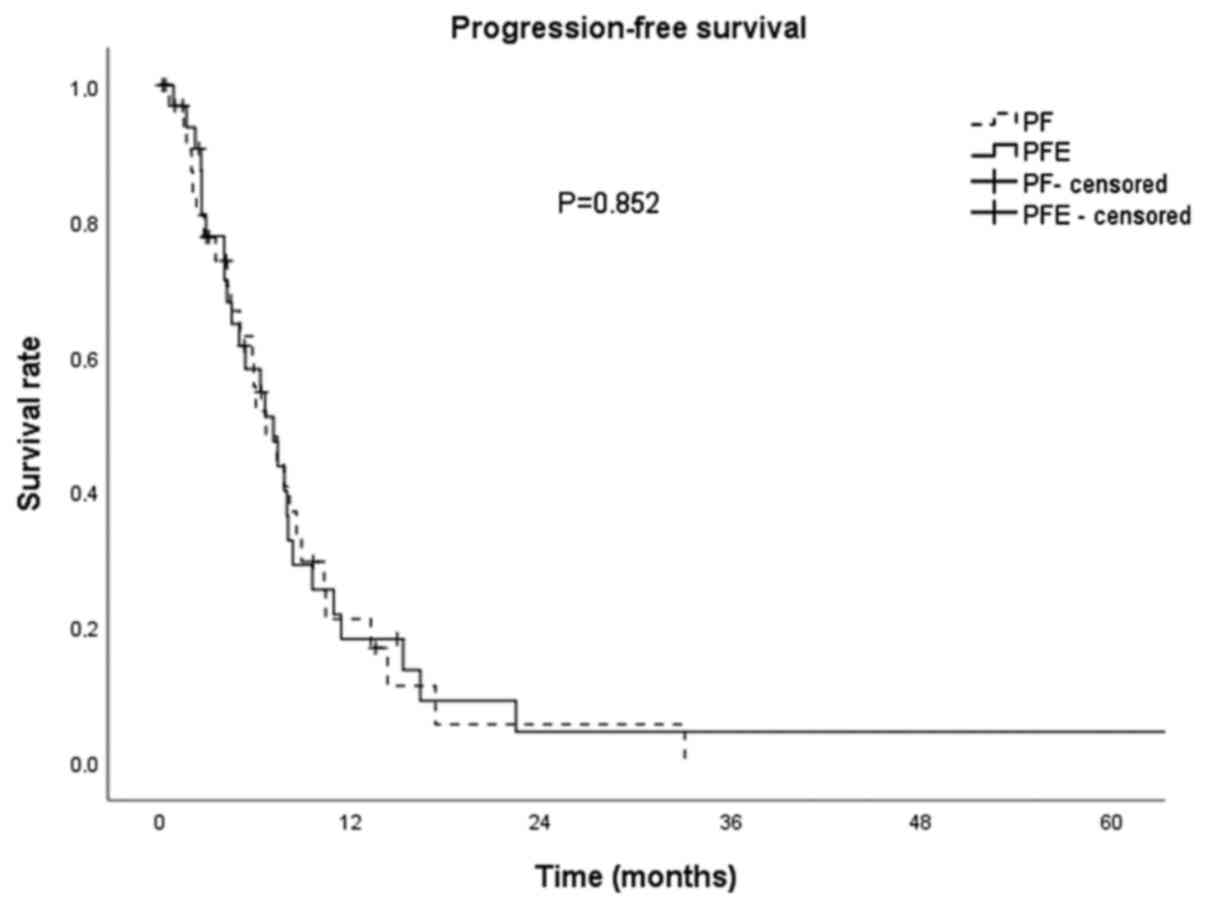
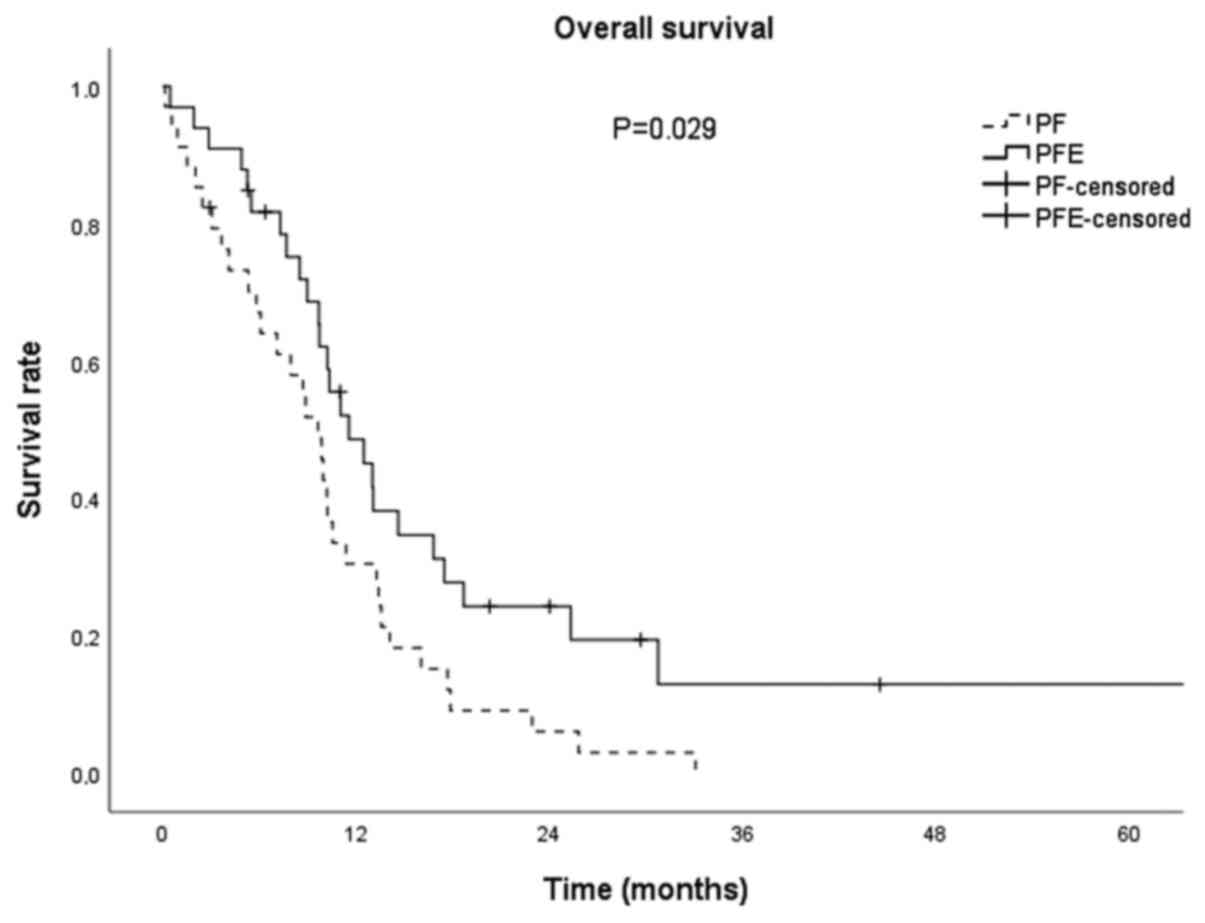
|
1
|
Cancer in Slovenia 2016. Available from:
https://www.onko-i.si/fileadmin/onko/datoteke/dokumenti/RRS/LP_2016.pdf.
|
|
2
|
Argiris A, Harrington KJ, Tahara M,
Schulten J, Chromette P, Ferreira Castro A and Licitra L:
Evidence-based treatment options in recurrent and/or metastatic
squamous cell carcinoma of the head and neck. Front Oncol.
7(72)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Vermorken J, Mesia R, Rivera F, Remenar E,
Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol D,
et al: Platinum-based chemotherapy plus cetuximab in head and neck
cancer. N Engl J Med. 339:1116–1127. 2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Price KA and Cohen EE: Current treatment
options for metastatic head and neck cancer. Curr Treat Options
Oncol. 13:35–46. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gatta G, Botta L, Sánchez MJ, Anderson LA,
Pierannunzio D and Licitra L: EUROCARE Working Group. Prognoses and
improvement for head and neck cancers diagnosed in Europe in early
2000s: The EUROCARE-5 population-based study. Eur J Cancer.
51:2130–2143. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gregoire V, Lefebvre JL, Licitra L and
Felip E: EHNS-ESMO-ESTRO Guidelines Working Group. Squamous cell
carcinoma of the head and neck: EHNS-ESMO-ESTRO clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol. 21
(Suppl 5):v184–v186. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Strojan P, Zakotnik B, Žumer B, Karner K,
Dremelj M, Jančar B, Jereb S and Grašič Kuhar C: Skin reaction to
cetuximab as a criterion for treatment selection in head and neck
cancer. Anticancer Res. 38:4213–4220. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
The RECIST Working Group. RECIST 1.1.
Available from: https://project.eortc.org/recist/wp-content/uploads/sites/4/2015/03/RECISTGuidelines.pdf.
|
|
9
|
US Department of Health and Human
Services: Common Terminology Criteria for Adverse Events. Version
5.0. Published November 27, 2017. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf.
Accessed December 12, 2020.
|
|
10
|
Depenni R, Cossu Rocca M, Ferrari D,
Azzarello G, Baldessari C, Alu M, Nole F, Codeca C, Boscolo G,
Piccininni M, et al: Clinical outcomes and prognostic factors in
recurrent and/or metastatic head and neck cancer patients treated
with chemotherapy plus cetuximab as first-line therapy in a
real-world setting. Eur J Cancer. 115:4–12. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Magnes T, Melchardt T, Weiss L, Mittermair
C, Neureiter D, Klieser E, Gampenrieder S, Moser G, Gaggl A, Griel
R and Egle A: Prognostic score in patients with recurrent or
metastatic carcinoma of the head and neck treated with cetuximab
and chemotherapy. PLoS One. 12(e180995)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sano D, Fujisawa T, Tokuhisa M, Shimizu M,
Sakagami T, Hatano T, Nishimura G, Ichikawa Y, Iwai H and Oridate
N: Real-world treatment outcomes of the EXTREME regimen as
first-line therapy for recurrent/metastatic squamous cell carcinoma
of the head and neck: A multi-center retrospective cohort study in
Japan. Anticancer Res. 39:6819–6827. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Le Tourneau C, Ghiani M, Cau MC, Depenni
R, Ronzino G, Bonomo P, Montesarchio V, Leo L, Schulten J,
Messinger D, et al: Cetuximab plus platinum-based therapy (PBT) as
a first-line treatment for patients with recurrent and/or
metastatic squamous cell carcinoma of the head and neck (R/M
SCCHN): An observational study (ENCORE). Ann Oncol. 29 (Suppl
8):viii372–viii399. 2018.
|
|
14
|
Grünwald V, Chirovsky D, Cheung WY,
Bertolini F, Ahn MJ, Yang MH, Castro G, Berrocal A, Sjoquist K,
Kuyas H, et al: Global treatment patterns and outcomes among
patients with recurrent and/or metastatic head and neck squamous
cell carcinoma: Results of the GLANCE H&N study. Oral Oncol.
102(104526)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Vermorken JB and Specenier P: Optimal
treatment for recurrent/metastatic head and neck cancer. Ann Oncol.
21 (Suppl 7):S252–S261. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Argiris A, Li Y and Forastiere A:
Prognostic factors and long-term survivorship in patients with
recurrent or metastatic carcinoma of the head and neck. Cancer.
101:2222–2229. 2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Anderson JR, Cain KC and Gelber RD:
Analysis of survival by tumor response and other comparisons of
time-to-event by outcome variables. J Clin Oncol. 26:3913–3915.
2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Alshadwi A, Nadershah M, Carlson ER, Young
LS, Burke PA and Daley BJ: Nutritional considerations for head and
neck cancer patients: A review of the literature. J Oral Maxillofac
Surg. 71:1853–1860. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Greenlee H, Unger JM, LeBlanc M, Ramsey S
and Hershman DL: Association between body mass index (BMI) and
cancer survival in a pooled analysis of 22 clinical trials. Cancer
Epidemiol Biomarkers Prev. 26:21–29. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gama RR, Song Y, Zhang Q, Brown MC, Wang
J, Habbous S, Tong L, Huang SH, O'Sullivan B, Waldron J, et al:
Body mass index and prognosis in patients with head and neck
cancer. Head Neck. 39:1226–1233. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Guigay J, Feyette J, Dillies AF, Sire C,
Kerger JN, Tennevet I, Machiels JP, Zanetta S, Pointreau Y, Bozec
Le Moal L, et al: Cetuximab, docetaxel and cisplatin in first-line
treatment in patients with recurrent or metastatic head and neck
squamous cell carcinoma: A multicenter, phase II GORTEC study. Ann
Oncol. 26:1941–1947. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hitt R, Irigoyen A, Cortes-Funes H, Grau
JJ, García-Sáenz JA and Cruz-Hernandez JJ: Spanish Head and Neck
Cancer Cooperative Group (TTCC). Phase II study of the combination
of cetuximab and weekly paclitaxel in the first-line treatment of
patients with recurrent an/or metastatic squamous cell carcinoma of
head and neck. Ann Oncol. 23:1016–1022. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cramer JD, Burtness B and Ferris RL:
Immunotherapy for head and neck cancer: Recent advances and future
directions. Oral Oncol. 99(104460)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Machiels JP, Rene Leemans C, Golunski W,
Grau C, Licitra L and Gregoire C: EHNS Executive Board. Squamous
cell carcinoma of the oral cavity, larynx, oropharynx and
hypopharynx: EHNS-ESMO-ESTO clinical practice guidelines for
diagnosis, treatment and follow-up. Ann Oncol. 31:1462–1475.
2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Burtness B, Harrington KJ, Greil R,
Soulières D, Tahara M, de Castro G Jr, Psyrri A, Baste N, Prakash
N, Bratland A, et al: Pembrolizumab alone or with chemotherapy
versus cetuximab with chemotherapy for recurrent or metastatic
squamous cell carcinoma of the head and neck (KEYNOTE-048): A
randomised, open-label, phase 3 study. Lancet. 394:1915–1928.
2019.PubMed/NCBI View Article : Google Scholar
|