Introduction
Poorly differentiated thyroid carcinoma (PDTC) is a
clinically and molecularly inchoate histologic variant of thyroid
carcinoma. PDTC occupies an intermediate position between
well-differentiated thyroid carcinoma (WDTC) and anaplastic thyroid
carcinoma (ATC) with regard to both histological features and
biologic aggressiveness and is often associated with locoregional
recurrence and distant disease leading to compromised survival
(1,2). PDTC was originally classified as a
distinct clinicopathologic variant in 2004 by the World Health
Organization (WHO), but it was not until 2006 that more stringent
criteria, known as the ‘Turin criteria’, were established to allow
diagnosis standardization (2,3). Turin
proposal stated that PDTC diagnosis should be rendered on
histologic specimens demonstrating solid/trabecular/insular growth
pattern, absence of conventional nuclear features of PTC, and at
least one of the following features: Convoluted nuclei, necrosis or
mitotic index ≥3/10 high power fields (HPFs) (2-4).
Although PDTC has distinct diagnostic criteria and
can develop de novo, in a significant proportion of patients,
PDTC-like features may occur simultaneously with follicular,
papillary or anaplastic carcinoma stressing the possibility that
PDTC may arise by dedifferentiation of pre-existing WDTC (5,6). The
mutational events that orchestrate this malignant progression are
ill-defined. Genetic alterations frequently detected in PDTC
include TP53, RAS, BRAF and CTNNB1 genes (7,8).
Environmental factors, such as iodine deficiency and
prior neck irradiation, have been implicated in the pathogenesis of
PDT (9). In chorus with other
thyroid carcinoma subtypes, PDTC demonstrates a female predominance
(9).
TP53 gene encodes a nuclear transcription factor
that regulates cell cycle by inducing arrest at the G1/S check
point and promotes apoptosis, hence functioning as a tumor
suppression gene. Inactivation of the p53 gene has often been
reported in undifferentiated thyroid carcinomas (10). Wild type p53 is an unstable protein
which is present at very low cellular levels due to murine double
minute gene 2 (MDM2)-mediated degradation by cytoplasmic and
nuclear proteasomes (11). On the
contrary mutated counterpart is resistant to MDM2-mediated
degradation, resulting in abnormal stabilization and
immunohistochemically detectable protein accumulation (12).
The aim of this case report presentation is to
clearly depict and confirm the hypothesis of the natural
progression of WDTC to ATC, showing the progression of tumor
dedifferentiation in a single patient, with progressive loss of p53
expression from the well differentiated to the dedifferentiated
components, confirming the hypothesis that p53 dysregulation may
contribute to the natural history of the disease.
Case report
A 60-year-old man presented with a palpable anterior
neck mass, which was firm and fixed to underlying structures. The
mass caused mild compressive symptoms including neck fullness and
dysphagia. The patient also manifested multiple cutaneous lesions
with histopathologic features compatible with neurofibroma. He did
not fulfill though at least one more of the seven cardinal
diagnostic criteria for von Recklinghausen disease, as he did not
have freckling of the groin or the axilla, café-au-lait macules,
skeletal abnormalities, Lisch nodules or a first-degree relative
with the disease. He refused imaging control to exclude an optic
glioma, although optic fields were normal with finger confrontation
test. He also refused mutational analysis. The diagnosis of von
Recklinghausen disease could therefore not be established. Cervical
radiation exposure was not elicited from past history. Iodine
status did not need to be evaluated, since the patient lives in an
iodine replete country.
The patient's medical record included
hypercholesterolemia, psychotic disorder, posttraumatic acute
subdural hematoma and nasal polyps. Family history was
unremarkable, except from a 46-year-old sister with breast cancer
and total thyroidectomy due to multinodular goiter.
Hormonal evaluation was conducted to evaluate
thyroid function: TSH was 2.5 µIU/ml (normal values 0.27-4.2) and
calcitonin 4.2 pg/ml (normal values <10 pg/ml). The patient was
also subjected to 24 h urine collection to rule out the presence of
a pheochromocytoma, on grounds of unproven von Recklinghausen
disease, at the prospect of surgery: Vanillylmandelic acid (VMA)
2.1 µg/24 h (normal values 1-11), catecholamines 220 µg/24 h
(normal values 80-515), metanephrines 108 µg/24 h (normal value
52-341), adrenaline 6 µg/24 h (normal values 4-25), noradrenaline
46.1 µg/24 h (normal values 20-105), dopamine 168.6 µg/24 h (normal
values 60-440).
Ultrasound revealed a 5.84x2.81 cm hypoechogenic
nodule on the left lobe with peripheral vascularity, which
comprised a 2.24x1.28 cm area with suspicious calcifications. Three
smaller hypoechogenic nodules with peripheral vascularity,
measuring 3.26, 3.18 and 0.83 cm respectively, were also observed
on the same lobe. On the right lobe three isoechogenic nodules with
cystic areas and no remarkable vascularity, dimensions 2.19, 1.47
and 0.73 cm, were found. No suspicious lymph nodes were detected by
ultrasound.
Cytological examination of a fine needle aspiration
(FNA) biopsy specimen was omitted from the diagnostic workup due to
patient refusal, and consequently the patient underwent total
thyroidectomy. Postoperative course was uneventful. The specimens
underwent histopathological examination by classic H&E staining
and immunohistochemical detection of p53 protein, as previously
described (13). Briefly, H&E
staining was performed according to routine hospital procedures,
while p53 staining was performed using a mouse monoclonal anti-p53
antibody (1:3,000; DO-7; Dako) as the primary antibody. The
staining intensity was graded as negative, weak, moderate and
strong. Histopathological examination revealed three foci at the
right lobe, dimensions 0.6, 0.8 and 2.4 cm respectively, which
showed predominantly papillary but also follicular growth pattern
with infiltrating margins and foci of extrathyroidal extension.
At the left lobe two lesions of 6.0 and 6.5 cm with
PDTC-like histologic features of insular and trabecular variant of
PDTC were described. The tumors were composed of small uniform
cells and of cells with large irregular nuclei, with partially
acidophil characteristics, and were surrounded by fibrous stroma.
The larger tumor showed foci of anaplastic transition, with diffuse
growth pattern, nuclear pleomorphism and areas of necrosis. Both
tumors invaded thyroid capsule and displayed extrathyroidal
extension reaching the inked surgical margins. Vascular emboli were
also identified. A dissected isthmic lymph node was free of
disease.
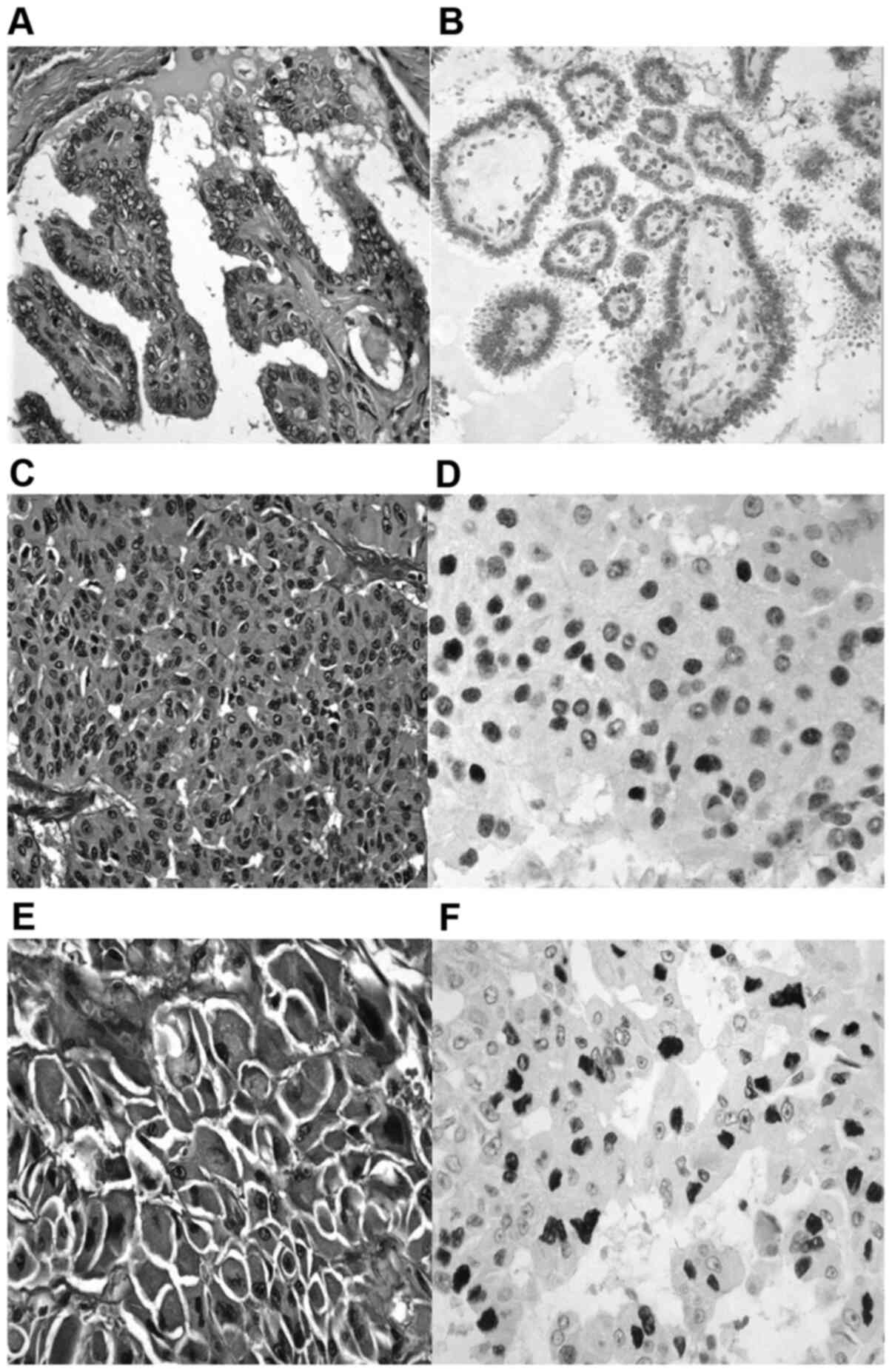
WDTC carcinoma demonstrated weak nuclear p53 protein
expression (Fig. 1A and B). PDTC appeared with diffuse, moderate to
strong nuclear p53 protein accumulation (Fig. 1C and D). ATC showed diffuse, strong nuclear p53
protein accumulation (Fig. 1E and
F). The patient was referred for
radioiodine remnant ablation.
Discussion
PDTC and ATC have been conjectured to arise from
WDTC due to frequently reported synchronous and metachronous
occurrence. The somatic molecular events and downstream pathways of
this dedifferentiation course have not been unraveled yet. In this
paper we demonstrate the simultaneous presence of the three
divergent histological subtypes in a single thyroid gland, with
progressive enhancement of nuclear p53 protein expression,
correlating with mutant p53 protein, in the more aggressive
variants.
PDTC is a rare thyroid carcinoma histotype that
occupies an intermediate position between WDTC and ATC in regards
of both morphological features and biologic behavior (1,2). PDTC
was initially designated as a distinct entity in 2004 by WHO, but
more refined criteria were established in the 2006 Turin proposal
(2-4).
In juxtaposition to WDTH, which usually has an indolent course,
PDTC diagnosis signals a steep increase in mortality and accounts
for a disproportionate number of deaths due to non-ATC (9).
The coexistence of WDTC or ATC with PDTC-like tumor
components has frequently been observed, suggesting that PDTC could
represent a bridge in the stepwise dedifferentiation from WDTC to
ATC (14). Although the molecular
pathways underlying this intricate procedure have not been
elucidated yet, loss of p53 tumor suppressor gene has been assumed
to convey to this anaplastic transformation, since p53 is well
expressed in WDTC but abated in PDTC and ATC (15).
Genetic analysis of thyroid malignancies has shown
varying correlations of specific mutations with tumor
aggressiveness. Although rearrangement of the RET proto-oncogene
are frequent even in the early stages of thyroid tumorigenesis, RAS
oncogene family mutations and BRAF600 gene mutations have failed to
date to show a distinct prognostic significance for recurrence and
overall survival. In the other hand, mutations in the TERT promoter
gene (especially in combination with BRAF600 gene mutations) and
p53 tumor suppressor gene mutations, have been associated with poor
prognosis and have been implicated in the progression of WDTC to
dedifferentiation (16). In detail,
mutations in p53 gene leads to either the expression of mutant
protein or to complete absence of expression. These mutations are
seen in up to 25% of patients with PDTC and in over 60% of patients
with ATC (17). However, various
studies have shown mutations in well differentiated tumors as well
(18,19). This reveals that p53 mutation is an
early event in the sequence of tumor dedifferentiation and
transformation to ATC.
There is no explicit consensus regarding the optimal
management of PDTC, partially due to its infrequent occurrence and
the previous heterogeneity of inclusion criteria (1,20,21).
The mainstay of treatment usually comprises total thyroidectomy
plus regional lymph dissection when required whereas the efficacy
of other modalities, such as radioactive iodine ablation (RAI),
external beam radiation therapy and chemotherapy is still
questionable (22). Although RAI
treatment is commonly implemented, PDTC may exhibit decreased radio
avidity and thus be refractory (6,22).
Finally, although our patient did not typically
fulfill the diagnostic criteria for von Recklinghausen disease, the
coexistence of neurofibromas is intriguing due to the scarcity of
reported coexistence of thyroid carcinoma with neurofibromatosis in
the literature (23-25).
Both PDTC and ATC demonstrate aggressive behavior
and forecast a robust increase in coherent mortality. Ιt is
consequently of key interest to illuminate the mechanisms of the
dedifferentiation process. Herein, we present a rare case of
gradual enhancement of nuclear mutated p53 protein expression in
multifocal thyroid tumor areas consisting of WDTC, ATC histology
and PDTC-like features. This observation strengthens the
postulation that ATC and PDTC could arise from progenitor foci of
WDTC through a multifactorial process involving loss of normal p53
function.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the current study, as
this is a report of an interesting case. Patient records are
available upon request from the corresponding author.
Authors' contributions
GB, KN, EK and EPi contributed to conception and
design of the manuscript. EPa, AL, DP, AnaP and AndP acquired,
analyzed and interpreted the data, and drafted the manuscript. GB,
KN and EPi revised the manuscript. DP, KN and EK confirm the
authenticity of all the raw data. All authors have read and
approved the final version of this manuscript.
Ethics approval and consent to
participate
No ethics approval was obtained as the patient
received standard treatment. Consent for treatment was obtained by
the patient per protocol.
Patient consent for publication
The patient has provided written informed consent
for the publication of this case report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
DeLellis RA, Lloyd VR, Heitz PU and Eng C
(eds): World Health Organization Classification of Tumours:
Pathology and Genetics of Tumours of Endocrine Organs. IARC Press,
Lyon, 2004.
|
|
2
|
Sadow PM and Faquin WC: Poorly
differentiated thyroid carcinoma: An incubating entity. Front
Endocrinol (Lausanne). 3(77)2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tallini G: Poorly differentiated thyroid
carcinoma. Are we there yet? Endocr Pathol. 22:190–194.
2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Volante M, Collini P, Nikiforov YE,
Sakamoto A, Kakudo K, Katoh R, Lloyd RV, LiVolsi VA, Papotti M,
Sobrinho-Simoes M, et al: Poorly differentiated thyroid carcinoma:
The turin proposal for the use of uniform diagnostic criteria and
an algorithmic diagnostic approach. Am J Surg Pathol. 31:1256–1264.
2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Garcia-Rostan G and Sobrinho-Simões M:
Poorly differentiated thyroid carcinoma: An evolving entity.
Diagnostic Histopathol. 17:114–123. 2011.
|
|
6
|
Volante M and Papotti M: Poorly
differentiated thyroid carcinoma: 5 years after the 2004 WHO
classification of endocrine tumours. Endocr Pathol. 21:1–6.
2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Eloy C, Ferreira L, Salgado C, Soares P
and Sobrinho-Simões M: Poorly differentiated and undifferentiated
thyroid carcinomas. Turk Patoloji Derg. 31 (Suppl 1):S48–S59.
2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Omur O and Baran Y: An update on molecular
biology of thyroid cancers. Crit Rev Oncol Hematol. 90:233–252.
2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Walczyk A, Kowalska A and Sygut J: The
clinical course of poorly differentiated thyroid carcinoma (insular
carcinoma)-own observations. Endokrynol Pol. 61:467–473.
2010.PubMed/NCBI
|
|
10
|
Quiros RM, Ding HG, Gattuso P, Prinz RA
and Xu X: Evidence that one subset of anaplastic thyroid carcinomas
are derived from papillary carcinomas due to BRAF and p53
mutations. Cancer. 103:2261–2268. 2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Levine AJ: p53, the cellular gatekeeper
for growth and division. Cell. 88:323–331. 1997.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Midgley CA and Lane DP: p53 protein
stability in tumour cells is not determined by mutation but is
dependent on Mdm2 binding. Oncogene. 15:1179–1189. 1997.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yemelyanova A, Vang R, Kshirsagar M, Lu D,
Marks MA, Shih IM and Kurman RJ: Immunohistochemical staining
patterns of p53 can serve as a surrogate marker for TP53 mutations
in ovarian carcinoma: An immunohistochemical and nucleotide
sequencing analysis. Mod Pathol. 24:1248–1253. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lam KY, Lo CY, Chan KW and Wan KY: Insular
and anaplastic carcinoma of the thyroid: A 45-year comparative
study at a single institution and a review of the significance of
p53 and p21. Ann Surg. 231(329)2000.PubMed/NCBI View Article : Google Scholar
|
|
15
|
McIver B, Hay ID, Giuffrida DF, Dvorak CE,
Grant CS, Thompson GB, van Heerden JA and Goellner JR: Anaplastic
thyroid carcinoma: A 50-year experience at a single institution.
Surgery. 130:1028–1034. 2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Penna GC, Vaisman F, Vaisman M,
Sobrinho-Simoes M and Soares P: Molecular markers involved in
tumorigenesis of thyroid carcinoma: Focus on aggressive histotypes.
Cytogenet Genome Res. 150:194–207. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Manzella L, Stella S, Pennisi MS, Tirro E,
Massimino M, Romano C, Puma A, Tavarelli M and Vigneri P: New
insights in thyroid cancer and p53 family proteins. Int J Mol Sci.
18(1325)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Marcello MA, Morari EC, Cunha LL, De Nadai
Silva AC, Carraro DM, Carvalho AL, Soares FA, Vassallo J and Ward
LS: P53 and expression of immunological markers may identify early
stage thyroid tumors. Clin Dev Immunol. 2013(846584)2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Shin MK, Kim JW, Min SK, Lee DJ, Kim JH,
Lee SC, Chung BW and Ju YS: Associations of the BRAF (V600E)
mutation and p53 protein expression with clinicopathological
features of papillary thyroid carcinomas patients. Oncol Lett.
10:1882–1888. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sanders EM Jr, LiVolsi VA, Brierley J,
Shin J and Randolph GW: An evidence-based review of poorly
differentiated thyroid cancer. World J Surg. 31:934–945.
2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Collini P, Mattavelli F, Pellegrinelli A,
Barisella M, Ferrari A and Massimino M: Papillary carcinoma of the
thyroid gland of childhood and adolescence: Morphologic subtypes,
biologic behavior and prognosis: A clinicopathologic study of 42
sporadic cases treated at a single institution during a 30-year
period. Am J Surg Pathol. 30:1420–1426. 2006.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hannallah J, Rose J and Guerrero MA:
Comprehensive literature review: Recent advances in diagnosing and
managing patients with poorly differentiated thyroid carcinoma. Int
J Endocrinol. 2013(317487)2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hashiba T, Maruno M, Fujimoto Y, Suzuki T,
Wada K, Isaka T, Izumoto S and Yoshimine T: Skull metastasis from
papillary thyroid carcinoma accompanied by neurofibromatosis type 1
and pheochromocytoma: Report of a case. Brain Tumor Pathol.
23:97–100. 2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kim BK, Choi YS, Gwoo S, Park YH, Yang SI
and Kim JH: Neurofibromatosis type 1 associated with papillary
thyroid carcinoma incidentally detected by thyroid ultrasonography:
A case report. J Med Case Rep. 6(179)2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Koksal Y, Sahin M, Koksal H, Esen H and
Sen M: Neurofibroma adjacent to the thyroid gland and a thyroid
papillary carcinoma in a patient with neurofibromatosis type 1:
Report of a case. Surg Today. 39:884–887. 2009.PubMed/NCBI View Article : Google Scholar
|