Introduction
Cutaneous angiosarcoma (CAS) is a rare neoplasm of
mesenchymal origin, accounting for less than 1% of all sarcomas
(1). While the pathogenesis of
angiosarcoma remains unclear, it has been associated with multiple
etiological risk factors, including radiotherapy, chronic
lymphedema, various chemicals, and immunodeficiency (2). In a population-based retrospective
cohort study, radiotherapy significantly increased the risk of
angiosarcoma (3). Angiosarcoma
arising in the base of chronic lymphedema is known as
Stewart-Treves syndrome. Some investigators have reported a
relationship between antecedent traumatic tissue injury and
pathogenesis (4). Recently,
ultraviolet light exposure has been associated with a common
etiological and genomic mutational basis for the pathogenesis of
angiosarcoma of the head and neck region (5).
The most commonly affected sites of CAS are the
scalp and facial skin (6), which
are associated with a poor prognosis compared to other sites
(7). The clinical presentation of
CAS is single or multiple bluish or violaceous nodules, and
occasionally ulceration or bleed (8). No definitive criteria have yet been
formulated for the staging of angiosarcoma. According to the 8th
edition of the American Joint Committee on Cancer staging manual on
the TNM staging system, angiosarcoma is excluded from the soft
tissue tumor chapter because of its aggressive infiltrative nature.
On immunohistochemistry, vascular endothelial markers, including
CD31, CD34, and von Willebrand factor-related antigens, are
informative for diagnosis (9). In
addition, lymphatic endothelial markers, such as D2-40, Prox-1, and
VEGFR-3, might be positively expressed, which indicates lymphatic
differentiation of angiosarcoma (10).
Although several treatment procedures have been
investigated, as yet, there is no consensus on the treatment of
angiosarcoma, due to a lack of statistically significant evidence.
Surgical resection with a free tumor margin is the primary
treatment of choice for angiosarcoma (11). The microscopic negative surgical
margin was significantly correlated with longer overall survival
(OS), which is considered an important prognostic factor (12). However, due to the diffuse
infiltrative and multifocal nature of CAS, preoperative medical
assessment of the lesion and careful selection of the patient are
critically important.
Radiotherapy is also considered a curative local
therapy for unresectable or incompletely resected tumors. The
effectiveness of postoperative radiotherapy for local control (LC)
and OS has been reported in several studies (13) however studies on the treatment of
angiosarcoma by radical radiotherapy without surgery are very
limited. The aim of the present study was to investigate the
clinical outcomes of radiotherapy without surgery for patients with
scalp CAS.
Materials and methods
Inclusion and exclusion criteria of
the patients
We conducted a retrospective analysis of consecutive
patients with scalp-localized CAS treated with radical radiotherapy
at the Department of Radiology in our institution from June 2008 to
January 2020. This retrospective study was performed in accordance
with the guidelines approved by the institutional review board (ID
number: 3372). All patients provided written informed consent. The
patients in this study satisfied the following inclusion criteria:
a) histologically confirmed angiosarcoma located in the scalp, b)
treated with radiotherapy with curative intent, c) no distant
metastasis, and d) no history of previous radiotherapy or surgery
of the scalp lesion. Retrospective patient data were obtained from
the medical records of our institution. Performance status of the
patients was assessed by Karnofsky Performance Status (14).
Radiation treatment planning
All patients underwent either electron beam
radiotherapy or helical tomotherapy-based intensity-modulated
radiotherapy (HT-IMRT). The radiation field design was either the
partial scalp or whole scalp, which was decided by a clinician at
the time of treatment. For partial scalp treatment, a 6-10 MeV
electron radiotherapy beam with a 5-mm bolus was administered to
the primary site with generous margins. In the HT-IMRT treatment
group, the planning computed tomography (CT) dataset was acquired
with a thermoplastic mask on a flat board for planning CT. The CT
image data were reconstructed with a slice thickness of 2 mm. These
data were then sent to a treatment planning system, such as
Pinnacle (Philips), Monaco (Elekta CMS), or TomoTherapy Planning
Station (Accuray). The clinical target volume (CTV) included the
primary tumor and total scalp. The planning target volume (PTV)
included the CTV with a 3-mm margin only on the inside of the CTV
and excluding the area outside the CTV. The prescribed dose was 70
Gy spread across 35 fractions targeted to 95% (exceeding 95% of the
volume) of the PTV. The treatment planning used a virtual bolus to
avoid hot spot doses; this process has been described in detail in
a previous report from our institution (15).
Statistical analysis
Data analysis was performed using the R Statistical
Software (Foundation for Statistical Computing). OS,
progression-free survival (PFS), LC, and distant metastasis control
(DMC) rates were calculated from the first day of radiotherapy
using the Kaplan-Meier method. OS was defined as the time interval
until death from any cause, and surviving patients were censored at
the date of the last follow-up examination. PFS was defined as the
time interval until progression or death from any cause, and living
patients without disease progression were censored at the date of
the last follow-up examination. LC was defined as the time interval
until intra-scalp recurrence, and patients free from local
recurrence were censored at the date of the last follow-up
examination or death. DMC was defined as the time interval until
metastatic recurrence, and patients free from metastatic recurrence
were censored at the date of the last follow-up examination or
death. Univariate Cox hazard analysis was used to calculate the
hazard ratios of the factors associated with OS and PFS.
Statistical significance was set at P<0.05.
Results
Background of patients
Fifteen patients were retrospectively analyzed in
this study; patient characteristics are shown in Table I. The median age was 75 years
(range, 59-84 years), and the majority (93.3%) of patients were
male. Six patients received partial radiotherapy, while the others
received total scalp radiotherapy. All but one patient received
radiotherapy with concurrent taxane regimen chemotherapy: Weekly
paclitaxel (7 patients, 46.7%) or 3-weekly docetaxel (7 patients,
46.7%). Intravenous administration of recombinant interleukin-2
(rIL-2) was administered to six patients (40.0%). Adjuvant therapy
was administered to 12 patients. The monthly docetaxel regimen was
administered to eight patients, weekly paclitaxel regimen to three
patients, and local intralesional injection to one patient.
 | Table ICharacteristics of 15 patients with
cutaneous angiosarcoma of the scalp treated by definitive
radiotherapy. |
Table I
Characteristics of 15 patients with
cutaneous angiosarcoma of the scalp treated by definitive
radiotherapy.
| Variables | Value |
|---|
| Age, years (median;
range) | 75 (59-84) |
| Sex, n (%) | |
|
Male | 14(93) |
|
Female | 1(7) |
| Karnofsky Performance
Status, n (%) | |
|
100 | 1(7) |
|
90 | 10(67) |
|
80 | 4(27) |
| Radiotherapy, n
(%) | |
|
Partial
scalp (electron) | 6(40) |
|
Total scalp
(electron) | 2(13) |
|
Total scalp
(tomotherapy) | 7(47) |
| Concurrent systemic
therapy, n (%) | |
|
rIL-2 | 1(7) |
|
DOC | 2(13) |
|
PTX | 6(40) |
|
DOC +
rIL-2 | 5(33) |
|
PTX +
rIL-2 | 1(7) |
| Adjuvant therapy, n
(%) | |
|
rIL-2
injection | 1(7) |
|
DOC | 8(53) |
|
PTX | 3(20) |
|
None | 3(20) |
Survival analysis
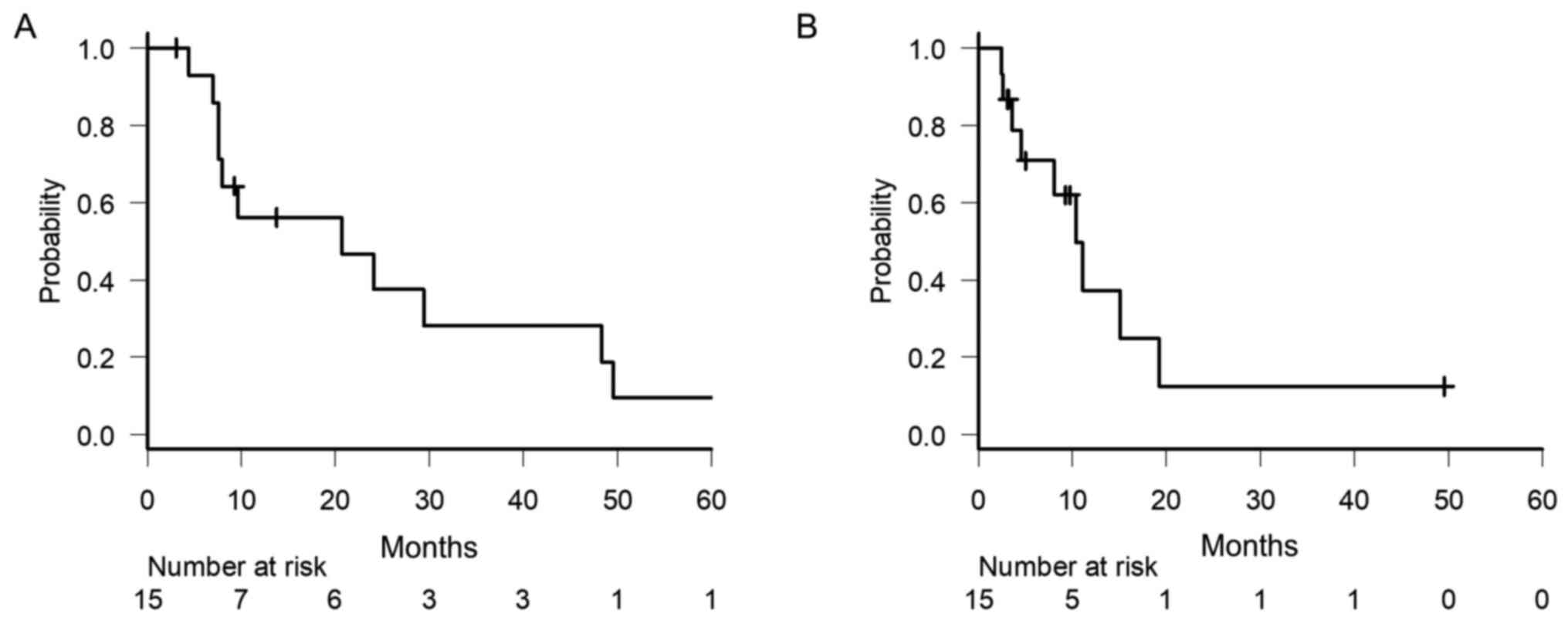
The median follow-up period for these patients was
9.7 months (range: 3.5-72.8 months). At the time of analysis, one
patient was lost to follow-up; one was alive with active disease;
one was alive with controlled disease; and the other 12 patients
had died, one of whom died from a myocardial infarction, which was
not directly associated with primary disease or its treatment. The
median OS time was 20.7 months [95% confidence interval (CI):
7.6-48.3 months], and the 1-, 3- and 5-year OS rates were 56.2,
28.1 and 9.4%, respectively (Fig.
1A). The median PFS time was 9.3 months (95% CI: 3.3-11.0
months), and the 1- and 3-year PFS rates were 21.7 and 7.2%,
respectively (Fig. 1B). The factors
associated with favorable OS or PFS were not identified in the
univariate analysis (Table
II).
 | Table IIRisk factors for overall survival and
progression-free survival time according to univariate Cox hazard
analysis. |
Table II
Risk factors for overall survival and
progression-free survival time according to univariate Cox hazard
analysis.
| | Overall survival | Progression-free
survival |
|---|
| Covariables | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Age (<75 years vs.
≥75 years) | 1.672
(0.449-6.221) | 0.444 | 1.948
(0.579-6.556) | 0.281 |
| Performance status
(>80% vs. ≤80%) | 3.788
(0.928-15.470) | 0.064 | 2.870
(0.751-10.960) | 0.123 |
| Radiation scalp field
(partial vs. total) | 4.993
(0.929-26.850) | 0.061 | 3.678
(0.927-14.590) | 0.064 |
| Chemotherapy regimen
(weekly PTX vs. others) | 0.292
(0.053-1.620) | 0.159 | 0.355
(0.105-1.204) | 0.097 |
| rIL-2 administration
(yes vs. no) | 3.449
(0.781-15.230) | 0.102 | 2.672
(0.756-9.445) | 0.127 |
| Adjuvant therapy (yes
vs. no) | 3.391
(0.614-18.730) | 0.161 | 3.259
(0.534-19.890) | 0.201 |
Recurrence and salvage treatment
At the time of analysis, 13 patients (86.7%)
developed recurrence. Among these, the first site of recurrence was
the scalp, as local recurrence in seven patients (46.7%), parotid
recurrence in two (13%), and distant metastasis in four (26.7%).
The 1-year LC and DMC rates were 37.8 and 61.5%, respectively. In
the seven patients with local recurrence, salvage surgery was
performed in one patient, additional electron radiotherapy in four
patients, and best supportive care in two patients. Two patients
with parotid recurrence were treated with EBRT. Among the patients
with distant metastasis, one received salvage chemotherapy (weekly
PTX) and the others selected the best supportive care.
Adverse event
The most common radiation-induced acute adverse
event was skin reaction. Moderate (G2) or severe (G3) acute skin
reactions were present in all patients, consisting of 12 patients
in G2 and three in G3. Severe (G3 or higher) radiation-induced late
complications, such as fistulas, strictures, or necrosis of the
bone, were not observed.
Discussion
The results of our study did not deviate
significantly from those of other studies. The clinical outcomes of
patients in our study were comparable to those of previous reports.
Hata et al (16) reported
that 17 patients with angiosarcoma of the scalp underwent total
scalp irradiation therapy with curative intent and found that the
3-year OS and disease-free survival rates for all patients were 22
and 6%, respectively. They adopted a two-step cone-down technique,
with an initial phase CTV including the entire scalp for a median
dose of 50 Gy in 25 fractions, followed by a tumor site with an
additional margin for a dose of 20 Gy in 10 fractions. However,
five patients experienced disease progression in the scalp distant
from the primary site, which had received prophylactic irradiation
at doses of 46-50 Gy. They, therefore, concluded that a dose of
less than 50 Gy in conventional fractions might be insufficient to
control microscopic tumors. Clear evidence to determine the
effective radiation dose and coverage field is debated, and the
optimal treatment strategy for radiotherapy remains
controversial.
Multimodal treatments, including surgery and
radiotherapy, were more effective than single-modality treatment in
improving clinical outcomes for patients with CAS. Ogawa et
al (17) revealed that patients
treated with combined therapy had a significantly more favorable OS
than patients treated with either surgery or radiotherapy alone
(2-year OS: 45.8% vs. 11.1%, P<0.001). Guadagnolo et al
(18) also reported that combined
modality local therapy was associated with improvement in LC, OS,
and disease-specific survival in 70 patients with angiosarcoma of
the face or scalp.
The role of adjuvant systemic therapy after initial
treatment has been investigated in previous retrospective studies.
Ihara et al (19) reported
that the administration of adjuvant chemotherapy consisting of a
taxane regimen after concurrent chemoradiotherapy was a significant
prognostic factor for PFS (P=0.036). Fujisawa et al
(20) showed that patients who
received taxane-based concurrent chemoradiotherapy (CCRT) with
maintenance chemotherapy showed a significant improvement in OS
compared to those receiving CCRT alone (P<0.01). In the present
study, while the OS and PFS were superior in the maintenance
chemotherapy group, the difference was not statistically
significant.
The most frequent metastatic site of angiosarcoma is
the lungs (21), as shown in our
report. Treatment options for the management of recurrent or
advanced CAS are limited. However, in recent years,
immunotherapeutic approaches have emerged as promising anti-cancer
systemic therapies. In particular, immune checkpoint inhibitors
have proven efficacious in various cancer entities. Florou et
al (22) treated seven
angiosarcoma patients with immune checkpoint inhibitors, such as
anti-cytotoxic T-lymphocyte antigen 4 or programmed cell death
protein 1 monoclonal antibody, five (71%) of whom had a partial
response, without severe toxicity.
In the present study, no statistically significant
prognostic factors were identified. However, local recurrence and
distant metastasis, especially in the lungs, were observed at a
considerable frequency. These pulmonary metastases could cause
fatal hemopneumothorax, and careful follow-up for distant
metastasis, including lung metastasis, after treatment is
considered crucial.
This study had several limitations. As the study was
conducted at a single institution, the sample size was small,
making it difficult to identify significant prognostic factors from
the data. Because of the retrospective nature of data collection,
our data had numerous risks of bias. Moreover, the retrospective
analysis of medical records makes it impossible to describe the
complete condition of patients at the time of treatment.
This retrospective study reported the clinical
outcomes of radiotherapy for CAS in our institution. Radiotherapy
combined with adjuvant chemotherapy showed a more favorable outcome
than radiotherapy alone; however, there were no statistically
significant differences between these groups. Further comprehensive
research is needed to clarify the optimal treatment strategy for
CAS.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
AK performed acquisition of data. HY and KN analyzed
and interpreted the clinical data. KN, AK and HY conceived the
study and participated in its design and coordination. AK was a
major contributor to the writing of this report. KN and HY
critically revised this report for important intellectual content.
The authenticity of all the raw data was confirmed by HY and AK.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of The University of Tokyo Hospital. Written informed
consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Conic RRZ, Damiani G, Frigerio A, Tsai S,
Bragazzi NL, Chu TW, Mesinkovska NA, Koyfman SA, Joshi NP, Budd GT,
et al: Incidence and outcomes of cutaneous angiosarcoma: A SEER
population-based study. J Am Acad Dermatol. 83:809–816.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Young RJ, Brown NJ, Reed MW, Hughes D and
Woll PJ: Angiosarcoma. Lancet Oncol. 11:983–991. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Huang J and Mackillop WJ: Increased risk
of soft tissue sarcoma after radiotherapy in women with breast
carcinoma. Cancer. 92:172–180. 2001.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Meis-Kindblom JM and Kindblom LG:
Angiosarcoma of soft tissue: A study of 80 cases. Am J Surg Pathol.
22:683–697. 1998.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Painter CA, Jain E, Tomson BN, Dunphy M,
Stoddard RE, Thomas BS, Damon AL, Shah S, Kim D, Gómez Tejeda
Zañudo J, et al: The angiosarcoma project: Enabling genomic and
clinical discoveries in a rare cancer through patient-partnered
research. Nat Med. 26:181–187. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gaballah AH, Jensen CT, Palmquist S,
Pickhardt PJ, Duran A, Broering G and Elsayes KM: Angiosarcoma:
Clinical and imaging features from head to toe. Br J Radiol.
90(20170039)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang L, Lao IW, Yu L and Wang J:
Clinicopathological features and prognostic factors in
angiosarcoma: A retrospective analysis of 200 patients from a
single Chinese medical institute. Oncol Lett. 14:5370–5378.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shon W and Billings SD: Cutaneous
malignant vascular neoplasms. Clin Lab Med. 37:633–646.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ohsawa M, Naka N, Tomita Y, Kawamori D,
Kanno H and Aozasa K: Use of immunohistochemical procedures in
diagnosing angiosarcoma. Evaluation of 98 cases. Cancer.
75:2867–2874. 1995.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mankey CC, McHugh JB, Thomas DG and Lucas
DR: Can lymphangiosarcoma be resurrected? A clinicopathological and
immunohistochemical study of lymphatic differentiation in 49
angiosarcomas. Histopathology. 56:364–371. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Morgan MB, Swann M, Somach S, Eng W and
Smoller B: Cutaneous angiosarcoma: A case series with prognostic
correlation. J Am Acad Dermatol. 50:867–874. 2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Fury MG, Antonescu CR, Van Zee KJ, Brennan
MF and Maki RG: A 14-year retrospective review of angiosarcoma:
Clinical characteristics, prognostic factors, and treatment
outcomes with surgery and chemotherapy. Cancer J. 11:241–247.
2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pawlik TM, Paulino AF, McGinn CJ, Baker
LH, Cohen DS, Morris JS, Rees R and Sondak VK: Cutaneous
angiosarcoma of the scalp: A multidisciplinary approach. Cancer.
98:1716–1726. 2003.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Karnofsky DA and Burchenal JH: Evaluation
of chemotherapeutic agents. In: The Clinical Evaluation of
Chemotherapeutic Agents in Cancer. MacLeod CM, editor. Columbia
University Press, New York, pp191-205, 1949.
|
|
15
|
Takenaka R, Haga A, Nawa K, Hideomi Y and
Nakagawa K: Improvement of the robustness to set up error by a
virtual bolus in total scalp irradiation with Helical tomotherapy.
Radiol Phys Technol. 12:433–437. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hata M, Wada H, Ogino I, Omura M, Koike I,
Tayama Y, Odagiri K, Kasuya T and Inoue T: Radiation therapy for
angiosarcoma of the scalp: Treatment outcomes of total scalp
irradiation with X-rays and electrons. Strahlenther Onkol.
190:899–904. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ogawa K, Takahashi K, Asato Y, Yamamoto Y,
Taira K, Matori S, Iraha S, Yagi N, Yogi A, Haranaga S, et al:
Treatment and prognosis of angiosarcoma of the scalp and face: A
retrospective analysis of 48 patients. Br J Radiol. 85:e1127–e1133.
2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Guadagnolo BA, Zagars GK, Araujo D, Ravi
V, Shellenberger TD and Sturgis EM: Outcomes after definitive
treatment for cutaneous angiosarcoma of the face and scalp. Head
Neck. 33:661–667. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ihara H, Kaji T, Katsui K, Miyake T, Waki
T, Katayama N, Matsuzaki H, Yamasaki O, Kuroda M, Morizane S and
Kanazawa S: Single institutional experience of radiation therapy
for angiosarcoma of the scalp without cervical lymph node
metastases: Impact of concurrent chemoradiation with maintenance
chemotherapy using taxanes on patient prognosis. Mol Clin Oncol.
11:498–504. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fujisawa Y, Yoshino K, Kadono T, Miyagawa
T, Nakamura Y and Fujimoto M: Chemoradiotherapy with taxane is
superior to conventional surgery and radiotherapy in the management
of cutaneous angiosarcoma: A multicentre, retrospective study. Br J
Dermatol. 171:1493–1500. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mark RJ, Poen JC, Tran LM, Fu YS and
Juillard GF: Angiosarcoma. A report of 67 patients and a review of
the literature. Cancer. 77:2400–2406. 1996.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Florou V, Rosenberg AE, Wieder E,
Komanduri KV, Kolonias D, Uduman M, Castle JC, Buell JS, Trent JC
and Wilky BA: Angiosarcoma patients treated with immune checkpoint
inhibitors: A case series of seven patients from a single
institution. J Immunother Cancer. 7(213)2019.PubMed/NCBI View Article : Google Scholar
|