Introduction
Nervous sheath tumors account for approximately 25%
of tumors of the intradural-extramedullary space, while
approximately 65% of them are schwannomas (1). Schwannomas represent slow-growing
benign tumors arising from Schwann cells of the nerve sheaths of
peripheral nerves and believed to originate from embryonic neural
crest cells (1,2).
The presenting symptoms depend on the tumor's
location and the degree of the spinal cord or the nerve root
compression. Patients usually complain about pain, motor deficits,
paresthesia and numbness. Symptoms are usually slowly progressive,
with some cases being asymptomatic and accidentally diagnosed by
imaging. Acute paraplegia is an extremely rare presenting symptom
usually associated with intratumoral hemorrhage with or without
related trauma (2). Furthermore,
the growth pattern of spinal schwannomas is not well known.
Therefore, although radical excision is recommended for symptomatic
tumors, the optimal treatment for the asymptomatic ones remains
unclear (2).
A case of 58-year-old male, suffering from
thoracolumbar schwannoma presenting with acute paraplegia, after a
fall is described. Histopathology did not reveal hemorrhage.
Taking into account the existing literature, the
present represents the first case of acute paraplegia, following
trauma, due to a thoracolumbar schwannoma, without intratumoral
hemorrhage, in a previously asymptomatic patient.
Case report
A 58-year-old male, with unremarkable medical
history, was admitted to the University Hospital of Heraklion
(Heraklion, Greece) due to acute paraplegia following a fall from a
tree (a height of 3 m).
The patient was oriented, afebrile (36.5˚C) and
hemodynamically stable (blood pressure=130/95 mmHg, heart rate=85
beats/min). He had no prior history of back pain or any other
symptoms.
On admission, motor examination revealed grade 0/5
power in both lower limbs. Sensation of lower limbs was impaired in
both: light touch, as well as pin prick, while patellar and
Achilles reflexes were absent. Furthermore, there was urinary and
bowel incontinence.
Emergency computer tomography (CT) scan showed
fracture of the right T12-L1 facet joint and of the right
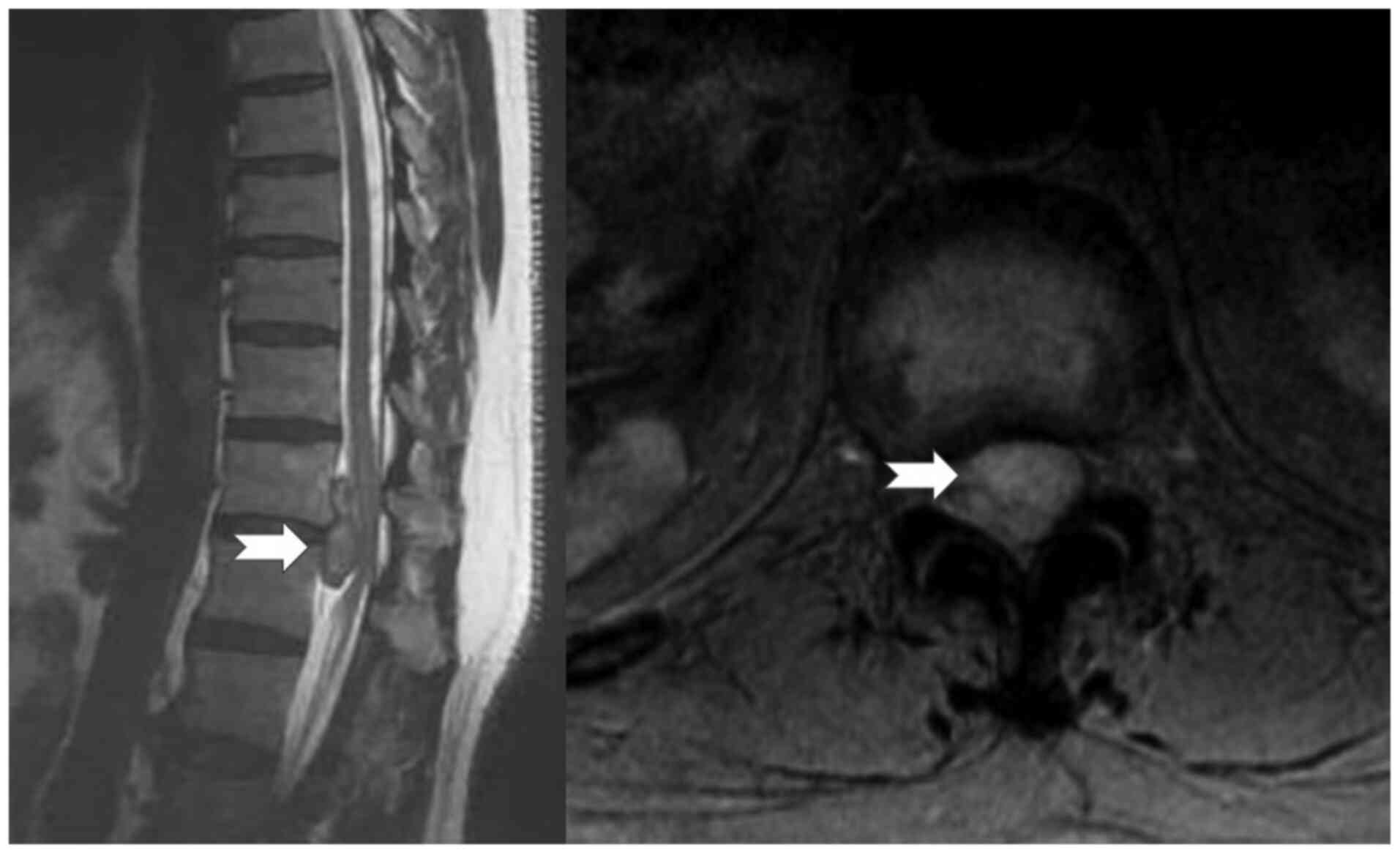
transverse process at the same level. A consequent magnetic
resonance imaging (MRI) revealed a well-defined, bilobular subdural
extramedullary mass, with a maximum diameter of 3.8 cm at the
vertical axis, compressing the spinal cord (Fig. 1).
He underwent emergency T11-L1 wide laminectomy,
combined with facetectomy on the affected levels, exploration of
the subdural space, debulking of the tumor and T10-L2
posterolateral transpedicular stabilization and fusion, while an
intradural, extramedullary mass, causing severe cord compression,
was found. The mass was completely removed through microsurgical
dissection from the surrounded nerve roots. The maternal root
failed to be identified. Hence, it was assumed that it had been
already damaged by the tumor, apparently without significant
functional value.
No intraoperative signs of peri-or intra-tumor
bleeding were observed. After the tumor's complete excision, the
spinal cord was sufficiently decompressed. The timeframe between
the injury and the surgical intervention was approximately 8 h.
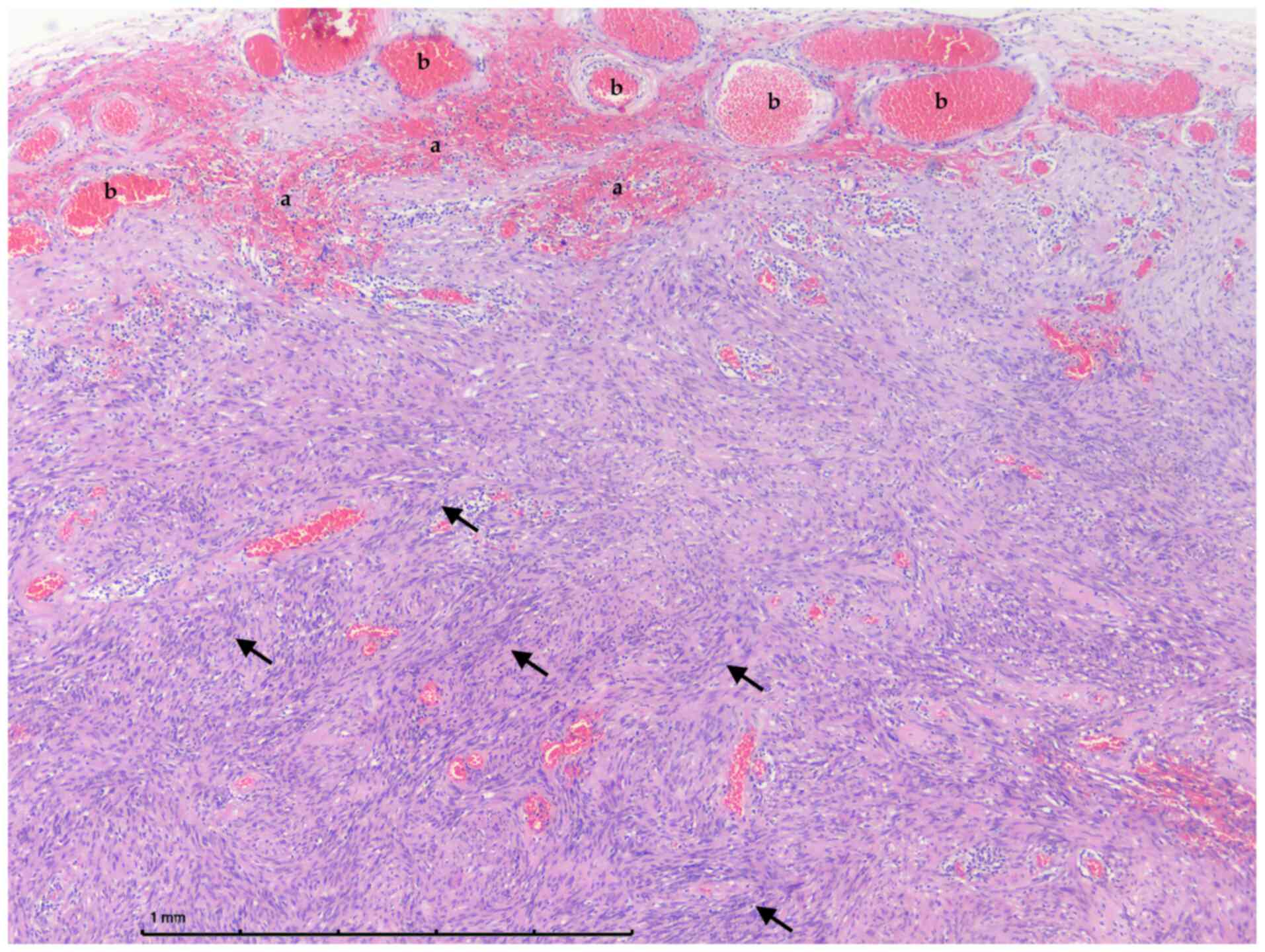
The excised tumor was stored in 10% buffered
formalin and sent for histopathological examination. Routine
histopathological analysis by hematoxylin/eosin stain revealed a
neuronal sheath tumor which was consist of alternatively by
cellular areas with numerous Verocay bodies (Antoni A) and few less
cellular areas (Antoni B) without any signs of atypia, necrosis and
intratumoral hemorrhage (Fig. 2).
The tumor was consistent with schwannoma.
The postoperative period was uneventful with rapid
recovery. Both motor and sensory functions improved gradually. At
the 4th postoperative day, he had regained muscle strength in both
lower limbs (2/5 power). He was discharged and he followed a
rehabilitation program strengthening his muscles for several
weeks.
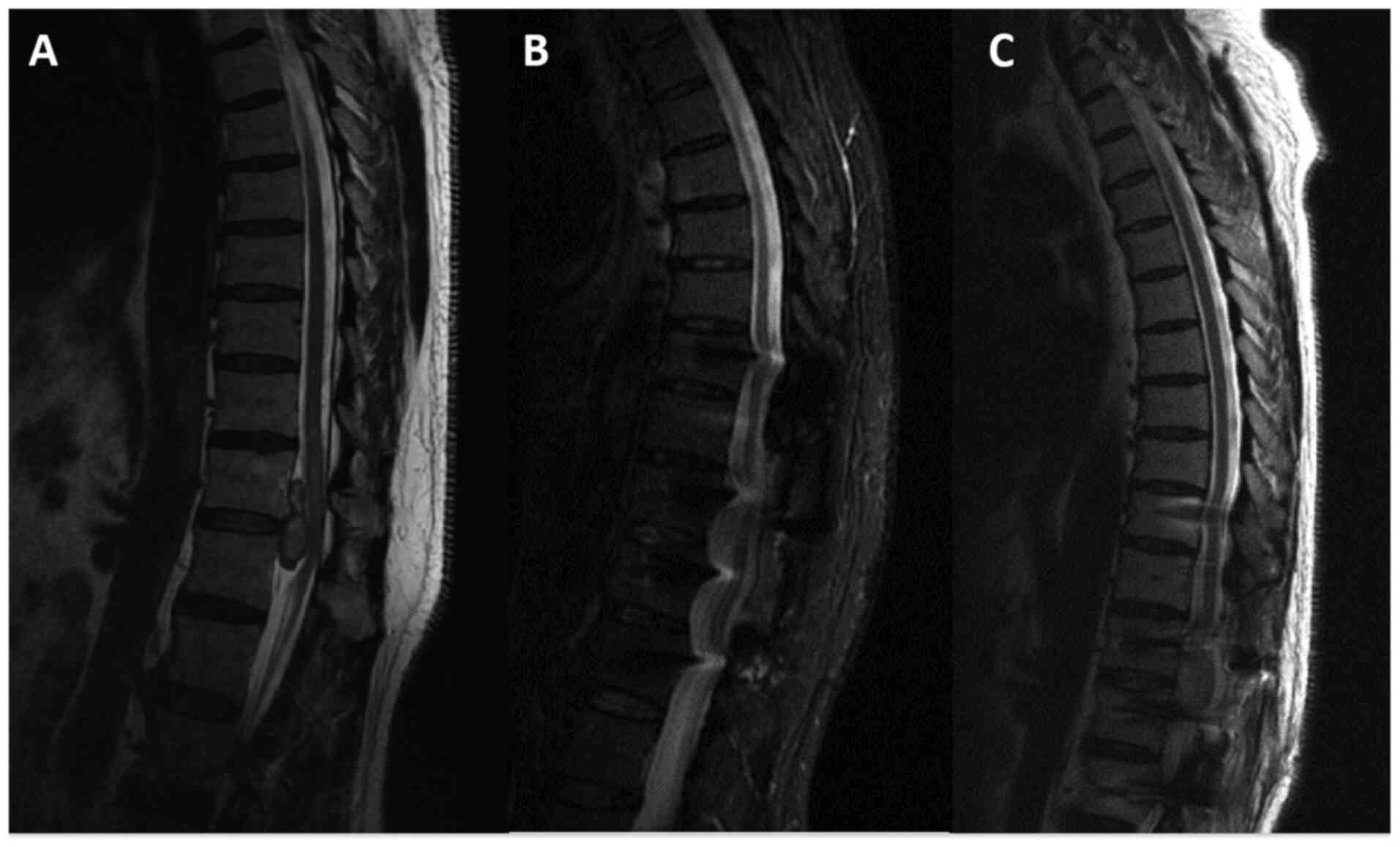
Six months later he had regained full strength of
both lower limbs, being ambulant without support. He was followed
up for a total of 44 months, being in excellent condition, fully
active, without neurological deficits. MRI scans at 6 and 44 months
after surgery, revealed normal spinal canal, in comparison with the
schwannoma occupied canal, revealed in the preoperative MRI scan
(Fig. 3).
Discussion
Spinal schwannomas account for one third of the
primary spinal neoplasms, while the clinical presentation of these
tumors is usually related to their location. They typically present
with symptoms and signs of myelopathy or nerve root compression, as
well as cauda equine syndrome (3).
Symptoms are insidious and slowly progressive, due to the slow
tumor growth. Early diagnosis and surgical removal are critical,
since long-term spinal cord compression may lead to permanent
neurological deficits (4). Thorough
physical examination in combination with MRI of the area are highly
diagnostic. Acute paraplegia as presenting symptom is extremely
rare and almost always associated with acute intratumoral
hemorrhage (4).
Table I summarizes
the reported cases of spinal schwannomas with intratumoral
hemorrhage (traumatic or spontaneous), presented with acute
neurologic deficit (3,5-24).
There is a limited number of cases with rapidly progressive
neurological deficits due to acute cord compression, caused by
tumor bleeding. History of prior symptoms is uncommon (8,13,14,16,20).
Sudden cord compression caused by tumor bleeding is similar to
spinal shock (20). Nine traumatic
cases with intratumoral bleeding have been reported so far
(8,13,16,19-24).
 | Table IReported cases of spinal schwannomas
with intratumoral hemorrhage (traumatic or spontaneous), presenting
with acute neurologic deficit. |
Table I
Reported cases of spinal schwannomas
with intratumoral hemorrhage (traumatic or spontaneous), presenting
with acute neurologic deficit.
| Authors, year | Study type | Presentation | Spine region | Trauma | Prior symptoms | Treatment | Histology | (Refs.) |
|---|
| Smith, 1985 | Case report | Cervical transverse
myelopathy | Cervical | No | Severe left scapular
pain for 2 weeks (night pain) | Surgical | Schwannoma
intratumoral hemorrhage | (5) |
| Lee and Lui,
1992 | 2 cases | Paraplegia | Thoracic and
thoracolumbar | No | i) 10-month history
of back pain ii) 2-year history of low back pain | Surgical | Neurofibroma with
focal haemorrhage | (6) |
| Uemura et al,
1998 | Case report | Paraparesis | Thoracic | No | Occasionally slight
sharp pain in right thigh | Surgical | Schwannoma
intratumoral hemorrhage | (7) |
| Cohen et al,
2000 | Case report | Paraplegia | Thoracic | Minor injury (fall
from a ladder) | None | Surgical | Neurinoma, severe
intra- and peritumoral hemorrhage | (8) |
| Ng, 2001 | Case report | Left hemiparesis | Cervical | No | 3-day history of
sudden onset of left shoulder pain radiating to the left forearm
and hand | Surgical | Schwannoma
intratumoral hemorrhage | (9) |
| Tanaka et al,
2002 | Case report | Paraparesis | Thoracic | No | 3-year history of
intermittent episodes of lower back pain | Surgical | Schwannoma intradural
hemorrhage | (10) |
| Parmar et al,
2004 | Case report | Intracranial
subarachnoid hemorrhage | Thoraco-lumbar
junction | No | History of vague
generalized backache for many years | Surgical | Schwannoma
intratumoral hemorrhage | (11) |
| Mahadewa et
al, 2005 | Case report | Paraplegia | Lumbar | No | Stable back pain and
a 4-year history of lower-extremity numbness bilaterally | Surgical | Schwannoma
intratumoral hemorrhage | (3) |
| Ciappetta et
al, 2008 | Case report | Myelopathy | Craniovertebral
junction | No | 3-day history of neck
pain | Surgical | Schwannoma
intratumoral hemorrhage | (12) |
| Sharifi et
al, 2009 | Case report | Severe neurological
deficit | Thoracolumbar | Motor-vehicle
accident | None | Surgical | Multiple
schwannomas | (13) |
| Yeh et al,
2011 | Case report | Paraplegia | Thoracic | No | None | Surgical | Schwannoma
intra-tumoral hematoma | (14) |
| Kukreja et
al, 2014 | Case report | Intracranial
subarachnoid hemorrhage | Cauda equina | No | 3-month history of
seizures A few days history of left leg pain | Surgical | Schwannoma
intratumoral hemorrhage | (15) |
| Jenkins et
al, 2015 | Case report | Paraplegia | Thoracic | Minor
(torsion) | None | Surgical | Schwannoma,
intratumoral hemorrhage | (16) |
| Sahoo et al,
2015 | Case report | Quadriparesis | Cervical | No | Weakness in both
upper and lower limbs for 1 day and neck pain radiating to shoulder
for 2 days | Surgical | Schwannoma
intra-tumoral hematoma | (17) |
| Zhang et al,
2015 | Case report | Paraplegia | Thoracic | No | 12-month back and
bilateral leg pain. Deterioration during the last 3 months | Surgical | Schwannoma
intratumoral hemorrhage | (18) |
| Hdeib et al,
2016 | Case report | Paraplegia | Thoracic | Minor (spinal
manipulation) | Back pain | Surgical | Schwannoma, spinal
intradural hematoma | (19) |
| Prasad et
al, 2016 | Case report | Paraplegia | C7-T3 | Minor fall | None | Surgical | Schwannoma
intratumoral hemorrhage | (20) |
| Vaibhav et
al, 2016 | Case report | Paraplegia | Thoracic | Jolting movement of
a speeding bus | Not known | Surgical | Schwannoma
intratumoral hemorrhage | (21) |
| Nadeem et
al, 2017 | Case report | Paraplegia | Cauda equina | Minor fall | Not known | Surgical | Schwannoma
intradural intramedullary hematoma | (22) |
| Jung et al,
2019 | Case report | Quadriparesis | Cervical | Physical
therapy | Neck pain | Surgical | Schwannoma with
intratumoral hemorrhage | (23) |
| Rahyussalim et
al, 2019 | Case report | Paraplegia
(previously paraparesis) | Thoracolumbar | Spinal manipulation
procedures | Difficulty standing
up from squatting position since 2 years ago | Surgical | Schwannoma with
intratumoral hemorrhage | (24) |
Spontaneous intratumoral hemorrhage is not uncommon
in nervous system tumors. Two main theories exist regarding the
hemorrhage's etiology. The mechanical theory supports that loading
causes traction of the tumor vessels resulting in bleeding, while
the vascular theory postulates that hemorrhage is caused by
spontaneous thrombosis of the tumor vessels, ischemic necrosis and
secondary bleeding (11,20,23,25).
Spinal injury after a traumatic event at the level
of the tumor could possibly lead to bleeding, resulting in acute
canal stenosis and nerve compression (19). In the present case hemorrhage within
the tumor or the spinal canal, although suspected and looked for,
was not identified.
Mahadewa et al (3) have also reported a patient with acute
onset of paraplegia without evidence of intratumoral bleeding.
However, in that case the patient had a history of stable back pain
and numbness in both legs for 4 years. MRI had revealed an
enhancing extra axial mass in the spinal canal, but the patient had
declined surgery. Sudden onset of paraplegia could be possibly due
to compression of neural elements due to infarction of a
significant artery. However, in cases of neurological recovery
without surgical intervention, this explanation cannot be supported
(3).
In the present case, a hypothesis about the sudden
onset of paraplegia could be made: mechanical loading caused by the
fall in an already, due to the tumor, narrow spinal canal, resulted
in acute neurological damage. Preexisting canal stenosis has been
proven to be independent risk factor for developing spinal cord
injury, even with minor trauma and without the presence of fracture
(26). Spinal canal stenosis may be
the reason for the discrepancy between the insignificance of the
trauma and the severity of its results, since acute spinal cord
injury following minor trauma has been reported (27). The spinal cord may become more
vulnerable against external force when the degree of cord
compression exceeds a certain threshold (27).
Little is known regarding the growth pattern of
spinal schwannomas. Although radical excision is recommended for
symptomatic tumors, the optimal treatment for the asymptomatic ones
remains unclear. Radiological features, such as heterogeneous
intensity on T2-weighted MRI images, may provide useful information
regarding the growth potential of spinal schwannomas. In a
retrospective study of 23 patients with 5 years mean follow-up, the
authors reported absolute relative tumors' growth rates of 139 mm³
and 5.3% per year, respectively. Homogeneously hyperintense or
heterogeneously intense on T2-weighted images tumors were
significantly larger than the isointense ones at the initial
examination. Tumors isointense on T2-weighted images increased very
little in volume. However, the schwannomas heterogeneously intense
on T2-weighted images had a significantly greater absolute growth
rate (28). Most asymptomatic
schwannomas have only minimal growth and do not need surgical
excision. Close clinical and imaging monitoring is required when
patients have large-volume tumors that are heterogeneously intense
on T2-weighted images. Surgical removal should be considered in
cases of constant tumor growth with significant compression of the
spinal cord and cauda equina (28).
The present patient retained normal spinal alignment
during the follow-up. Spinal deformities arise in up to 18% of
adults and 100% of children after laminectomy for spinal cord tumor
excision. Although the necessity of fixation in children has been
already documented, evidence supporting concomitant fusion after
spinal cord tumors removal in adults is limited (29).
Kobayashi et al (30) recently examined the records for 32
adults who underwent excision of thoracic spinal cord tumors by
multilevel laminectomies without fixation. They concluded that even
without fixation, sagittal alignment remained unchanged following
tumors' excision in the middle and lower thoracic spine, suggesting
that fixation may not be necessary. On the contrary, when the tumor
is located at the upper thoracic spine, postoperative kyphosis may
increase. However, this study had several limitations including the
retrospective study design, the small sample size and the lack of a
control group treated by laminectomy for a different thoracic
spinal disorder (30).
Avila et al (29) reviewed the criteria for fusion
following spinal cord tumor resection in adults. The main criteria
for fusion were: Preoperative deformity, three or more levels of
laminectomy, laminectomy encompassing a spinal junction, young age,
facectomy >50%, persistent deformity 1 year after surgery and C2
laminectomy. Based on these data, in the present case, posterior
transpendicular fixation following tumor removal was performed.
Taking into account the existing literature, this is
the first case describing a spinal intradural schwannoma causing
acute paraplegia in a previously asymptomatic patient in the
setting of trauma, without evidence of bleeding. Acute neurological
deterioration, such as paraplegia, may not only be the result of
intratumoral hemorrhage but may also occur as a post-traumatic
event, possibly due to violent movement or edema formation within a
marginally narrowed spinal canal. This possibility should be
seriously considered in the management of patients with such tumors
and represents additional reason for early surgery. Furthermore, in
cases of acute post-traumatic neurological deficits that are not
sufficiently justified by the injury per se, the presence of
intracanal tumor should be included in the differential diagnosis.
MRI remains the main diagnostic imaging technique in such cases.
Urgent canal decompression and surgical excision of the tumor is
the treatment of choice.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
DAK, CT and CK made substantial contributions to the
conception and design of the current study. VC, AV, GS and KA
acquired and analyzed the data. DAK, CT and CK drafted the
manuscript. VC, AV, GS and KA critically revised the manuscript. KA
and CK confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent for publication was
received from the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Emel E, Abdallah A, Sofuoglu OE, Ofluoglu
AE, Gunes M, Guler B and Bilgic B: Long-term surgical outcomes of
spinal schwannomas: Retrospective analysis of 49 consecutive cases.
Turk Neurosurg. 27:217–225. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kondziolka D, Bernstein M, Resch L, Tator
CH, Fleming JF, Vanderlinden RG and Schutz H: Significance of
hemorrhage into brain tumors: Clinicopathological study. J
Neurosurg. 67:852–857. 1987.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mahadewa T, Harsan H, Nugroho S and
Bernstein M: Postoperative recovery of complete sudden paraplegia
due to lumbar schwannoma. Case report. J Neurosurg Spine.
2:601–603. 2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Li H, Weng Y, Zhou D, Nong L and Xu N:
Experience of operative treatment in 27 patients with intraspinal
neurilemmoma. Oncol Lett. 14:4817–4821. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Smith RA: Spinal subdural hematoma,
neurilemmoma, and acute transverse myelopathy. Surg Neurol.
23:367–370. 1985.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lee ST and Lui TN: Acute paraplegia
resulting from haemorrhage into a spinal neurofibroma. Paraplegia.
30:445–448. 1992.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Uemura K, Matsumura A, Kobayashi E, Tomono
Y and Nose T: CT and MR presentation of acute hemorrhage in a
spinal schwannoma. Surg Neurol. 50:219–220. 1998.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cohen ZR, Knoller N, Hadani M, Davidson B,
Nass D and Ram Z: Traumatic intratumoral hemorrhage as the
presenting symptom of a spinal neurinoma. J Neurosurg. 93 (Suppl
2):S327–S329. 2000.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ng PY: Schwannoma of the cervical spine
presenting with acute haemorrhage. J Clin Neurosci. 8:277–278.
2001.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tanaka H, Kondo E, Kawato H, Kikukawa T,
Ishihara A and Toyoda N: Spinal intradural hemorrhage due to a
neurinoma in an early puerperal woman. Clin Neurol Neurosurg.
104:303–305. 2002.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Parmar H, Pang BC, Lim CC, Chng SM and Tan
KK: Spinal schwannoma with acute subarachnoid hemorrhage: A
diagnostic challenge. AJNR Am J Neuroradiol. 25:846–850.
2004.PubMed/NCBI
|
|
12
|
Ciappetta P, D'Urso PI and Colamaria A:
Giant craniovertebral junction hemorrhagic Schwannoma: Case report.
Neurosurgery. 62(E1166)2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sharifi G, Mortaz M and Parsaei B:
Multiple intradural extramedullary tumours presenting with
paraplegia after trauma. Acta Neurochir (Wien). 151:697–698.
2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yeh HM, Leung JH, Huang KC, Tung CL, Huang
CL and Huang KM: A long segmental hemorrhagic spinal schwannoma
with atypical presentation. J Radiol Sci. 36:191–194. 2011.
|
|
15
|
Kukreja S, Ambekar S, Sharma M and Nanda
A: Cauda equina schwannoma presenting with intratumoral hemorrhage
and intracranial subarachnoid hemorrhage. J Neurosurg Spine.
21:357–360. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jenkins AL III, Ahuja A, Oliff AH and
Sobotka S: Spinal Schwannoma presenting due to torsion and
hemorrhage: Case report and review of literature. Spine J.
15:e1–e4. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sahoo RK, Das PB, Sarangi GS and Mohanty
S: Acute hemorrhage within intradural extramedullary schwannoma in
cervical spine presenting with quadriparesis. J Craniovertebr
Junction Spine. 6:83–85. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang HZ, Li Y, Han Y, Wang X, She L, Yan
Z and Dong L: Spontaneous acute hemorrhage of intraspinal canal
cellular schwannoma with paraplegia: A case report. Br J Neurosurg.
29:425–427. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hdeib A, Goodwin CR, Sciubba D, Bydon A,
Wolinsky JP, Witham T and Gokaslan ZL: Hemorrhagic thoracic
schwannoma presenting with intradural hematoma and acute paraplegia
after spinal manipulation therapy. Int J Spine Surg.
20(42)2016.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Prasad GL, Kongwad LI and Valiathan MG:
Spinal intradural schwannoma with acute intratumoural haemorrhage:
Case report and review. J Clin Diagnostic Res. 10:PD01–3.
2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Vaibhav N, Mahalingam SS and Parthiban
JKBC: Hemorrhage within the schwannoma of thoracic spinal cord
presenting as acute rapidly progressive paraplegia: A rare case. J
Spinal Surg. 3:160–162. 2016.
|
|
22
|
Nadeem M, Mansoor S, Assad S, Ilyas F,
Qavi AH and Saadat S: Spinal schwannoma with intradural
intramedullary hemorrhage. Cureus. 9(e1082)2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jung GS, Lee YM, Kim YZ and Kim JS:
Intratumoral hemorrhage of the cervical spinal schwannoma
presenting: Acute quadriparesis. Brain Tumor Res Treat. 7:160–163.
2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Rahyussalim AJ, Wisnubaroto RP, Kurniawati
T, Latsarizul ASB and Chairani N: Hemorrhagic spinal schwannoma in
thoracolumbar area with total paraplegia. Case Rep Med.
2019(7190739)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Koyanagi I, Iwasaki Y, Hida K, Akino M,
Imamura H and Abe H: Acute cervical cord injury without fracture or
dislocation of the spinal column. J Neurosurg. 93 (Suppl
1):S15–S20. 2000.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Aebli N, Rüegg TB, Wicki AG, Petrou N and
Krebs J: Predicting the risk and severity of acute spinal cord
injury after a minor trauma to the cervical spine. Spine J.
13:597–604. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Oichi T, Oshima Y, Okazaki R and Azuma S:
Preexisting severe cervical spinal cord compression is a
significant risk factor for severe paralysis development in
patients with traumatic cervical spinal cord injury without bone
injury: A retrospective cohort study. Eur Spine J. 25:96–102.
2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ando K, Imagama S, Ito Z, Kobayashi K,
Yagi H, Hida T, Ito K, Tsushima M, Ishikawa Y and Ishiguro N: How
do spinal schwannomas progress? The natural progression of spinal
schwannomas on MRI. J Neurosurg Spine. 24:155–159. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Avila MJ, Walter CM, Skoch J, Abbasifard
S, Patel AS, Sattarov K and Baaj AA: Fusion after intradural spine
tumor resection in adults: A review of evidence and practices Clin
Neurol. Neurosurg. 138:169–173. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kobayashi Y, Kawabata S, Nishiyama Y,
Tsuji O, Okada E, Fujita N, Yagi M, Watanabe K, Matsumoto M,
Nakamura M and Nagoshi N: Changes in sagittal alignment after
surgical excision of thoracic spinal cord tumors in adults. Spinal
Cord. 57:380–387. 2019.PubMed/NCBI View Article : Google Scholar
|