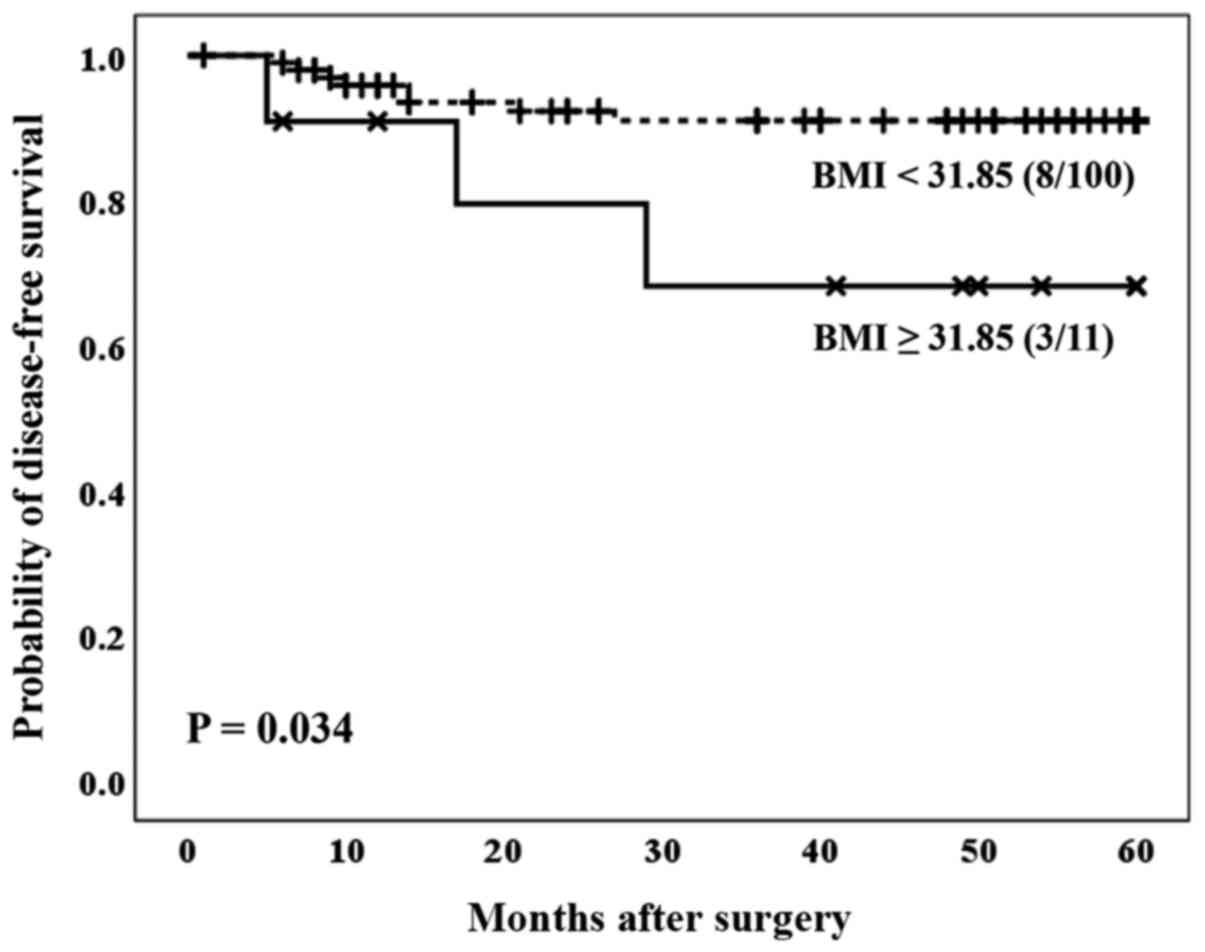
Concerning BMI, the optimal cut-off values for the
BMI were calculated using the Cutoff Finder Web Application
(24). Patients with a BMI ≥31.85
showed a significantly worse prognosis compared to the group of
patients with a BMI <31.85 (P=0.034) (Fig. 3). These results were not independent
of the gender of the patients included in this study.
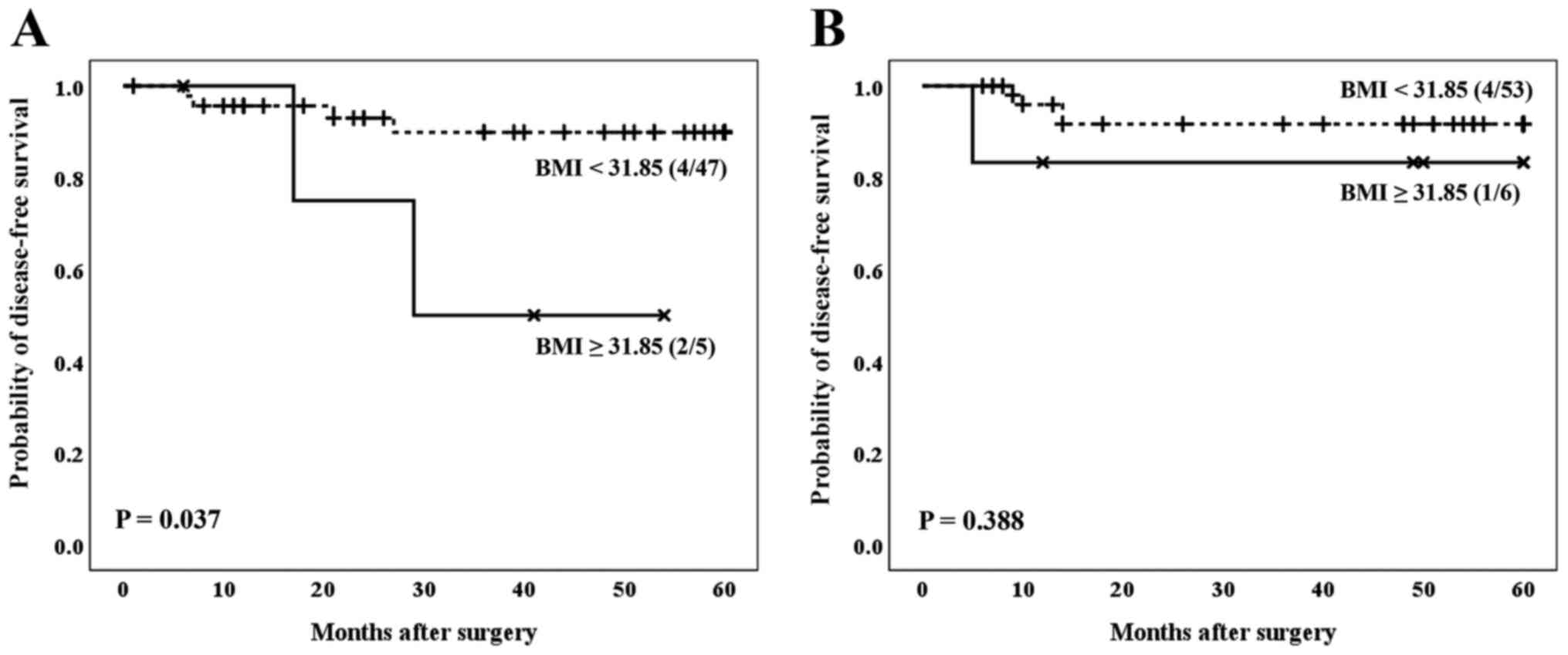
As shown in Fig. 4,
males with a BMI greater than or equal to 31.85 showed a
significantly worse prognosis compared to males with a BMI lower
than 31.85 (P=0.037) (Fig. 4A). For
females, however, such differences were not evident (P=0.388)
(Fig. 4B).
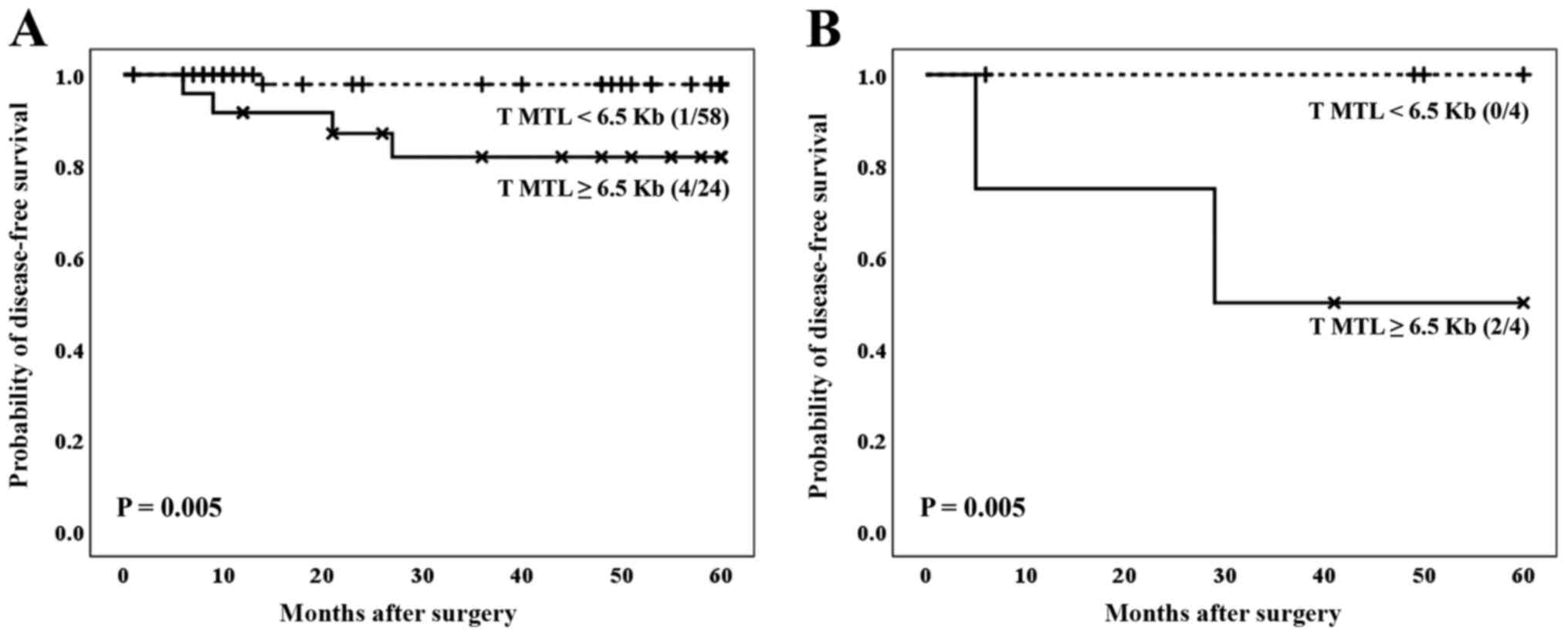
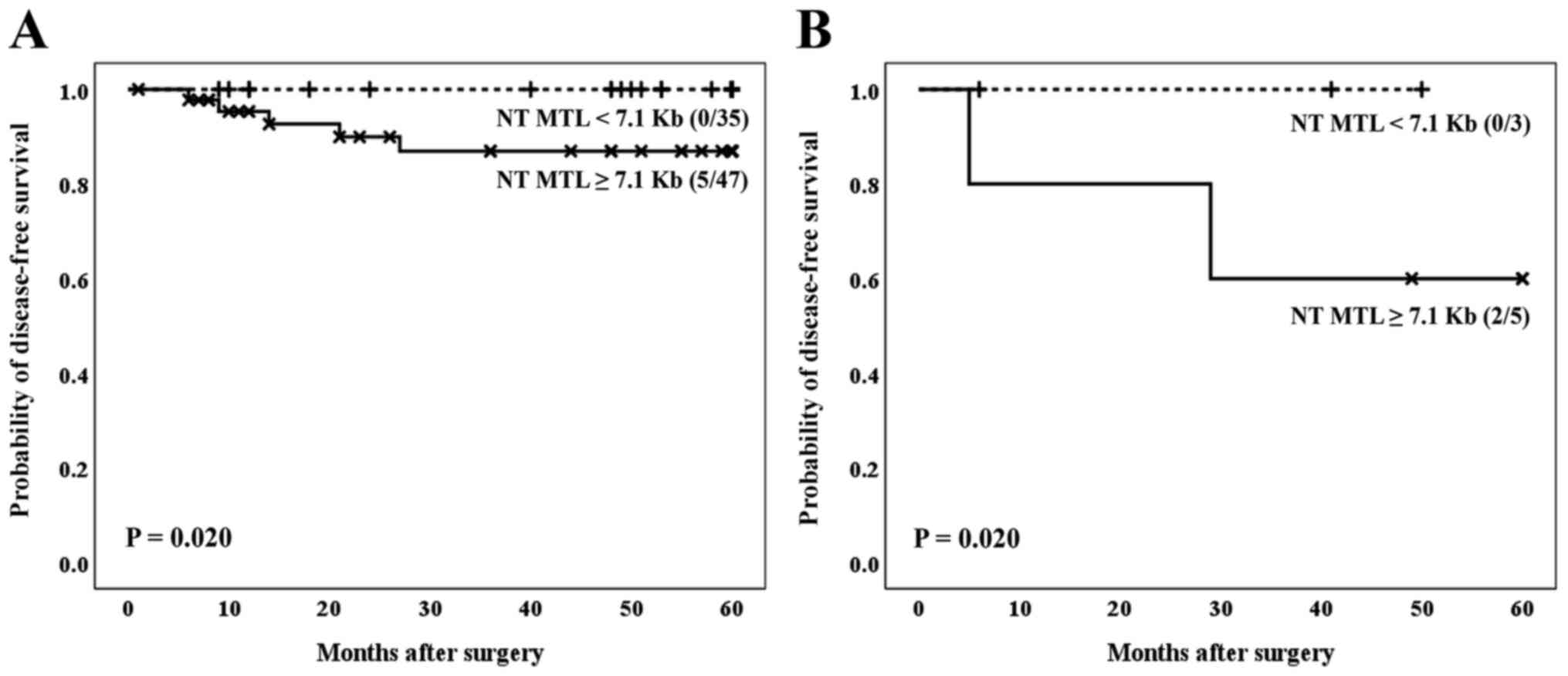
When the two variables (telomere status and BMI)
were considered together in relation to the prognosis of the
patients affected by the CRC, our results indicated that the
telomere status acts as a definitive molecular parameter to
establish the prognosis of patients. In fact, independently of the
BMI, patients affected by cancer and showing the lowest values of
average telomere length, both in tumor and non-tumor samples,
showed the best clinical evolution (Fig. 5A and B, for tumor tissues, P=0.005; and Fig. 6A and B, for non-tumor tissues, P=0.020).
Discussion
There is growing evidence that inflammation is a
central and reversible mechanism through which obesity promotes
cancer risk and progression. The tumor promoting effects of obesity
occur locally through the inflammation of adipose tissue and the
associated alterations in the microenvironment (2). Therefore, the characterization of
biomarkers to identify patients with obesity with high-risk CRC
seems paramount for an early diagnosis and improvement in the
election of the most appropriate therapeutic protocols.
In this context, considering that telomere
shortening has been associated with obesity in several studies, and
that telomere maintenance is critical for the progression of
cancer, our study was carried out considering a large population of
CRC patients, with and without obesity, submitted to surgery with
curative intention. Results from this work allowed us to
demonstrate that telomere status is related to obesity and clinical
prognosis.
Given the technical requirements of the
methodologies used in this study, it is necessary to highlight some
limitations. On the one hand, not all the usual weight data from
patients were available; therefore, there are some cases where the
usual BMI could not be calculated. Furthermore, the technique used
to evaluate telomere length requires a significant amount of DNA.
There were samples in which it was not possible to obtain enough
DNA from the available tissues. The number of cases submitted to
analysis in each of the determinations included in this work, as
well as the reasons for case exclusions, are detailed in the flow
diagram in Fig. 1.
Although the activity of telomerase was positive in
most of the tumors considered in our study, we detected a
significant shortening of telomeres in the tumor samples compared
to the mean values of the telomere length observed in the non-tumor
paired tissues, as previously reported by others (16,30).
Furthermore, according to the results obtained in the present
investigation, the lower mean values of telomere length, both in
tumors and in their non-tumor paired tissues, were associated with
earlier stages of Dukes, in agreement with other groups (31). Thus, these data prove that shorter
telomeres are associated with cancers that would, a priori, confer
a better prognosis to patients with CRC. In addition, our results
indicate that cancers displaying lower values of shortening of
telomere length occur in patients with obesity. We consider these
data of interest since, although several studies have established a
link between obesity and the risk of colon cancer, little is known
about the effect of obesity on the outcomes after diagnosis
(7).
It has been reported that a BMI greater than 35.0
kg/m2 at the time of diagnosis in patients with colon
cancer is associated with an increased risk of recurrence (7). However, other authors have not
confirmed these data, nor a significant correlation between BMI and
an increased risk of death in patients with CRC (32). It has also been suggested that BMI
prior to diagnosis is an important predictor of survival among
patients with non-metastatic CRC (6). More recently, Bhaskaran et al
(5) have reported that
heterogeneity in the effects of BMI suggests different mechanisms
or combinations of mechanisms associated with different tumor
locations and in different subgroups of patients.
In our study, the group of patients with BMI values
greater than 31.85 kg/m2, showed a significantly worse
clinical prognosis. However, Cox multivariate regression analyses
did not demonstrate that these results were independent of the
Dukes tumor stage.
Interestingly, our results indicate significant
differences according to the gender: When the effect of obesity on
the prognosis of patients with CRC was analyzed, these differences
were only evident in the male population. Although this fact could
be explained by considering the relationship between gender and fat
distribution (33), other aspects
might be further investigated, including additional genetic,
hormonal or molecular mechanisms, in order to explain the effect of
obesity on the prognosis of CRC in relation to the gender of
patients (34).
As for the telomere status, our data support that
the prognosis of patients with CRC, whether with or without
obesity, is strongly related to the length of the tumor telomere,
being these results independent of the stage of the tumor.
In the present study we have identified a specific
length of telomere in non-tumor tissues that seems critical to
predict the prognosis of cancer. Therefore, patients with a
telomere length less than 7.1 kb in non-tumor tissues present a
better clinical evolution, considering both subjects with and
without obesity. At this point, our results would support the idea
that carcinogenic cells have a common biological history with
normal tissue (35).
These data allow us to hypothesize the possibility
that tumor cells with shorter telomeres activate cellular
senescence, thereby conferring a more favorable clinical prognosis
to patients affected by CRC. In fact, the progressive shortening of
telomeres results in the formation of dysfunctional telomeres that
compromise tissue proliferation (19).
The shortening of human telomeres has two opposite
effects during the development of cancer. On the one hand, the
shortening of telomeres can exert a tumor suppressive effect
through the arrest of proliferation. On the other hand, the loss of
telomere protection can lead to a telomere crisis, which is a state
of extensive genomic instability that can promote the progression
of cancer (17). Our research team
has previously reported data in CRC that support a worse clinical
evolution in patients with tumor telomere maintenance (16).
In conclusion, in the present study we have jointly
evaluated the prognostic relevance of obesity and telomere status
in patients affected by CRC who had undergone surgery of curative
intention. Our results demonstrate that the length of telomeres is
a useful biomarker to predict the clinical outcome in these
subjects. Patients with shorter telomeres, both in the tumor and
their non-tumor paired tissues, had the best clinical evolution,
independently of the Dukes' stage. Our data allow us to conclude
that patients with obesity had a poorer prognosis, however, these
results were not independent from the tumor Dukes' stage.
Further investigations are needed to analyze the
effect of obesity on the clinical course of CRC in the context of
other factors, such as the gender of the patients.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants PI15/01199 and
PI19/00073 from the Carlos III Institute of Health (Ministerio de
Economía y Competitividad), Spain and co-funded by the European
Union through the European Regional Development Fund (ERDF) ‘A way
to make Europe’. Funders did not participate in study design, data
collection and analysis, decision to publish, nor preparation of
the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ATG and PI conceived and designed the study. SGM,
DGG, TFM and ST performed the assays. SDLS, IS, OCV, AB and ATG
participated in the acquisition and interpretation of data. SGM,
DGG, TFM, AB, CDJ, ATG and PI assessed the authenticity of all raw
data and analyzed the results. SGM, DGG, SDLS and PI wrote the
paper. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Committee of the San Carlos Hospital (Madrid, Spain). Written
informed consent was obtained from patients prior to investigation
assuring the confidentiality of their data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kolb R, Sutterwala FS and Zhang W: Obesity
and cancer: Inflammation bridges the two. Curr Opin Pharmacol.
29:77–89. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Iyengar NM, Gucalp A, Dannenberg AJ and
Hudis CA: Obesity and cancer mechanisms: Tumor microenvironment and
inflammation. J Clin Oncol. 34:4270–4276. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Iyengar NM, Hudis CA and Dannenberg AJ:
Obesity and cancer: Local and systemic mechanisms. Annu Rev Med.
66:297–309. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Avgerinos KI, Spyrou N, Mantzoros CS and
Dalamaga M: Obesity and cancer risk: Emerging biological mechanisms
and perspectives. Metabolism. 92:121–135. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bhaskaran K, Douglas I, Forbes H,
dos-Santos-Silva I, Leon DA and Smeeth L: Body-mass index and risk
of 22 specific cancers: A population-based cohort study of 5·24
million UK adults. Lancet. 384:755–765. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Campbell PT, Newton CC, Dehal AN, Jacobs
EJ, Patel AV and Gapstur SM: Impact of body mass index on survival
after colorectal cancer diagnosis: The cancer prevention study-II
nutrition cohort. J Clin Oncol. 30:42–52. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dignam JJ, Polite BN, Yothers G, Raich P,
Colangelo L, O'Connell MJ and Wolmark N: Body mass index and
outcomes in patients who receive adjuvant chemotherapy for colon
cancer. J Natl Cancer Inst. 98:1647–1654. 2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wu RA, Upton HE, Vogan JM and Collins K:
Telomerase mechanism of telomere synthesis. Annu Rev Biochem.
86:439–460. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Aviv A, Anderson JJ and Shay JW:
Mutations, cancer and the telomere length paradox. Trends Cancer.
3:253–258. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Luu HN, Qi M, Wang R, Adams-Haduch J,
Miljkovic I, Opresko PL, Jin A, Koh WP and Yuan JM: Association
between leukocyte telomere length and colorectal cancer risk in the
singapore Chinese health study. Clin Transl Gastroenterol. 10:1–9.
2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cleal K, Norris K and Baird D: Telomere
length dynamics and the evolution of cancer genome architecture.
Int J Mol Sci. 19(482)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tomasova K, Kroupa M, Forsti A, Vodicka P
and Vodickova L: Telomere maintenance in interplay with DNA repair
in pathogenesis and treatment of colorectal cancer. Mutagenesis.
35:261–271. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Schmidt JC and Cech TR: Human telomerase:
Biogenesis, trafficking, recruitment, and activation. Genes Dev.
29:1095–1105. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Garcia-Aranda C, de Juan C, Diaz-Lopez A,
Sanchez-Pernaute A, Torres AJ, Diaz-Rubio E, Balibrea JL, Benito M
and Iniesta P: Correlations of telomere length, telomerase
activity, and telomeric-repeat binding factor 1 expression in
colorectal carcinoma. Cancer. 106:541–551. 2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fernández-Marcelo T, Sánchez-Pernaute A,
Pascua I, De Juan C, Head J, Torres-García AJ and Iniesta P:
Clinical relevance of telomere status and telomerase activity in
colorectal cancer. PLoS One. 11(e0149626)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Maciejowski J and de Lange T: Telomeres in
cancer: Tumour suppression and genome instability. Nat Rev Mol Cell
Biol. 18:175–186. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Feldser DM and Greider CW: Short telomeres
limit tumor progression in vivo by inducing senescence. Cancer
Cell. 11:461–469. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang Y, Wang X, Flores ER, Yu J and Chang
S: Dysfunctional telomeres induce p53-dependent and independent
apoptosis to compromise cellular proliferation and inhibit tumor
formation. Aging Cell. 15:646–660. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Roger L, Jones RE, Heppel NH, Williams GT,
Sampson JR and Baird DM: Extensive telomere erosion in the
initiation of colorectal adenomas and its association with
chromosomal instability. J Natl Cancer Inst. 105:1202–1211.
2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mundstock E, Sarria EE, Zatti H, Mattos
Louzada F, Kich Grun L, Herbert Jones M, Guma FT, Mazzola In
Memoriam J, Epifanio M, Stein RT, et al: Effect of obesity on
telomere length: Systematic review and meta-analysis. Obesity
(Silver Spring). 23:2165–2174. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
An R and Yan H: :Body weight status and
telomere length in U.S. middle-aged and older adults. Obes Res Clin
Pract. 11:51–62. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Correia-Melo C, Hewitt G and Passos JF:
Telomeres, oxidative stress and inflammatory factors: Partners in
cellular senescence? Longev Healthspan. 3(1)2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Masi S, Salpea KD, Li K, Parkar M, Nibali
L, Donos N, Patel K, Taddei S, Deanfield JE, D'Aiuto F and
Humphries SE: Oxidative stress, chronic inflammation, and telomere
length in patients with periodontitis. Free Radic Biol Med.
50:730–735. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Nikolouzakis TK, Vassilopoulou L,
Fragkiadaki P, Sapsakos T, Papadakis GZ, Spandidos DA, Tsatsakis AM
and Tsiaoussis J: Improving diagnosis, prognosis and prediction by
using biomarkers in CRC patients. Oncol Rep. 42:2228–2244.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Turnbull RB Jr, Kyle K, Watson FR and
Spratt J: Cancer of the colon: The influence of the no-touch
isolation technic on survival rates. Ann Surg. 166:420–427.
1967.PubMed/NCBI View Article : Google Scholar
|
|
27
|
González-Quevedo R, Iniesta P, Morán A, de
Juan C, Sánchez-Pernaute A, Fernández C, Torres A, Díaz-Rubio E,
Balibrea JL and Benito M: Cooperative role of telomerase activity
and p16 expression in the prognosis of non-small-cell lung cancer.
J Clin Oncol. 20:254–262. 2002.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Frías C, García-Aranda C, De Juan C, Morán
A, Ortega P, Gómez A, Hernando F, López-Asenjo JA, Torres AJ,
Benito M and Iniesta P: Telomere shortening is associated with poor
prognosis and telomerase activity correlates with DNA repair
impairment in non-small cell lung cancer. Lung Cancer. 60:416–425.
2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Budczies J, Klauschen F, Sinn BV, Győrffy
B, Schmitt WD, Darb-Esfahani S and Denkert C: Cutoff finder: A
comprehensive and straightforward Web application enabling rapid
biomarker cutoff optimization. PLoS One. 7(e51862)2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Bertorelle R, Rampazzo E, Pucciarelli S,
Nitti D and De Rossi A: Telomeres, telomerase and colorectal
cancer. World J Gastroenterol. 20:1940–1950. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Jia H and Wang Z: Telomere length as a
prognostic factor for overall survival in colorectal cancer
patients. Cell Physiol Biochem. 38:122–128. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Meyerhardt JA, Niedzwiecki D, Hollis D,
Saltz LB, Mayer RJ, Nelson H, Whittom R, Hantel A, Thomas J and
Fuchs CS: Cancer and Leukemia Group B 89803. Impact of body mass
index and weight change after treatment on cancer recurrence and
survival in patients with stage III colon cancer: Findings from
cancer and leukemia group B 89803. J Clin Oncol. 26:4109–4115.
2008.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Doleman B, Mills KT, Lim S, Zelhart MD and
Gagliardi G: Body mass index and colorectal cancer prognosis: A
systematic review and meta-analysis. Tech Coloproctol. 20:517–535.
2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Cheung WY, Shi Q, O'Connell M, Cassidy J,
Blanke CD, Kerr DJ, Meyers J, Van Cutsem E, Alberts SR, Yothers G,
et al: The predictive and prognostic value of sex in early-stage
colon cancer: A pooled analysis of 33,345 patients from the ACCENT
database. Clin Colorectal Cancer. 12:179–187. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Nakamura K, Furugori E, Esaki Y, Arai T,
Sawabe M, Okayasu I, Fujiwara M, Kammori M, Mafune K, Kato M, et
al: Correlation of telomere lengths in normal and cancers tissue in
the large bowel. Cancer Lett. 158:179–184. 2000.PubMed/NCBI View Article : Google Scholar
|