Introduction
Lung cancer is a leading cause of cancer-related
deaths worldwide and number of lung cancer deaths is estimated to
increase by 86% by 2035 (1,2).
For years, standard treatments for the patients with
advanced metastatic non-small cell lung cancer (NSCLC) included
cytotoxic chemotherapy and specific inhibitors for NSCLC subtypes,
harboring epidermal growth factor receptor (EGFR) mutations or
chromosomal rearrangements of anaplastic lymphoma kinase (ALK)
(3). As a result, the patient with
advanced NSCLC had a median survival of approximately 1 year after
chemotherapy and 2-3 years after the treatment with EGFR tyrosine
kinase inhibitors (4,5).
Recently, immunotherapy with immune checkpoint
inhibitors has demonstrated durable responses and significantly
improved survival rate in the patients with NSCLC. Programmed death
protein 1 (PD-1) and its ligand (PD-L1) are the first introduced
checkpoints being targeted in NSCLC and according to several
clinical trials, antibodies against PD-1 and PD-L1 have significant
efficacy in both, the first line and the second line treatment of
metastatic NSCLC (6-9).
Despite of demonstrated successes, response to the immunotherapy
interventions is seen only in a subset (≤5%) of patients (10). Therefore, a substantial amount of
current research is focused on improved performance of
immunotherapies with novel combinations (including cytotoxic
agents) together with biomarker optimization (11,12).
In fact, the immunotherapy and chemotherapy combinations that are
already proven to be more effective are now entered into clinical
practice of metastatic NSCLC management (9,13).
To date, the most advanced biomarker for NSCLC is an
immunohistochemical expression level of PD-L1 on the tumor cells.
In different NSCLC cell line models and between patients, the
expression levels of PD-L1 may vary significantly (14), whereas several phase-III clinical
studies have reported better treatment responses in patients with
higher tumor proportion score of PD-L1 (7-9).
Previous reports additionally showed that the treatment efficacy of
immune checkpoint inhibitors also correlated with the molecular
smoking signature, higher neoantigen burden, and DNA repair pathway
mutations that all lead to high mutational burden (15,16).
Furthermore, it has been reported that pathogenic DNA damage
response (DDR) and repair mutations are associated with improved
treatment response rate, progression-free survival, and overall
survival in patients with NSCLC treated with programmed death
ligand 1 [PD-(L)1] inhibitor therapy (17). Therefore, it is suggested that
combining DDR inhibitors with DNA damaging agents, or PD-1 blockade
can enhance the overall DNA damage and induce a more sustained
upregulation of the cGAS-STING pathway and consequently the
production of Th1 cytokines (18).
Changes in DNA repair machinery are especially
important, when combinations of immune checkpoint inhibitors and
conventional cytotoxic anti-cancer therapies (chemotherapy,
radiotherapy) are in consideration. It has been demonstrated that
an ability of cancer cells to repair therapeutically induced DNA
damage has extensive impacts on anti-cancer therapeutic efficacy
(19). Moreover, since DDR
inhibition can also induce and amplify DNA damage in cancer cells,
combining DDR inhibitors with immune checkpoint inhibitors and/or
cytotoxic therapies represents an attractive strategy to improve
patient outcomes (18).
DNA-dependent protein kinase catalytic subunit
(DNA-PKcs), encoded by PRKDC/XRCC7 gene, is a pivotal DNA damage
response (DDR) player (20). Along
with Ku heterodimer, DNA-PKcs forms the DNA-PK holoenzyme complex.
Of note, Ku heterodimer consists of Ku70 (XRCC6) and Ku80 (XRCC5)
subunits. DNA-PK holoenzyme complex is a core component of
non-homologous end joining (NHEJ) machinery, which alongside
homologous recombination (HR), comprise the two major canonical
pathways for DNA double strand breaks (DSBs) repair (21,22).
Expression and functioning of DNA-PK in several NSCLC cell lines
has been reported (23), whereas
the inhibition of DNA-PK was shown to enhance chemosensitivity as
well as radiosensitivity in these cells (24,25).
Despite of that, interactions between PD-1/PD-L1 axis and DNA-PK
are not fully explored.
The aim of our retrospective study was to describe
the relationship between the expression of immune checkpoints PD-1
and PD-L1 and an enzyme DNA-PK, which is a part of key pathway for
a repair of cancer therapy induced damage. For that, we carried out
the immunohistochemical staining of the proteins of interest in the
biopsy specimen obtained from patients thus providing a unique
insight into the tumor and its microenvironment which includes the
components of immune system.
Materials and methods
Patients and tissue samples
Tumor tissue samples were obtained from 121 patients
with NSCLC, who were operated at Tartu University Hospital. None of
patients received any cytotoxic drugs before surgery.
Clinicopathological features of the recruited patients including
age, sex, histologic type, and smoking status were collected from
their medical records. The study was approved by the Reseach Ethics
Committee of University of Tartu.
Microscopy and immunohistochemistry
(IHC)
Surgically excised NSCLC specimens were immediately
fixed in buffered 10% formaline (pH 7.4) for 24 h and was
subsequently embedded into paraffin wax (as routinely performed).
Using the embedded tissue blocks, serial paraffin sections of 4 µm
were cut and were placed on glass slides for standard IHC.
Haematoxylin and eosin-stained sections were used
for primary diagnosis of NSCLC. The diagnosis was confirmed by two
independent pathologists. Afterwards, overall extent of
inflammation was estimated in the tumor tissue. This was based on
typical visual appearance of inflammation, including presence of
edema and inflammatory cellular infiltration. For this analysis, an
arbitrary score ranging from 0 to 3 (0, no inflammation; 1, weak;
2, moderate; 3, strong inflammatory reaction) was used.
For immunostaining, solutions and buffers purchased
from Dako GmbH were used. The sections were deparaffinized and were
incubated in target retrieval solution (pH 9.0) in 96˚C
thermostated water bath for 40 min and afterwards in peroxidase
blocking solution for 5 min at room temperature. Subsequently, the
tissue sections were incubated with specific anti-human PD-1
(1:100; Thermo Fisher Scientific, Inc., #PA5-32543), PD-L1 (1:50;
Thermo Fisher Scientific, Inc., #PA5-20343) or DNA-PK (1:100; Santa
Cruz Biotechnology; #sc-390849) antibody at room temperature for 1
h under humid conditions. After several washings, the
antigen-antibody complex was visualized by using Dako REAL™
EnVision Detection System, Peroxidase/DAB+, Rabbit/Mouse. The
slides were counterstained with hematoxyline, dehydrated and
cover-slipped for light microscopy.
Since a number of studies showed significant
discrepancies in PD-L1 detection using different antibodies
(26), we decided to perform
additional immunostaining with clinically validated PD-L1 antibody.
For this, we used PD-L1 ICH 22C3 pharmDx (Dako; Agilent
Technologies, Inc.) kit which is a qualitative immunohistochemical
assay with monoclonal mouse anti-PD-L1; a clone 22C3 intended for
use in the detection of PD-L1 in formalin-fixed, paraffin-embedded
NSCLC tissue. This particular staining was performed in accredited
hospital laboratory using EnVision FLEX visualization system on
Autostainer Link 48 according to the PD-L1 ICH 22C3 pharmDx (Dako;
Agilent Technologies, Inc.) protocol.
Staining interpretation of PD-1, PD-L1
(PA5-20343, 22C3 pharmDx) and DNA-PK expression
Evaluation of IHC stained slides was carried out in
a blinded fashion by the two authors independently in 10 randomly
taken high power microscopic fields (magnification, x400).
At first, immunohistochemical expression of PD-1 was
determined as a number of PD-1 positive cells per microscopic
field. Expression of PD-L1 (PA5-20343) on tumor cells was evaluated
using an arbitrary score (0, no staining; 1, weak staining; 2,
moderate staining; 3, strong staining). Proportion of DNA-PK
positive tumor cells (%) was calculated per microscopic field.
An experienced and qualified pathologist performed
the evaluation of clinically relevant PD-L1 antibody. The
expression of PD-L1 (22C3 pharmDx) was determined by using tumor
proportion score (TPS, %), which is a percentage of viable tumor
cells showing partial or complete membrane staining at any
intensity.
Statistical analysis
SPSS statistical software was used to calculate
individual means, group means, and standard deviations of the mean.
To determine statistical significance between the two data sets, we
used Student's t-test. For all the selected parameters (PD-1,
PD-L1, DNA-PK), we compared the means of following groups: Male vs.
female, adenocarcinoma vs. squamous cell cancer, current smoker vs.
non-smoker, no or trivial inflammation (score 0-2) vs. strong
inflammation (score 3). Additionally, a nonparametric Spearman
correlation analysis was utilized to check an association between
PD-1/PD-L1 and DNA-PK. P-value <0.05 was considered as
statistically significant.
Analysis of publicly available
information regarding DNA-PK, PD-L1 and PD-1 mRNA and protein
expression in lung cancer tissue
For comparison of our IHC data obtained from patient
biopsy specimen to the publicly available mRNA sequencing data from
The Cancer Genome Atlas (TCGA) database, we carried out correlation
analysis on the following webpage: http://gepia.cancer-pku.cn/detail.php?clicktag=correlation
(27). The chosen parameters were
as follows: gene names - PRKDC for DNA-PK, CD-274 for PD-L1, PDCD1
for PD-1; cancer name - LUAD for lung adenocarcinoma, LUSC for lung
squamous carcinoma; correlation coefficient: Spearman. The results
are presented in the Figs. S1 and
S2. The information regarding the
immunostaining of proteins of interest in the lung cancer-derived
tissue slices was retrieved from the Human Protein Atlas (HPA)
database: for DNA-PK, https://www.proteinatlas.org/ENSG00000253729-PRKDC/pathology/lung+cancer#img;
for PD-L1, https://www.proteinatlas.org/ENSG00000120217-CD274/pathology/lung+cancer#img;
for PD-1, https://www.proteinatlas.org/ENSG00000188389-PDCD1/pathology/lung+cancer#img.
Results
The clinicopathological characteristics of the
recruited patients are summarized in Table I. Among 121 patients, 89 patients
were males (74%) and 32 were females (26%), with a mean age of 67
years (range, 36-81 years). Fifty-six patients (46%) had
adenocarcinoma and 65 patients (54%) were diagnosed of squamous
cell cancer. Majority of the patients (79%) were current
smokers.
 | Table ICharacteristics of patients with
non-small cell lung cancer (n=121). |
Table I
Characteristics of patients with
non-small cell lung cancer (n=121).
| Variable | No of patients
(n=121) | Percentage (%) |
|---|
| Sex | | |
|
Male | 89 | 74 |
|
Female | 32 | 26 |
| Mean age, years
(range)a | 67 (36-81) | - |
| Histology | | |
|
Adenocarcinoma | 56 | 46 |
|
Squamous
cell cancer | 65 | 54 |
| Smoking status | | |
|
Current
smoker | 95 | 79 |
|
Non-smoker | 17 | 14 |
|
Unknown | 9 | 7 |
Extent of inflammation in NSCLC tumor
tissues
Overall extent of inflammation estimated in the
tumor tissue was based on a typical visual appearance of
inflammation, including presence of edema and inflammatory cellular
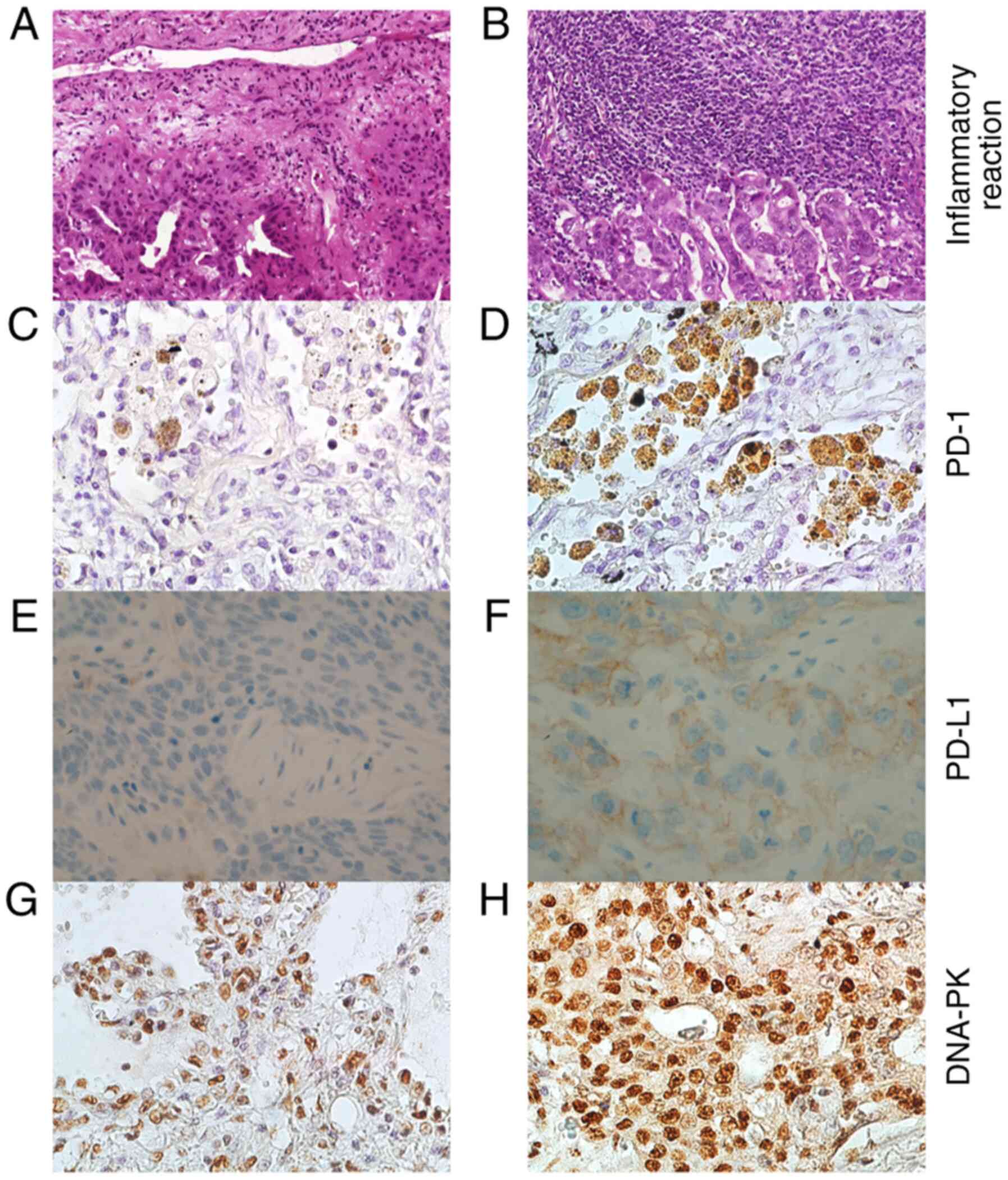
infiltration (score 0-3). Fig. 1
illustrates NSCLC tissue with trivial (Fig. 1A) and strong (Fig. 1B) inflammation features.
Predominantly, the tissue sections showed moderate inflammatory
features, which was followed by strong and trivial or no
inflammation (moderate: 36%, strong: 30%, trivial: 29% and no
inflammation: 5%, respectively).
Expression of PD-1, PD-L1 and DNA-PK
in NSCLC tissues
Almost all immunohistochemical parameters were
examined by two independent researchers and showed good accordance
to each other (P<0.0005). Experienced and qualified pathologist
performed the evaluation of clinically relevant PD-L1 antibody
(22C3 pharmDx) staining.
Fig. 1 represents
NSCLC tissues with a few (Fig. 1C)
and numerous (Fig. 1D) PD-1
positive cells, tumors with negative (Fig. 1E) and positive PD-L1 (Fig. 1F) expression (22C3 pharmDx) as well
as NSCLC samples with trivial (Fig.
1G) and noteworthy (Fig. 1H)
DNA-PK expression. According to IHC, the PD-1 expression originated
from the lymphocytes present in the biopsy specimen.
Expression levels of PD-1, PD-L1 (22C3 pharmDx) and
DNA-PK are depicted in Table II.
In the evaluated parameters, we found significant differences in
the subgroups for PD-1 and PD-L1. As shown in the Table II, there were more PD-1 positive
cells in the tumor tissues collected from males than female
patients (P=0.03). In addition, PD-1 positive cells were low in
number, when strong inflammation was present (P=0.03). Furthermore,
we found that PD-L1 was strongly expressed in ‘current smokers’
patients than non-smokers (P=0.025). For DNA-PK expression, we did
not find any notable differences in the selected clinical
parameters (male vs. female, current vs. non-smoker), histological
form (adenocarcinoma vs. squamous cell cancer) or the extent of
inflammation.
 | Table IIExpression of PD-1, PD-L1 (22C3
pharmDx) and DNA-PK in non-small cell lung cancer (n=121). |
Table II
Expression of PD-1, PD-L1 (22C3
pharmDx) and DNA-PK in non-small cell lung cancer (n=121).
| | PD-1 | PD-L1 | DNA-PK |
|---|
| Variable | Mean ± SEM | P-value | Mean ± SEM | P-value | Mean ± SEM | P-value |
|---|
| Sex | | 0.030 | | 0.667 | | 0.733 |
| Male | 17.6±6.0 | | 18.5±3.3 | | 86.1±1.0 | |
| Female | 5.9±2.0 | | 13.5±4.2 | | 86.7±1.6 | |
| Histology | | 0.127 | | 0.500 | | 0.157 |
|
Adenocarcinoma | 20.2±11.1 | | 17.6±5.2 | | 88.0±1.4 | |
|
Squamous
cell cancer | 11.6±4.2 | | 18.4±3.6 | | 85.4±1.2 | |
| Smoking status | | 0.259 | | 0.025 | | 0.814 |
|
Current
smoker | 16.4±5.5 | | 19.5±3.1 | | 86.3±1.0 | |
|
Non-smoker | 6.6±3.2 | | 1.4±0.8 | | 86.1±2.1 | |
| Inflammation
score | | 0.030 | | 0.212 | | 0.149 |
| No or weak
inflammation (score 0-2) | 18.2±6.2 | | 19.0±3.4 | | 86.3±1.0 | |
| Strong inflammation
(score 3) | 5.0±1.5 | | 13.9±4.8 | | 85.8±1.6 | |
Correlation analyses
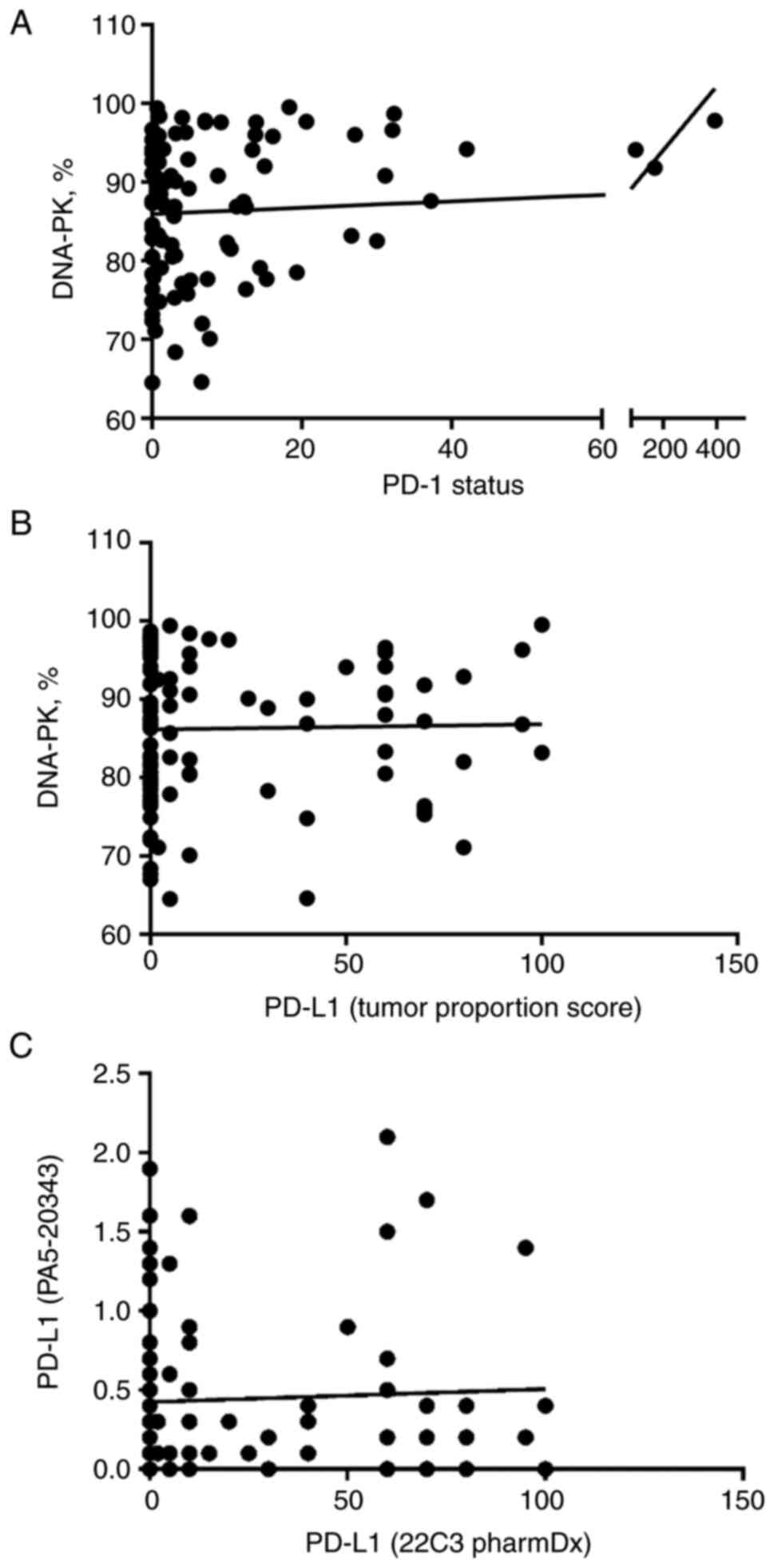
The results of the correlation analysis are depicted
in Fig. 2.
The analysis was based on patient's individual
values and revealed a significant association between one of the
targets of immune checkpoint inhibitors and tumor cell DNA-PK. Most
importantly, we found a positive correlation between the number of
PD-1 positive cells and tumor cell DNA-PK expression (P=0.027). The
biopsies from three patients had extremely high levels of PD-1
expression; still, we found no physiologically relevant criteria to
exclude these patients from the study.
We compared the results of two different PD-L1
immunohistochemistry assays before performing PD-L1 correlation
analyses. This is because recent studies raised questions about
analytical and clinical comparability of different PD-L1 assays
used in clinical trials and practice as the assays utilize various
staining platforms and may have their own scoring systems (26,28,29).
Indeed, we found that immunohistochemical stainings of the two
PD-L1 antibodies used in our study did not correlate (P=0.651).
Therefore, we used the results of clinically validated PD-L1
antibody (22C3 pharmDx) for PD-L1 and DNA-PK correlation analysis.
In contrast to PD-1, no correlation between PD-L1 and DNA-PK was
detected (P=0.926).
Correlation analysis of mRNA
sequencing data available in the cancer genome atlas (TCGA)
database
Finally, we explored whether the correlations
established in this study could be derived based on mRNA or
proteomic data available in the public databases, The Cancer Genome
Atlas (TCGA) and Human Protein Atlas (HPA).
As per TCGA database, a positive correlation was
observed in mRNA levels of DNA-PK (PRKDC) and PD-L1 (CD274)
(Spearman P=0.039; Fig. S1) and a
negative correlation was observed in mRNA levels of DNA-PK (PRKDC)
and PD-1 (PDCD1) (Spearman P=0.0088; Fig. S2). According to HPA, staining of
all three proteins of interest (DNA-PK, PD-L1, and PD-1) can
observed in lung cancer tissue samples, yet the patient-dependent
variability is remarkably high for all cases.
Discussion
Blockade of inhibitory immune checkpoints PD-1/PD-L1
has become a valid treatment option for several tumors including
advanced NSCLC (7,8,30).
Moreover, immunotherapy is increasingly used in combination with
cytotoxic treatments, such as chemotherapy (9,13).
Although the combined treatments in NSCLC are more effective, the
underling mechanisms that lead to higher antitumor activity are not
fully understood. Therefore, the aim of our retrospective study was
to describe the relationship between the expression of immune
checkpoints (PD-1, PD-L1) and DNA-PK, which is a part of key
pathway involved in repairing cytotoxic cancer therapy induced
damage.
Till today, immunohistochemical expression level of
PD-L1 on tumor cells is the most advanced biomarker in NSCLC.
Several phase-III clinical studies have reported better treatment
responses in the patients with higher tumor proportion score of
PD-L1. The latter has been clearly shown in monotherapy studies in
both, first- and second-line treatment of NSCLC patients (7,8,30).
Similarly, in the recently published study on lung adenocarcinoma
patients, a positive relation was found between higher PD-L1
expression and longer overall survival wherein pembrolizumab plus
pemetrexed and a platinum-based drug combination was used (9). Nevertheless, in patients with squamous
cell carcinoma, the relationship between higher PD-L1 expression
and longer overall survival was not detected (13). The latter indicates that the
clinical usefulness of PD-L1 as a biomarker in patients receiving
immunotherapy with cytotoxic agents may be less clear given that
the combination treatments improved outcomes over chemotherapy
across all categories of PD-L1 tumor proportion scores (9,13).
Therefore, the development of biomarkers for anti-PD-1/PD-L1 and
chemotherapy combinations requires better understanding of PD-1 and
PD-L1 expression profiles that is complemented with the markers,
which are important in cytotoxic cancer therapy.
In our retrospective study, we first analyzed the
expression of PD-1, PD-L1 and DNA-PK according to the clinical
parameters (male vs. female, current vs. non-smoker), histological
form (adenocarcinoma vs. squamous cell cancer) and the extent of
inflammation. In all the evaluated parameters, we detected
significant differences for PD-1 and PD-L1. Significantly higher
number of positive PD-1 cells were seen in the tumor tissues from
males, which supported the observations in the previous studies,
wherein higher PD-1 scores were found in males (31). Indeed, it was shown that
immunotherapy tends to be more effective in male cancer patients,
when compared to female patients, whereas higher expression of PD-1
and PD-L1 was suggested as one of the reasons for the increased
efficacy (32,33).
Our study also revealed that NSCLC tissues with no
or trivial visual inflammation had higher number of PD-1 positive
cells. It is well known that there are key histological differences
between acute and chronic inflammation, especially in terms of
leukocyte types that are predominantly present in the tissue
(polymorphonuclear neutrophils vs. macrophages and lymphocytes)
(34). Additionally, numerous
studies showed that PD-1 expression can be found in multiple immune
cell types, including T cells, B cells, macrophages as well as
dendritic cells (35). In tumors,
chronic inflammation leads to permanent stimulation of T cells,
which promotes T cell exhaustion that finally results in
upregulation of co-inhibitory receptors such as PD-1(36). Therefore, it is reasonable to
believe that higher number of PD-1 positive cells in the tumor's
tissues with no or trivial inflammation as seen in our study was
mainly due to inhibitory tumor microenvironment.
Recent studies have raised questions about
analytical and clinical comparability of different PD-L1 assays
used in clinical trials and practice because the assays utilize
various IHC antibodies, staining platforms and may have their own
scoring systems (26,28,29).
Here, we showed that the results of different IHC PD-L1 antibodies
do not correlate. Therefore, it is very important to use clinically
relevant antibodies even in retrospective studies.
PD-L1 immunohistochemistry with clinically validated
antibody also revealed that the expression of PD-L1 was
significantly higher in the NSCLC patients with ‘current smokers’
status. Several meta-analyses and other studies showed that high
PD-L1 expression was positively correlated with different clinical
and pathological features in lung cancer patients, such as male
gender, smoking status, higher histological grade, larger tumor
size, positive lymph nodal metastasis and TNM stage (37-39).
Our study did not find any association between PD-L1 and other
clinical and the selected pathological characteristics. It could be
due to several reasons; first, our patient groups were not well
balanced. Additionally, we were unable to rule out tumor
heterogeneity, which was showed influential for PD-L1 expression in
NSCLC as reported recently (40).
Although we did not detect significant differences
in tumor cell DNA-PK expression in the selected clinical and
pathological characteristics, the most intriguing finding of this
study was, a significant positive correlation between PD-1/PD-L1
axis and DNA-PK expression based on individual patient values. The
patients whose tumors had higher number of PD-1 positive cells,
also showed higher proportion of DNA-PK positive tumor cells.
As mentioned earlier, DNA-PK, a serine/threonine
protein kinase, consists of a catalytic subunit (DNA-PKcs) and Ku
heterodimer that consists of Ku70 and Ku80 subunits (41). DNA-PK is involved in repairing DNA
double-strand breaks (DSBs) via three main pathways: classical
non-homologous end-joining NHEJ (C-NHEJ), alternative NHEJ (A-NHEJ)
and homologous recombination (HR) (42). It has been widely accepted that the
ability of cancer cells to repair therapeutically induced DNA
damage impacts anti-cancer therapeutic efficacy to a major extent
(19,43,44).
In our study, more than 80% of cancer cells (on average) were seen
to express DNA-PK. Accordingly, in previously published studies,
significantly higher DNA-PK expression was detected in NSCLC than
adjacent normal tissues (45).
Moreover, DNA-PK expression level serves as a biomarker for
predicting a response to cytotoxic therapy and thereby survival,
since worse treatment outcome was reported in the patients with
high levels of DNA-PK (45,46). Furthermore, the inhibition of DNA-PK
was shown to enhance chemosensitivity as well as radiosensitivity
in NSCLC (24,25).
Interactions between DNA-PK and PD-1/PD-L1 axis are
not fully explored. Previously, it was showed that nuclear γH2AX
(unique histone subunit, which serves as a sensor of
double-stranded DNA damage) expression was positively associated
with PD-L1 expression in squamous cell lung carcinoma (47). Similarly, upregulation of PD-L1
expression in cancer cells was reported in response to DSBs
(48). Moreover, as per TCGA
database, a positive correlation was observed in mRNA levels of
DNA-PK (PRKDC) and PD-L1 (CD274) (Fig.
S1) (27). These evidences
suggested that DNA damage response in tumor cells generates an
immune response that further upregulates PD-L1 expression in tumor
cells. Higher PD-L1 expression in the tumors cells consecutively
sensitizes them to anti PD-L1 therapy. According to TCGA database,
there is a negative correlation between the DNA-PK (PRKDC) and PD-1
(PDCD1) (Fig. S2), yet this does
not directly contradict our findings: the biopsy samples used for
IHC contain several types of cells, and hence protein staining can
reveal correlations that are intrinsically different to those
provided by mRNA sequencing in tumor cells only. This fact
underlines the ultimate strength of our study.
Here, we showed that the patients, whose tumors had
higher number of PD-1 positive cells, also had a higher proportion
of DNA-PK positive tumor cells. Although the most well-known role
of DNA-PK is, its involvement in DNA damage response pathways, this
kinase also participates in other functions, e.g., promotion of
genomic stability, regulation of hypoxic response via hypoxia
inducible factor (HIF) dependent and HIF-independent mechanisms. In
addition, DNA-PK affects metabolism, participates in
transcriptional regulation of hormone receptors, and activates
innate immune system (41,42,49).
Various studies have established the role of DNA-PK in
transcriptional programs that operate biological processes, such as
epithelial to mesenchymal transition (EMT), nuclear receptor
signaling and inflammatory responses (22). However, importance of PD-1 and
DNA-PK association in NSCLC is not known. Therefore, detailed
studies are required regarding the mechanisms that are responsible
for higher expression of PD-1 and DNA-PK in tumors and further
their effect on the efficacy of combined treatments in NSCLC.
This report has several limitations, including
retrospective nature of the study and absence of equally balanced
study groups in terms of patient number. Furthermore, we used tumor
tissue samples in our study. As opposed to NSCLC cell lines, tissue
samples may contain not only cancerous cells but also other
components native to the tumor microenvironment which may affect
the results of the study. In spite of the limitations, the authors
showed that, there is a significant positive correlation between
PD-1/PD-L1 axis and DNA-PK expression in NSCLC. Clear understanding
of underlying mechanisms is of high importance to develop
predictive and prognostic molecular markers for the combination
treatment of immune checkpoint inhibitors and cytotoxic cancer
therapies. In our further studies, we will focus on the tumor side
of the PD-1/PD-L1 signaling axis to investigate combined effects of
immunotherapy and chemotherapy in a simplified system, represented
by the PD-L1 positive vs. PD-L1 negative cell lines.
Supplementary Material
Correlation between mRNA levels of
DNA-dependent protein kinase (PRKDC) and programmed cell death
ligand 1 (CD274) in lung adenocarcinoma and lung squamous carcinoma
according to the data obtained from The Cancer Genome Atlas
database. Spearman P-value is presented; log2 stands for binary
logarithm. TPM, transcripts per kilobase million.
Correlation between mRNA levels of
DNA-dependent protein kinase (PRKDC) and programmed cell death
protein 1 (PDCD1) in lung adenocarcinoma and lung squamous
carcinoma according to the data obtained from The Cancer Genome
Atlas database. Spearman P-value is presented. log2 stands for
binary logarithm. TPM, transcripts per kilobase million.
Acknowledgements
Not applicable.
Funding
The study was supported by the Estonian Society of Clinical
Oncologists (grant no. A171063).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MS, JJ, MK and DL planned and guided the current
study, analyzed the data and wrote the manuscript. JN, LM, HA, AV,
AM, MB, TV, MS and HT were responsible for histology, IHC and
scoring. All authors read and approved final draft of the
manuscript. MS and JJ confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Research Ethics Committee of University of Tartu
approved the study.
Patient consent for publication
Not applicable.
Competing interests
JJ is an advisory board member for AstraZeneca and
MSD, and has received research funding from AstraZeneca. The other
authors declare that they have no competing interests.
References
|
1
|
Didkowska J, Wojciechowska U, Manczuk M
and Łobaszewski J: Lung cancer epidemiology: Contemporary and
future challenges worldwide. Ann Transl Med. 4(150)2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Planchard D, Popat S, Kerr K, Novello S,
Smit EF, Faivre-Finn C, Mok TS, Reck M, Van Schil PE, Hellmann MD,
et al: Metastatic non-small cell lung cancer: ESMO clinical
practice guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 29 (Suppl 4):iv192–iv237. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yang JC, Wu YL, Schuler M, Sebastian M,
Popat S, Yamamoto N, Zhou C, Hu CP, O'Byrne K, Feng J, et al:
Afatinib versus cisplatin-based chemotherapy for EGFR
mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6):
Analysis of overall survival data from two randomised, phase 3
trials. Lancet Oncol. 16:141–151. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Paz-Ares L, de Marinis F, Dediu M, Thomas
M, Pujol JL, Bidoli P, Molinier O, Sahoo TP, Laack E, Reck M, et
al: Maintenance therapy with pemetrexed plus best supportive care
versus placebo plus best supportive care after induction therapy
with pemetrexed plus cisplatin for advanced non-squamous
non-small-cell lung cancer (PARAMOUNT): A double-blind, phase 3,
randomised controlled trial. Lancet Oncol. 13:247–255.
2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Reck M, Rodriguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus chemotherapy for PD-L1-positive
non-small-cell lung cancer. N Engl J Med. 375:1823–1833.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gandhi L, Rodriguez-Abreu D, Gadgeel S,
Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ,
Powell SF, et al: Pembrolizumab plus chemotherapy in metastatic
non-small-cell lung cancer. N Engl J Med. 378:2078–2092.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Attili I, Passaro A, Pavan A, Conte P, De
Marinis F and Bonanno L: Combination immunotherapy strategies in
advanced non-small cell lung cancer (NSCLC): Does biological
rationale meet clinical needs? Crit Rev Oncol Hematol. 119:30–39.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Langer CJ: Emerging immunotherapies in the
treatment of non-small cell lung cancer (NSCLC): The role of immune
checkpoint inhibitors. Am J Clin Oncol. 38:422–430. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rocco D, Della Gravara L, Battiloro C and
Gridelli C: The role of combination chemo-immunotherapy in advanced
non-small cell lung cancer. Expert Rev Anticancer Ther. 19:561–568.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Paz-Ares L, Luft A, Vicente D, Tafreshi A,
Gümüş M, Mazières J, Hermes B, Çay Şenler F, Csőszi T, Fülöp A, et
al: Pembrolizumab plus chemotherapy for squamous non-small-cell
lung cancer. N Engl J Med. 379:2040–2051. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yu W, Hua Y, Qiu H, Hao J, Zou K, Li Z, Hu
S, Guo P, Chen M, Sui S, et al: PD-L1 promotes tumor growth and
progression by activating WIP and β-catenin signaling pathways and
predicts poor prognosis in lung cancer. Cell Death Dis.
11(506)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rizvi NA, Hellmann MD, Snyder A, Kvistborg
P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al: Cancer
immunology. Mutational landscape determines sensitivity to PD-1
blockade in non-small cell lung cancer. Science. 348:124–128.
2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mouw KW, Goldberg MS, Konstantinopoulos PA
and D'Andrea AD: DNA damage and repair biomarkers of immunotherapy
response. Cancer Discov. 7:675–693. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ricciuti B, Recondo G, Spurr LF, Li YY,
Lamberti G, Venkatraman D, Umeton R, Cherniack AD, Nishino M, Sholl
LM, et al: Impact of DNA damage response and repair (DDR) gene
mutations on efficacy of PD-(L)1 immune checkpoint inhibition in
non-small cell lung cancer. Clin Cancer Res. 26:4135–4142.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lamberti G, Andrini E, Sisi M, Federico AD
and Ricciuti B: Targeting DNA damage response and repair genes to
enhance anticancer immunotherapy: Rationale and clinical
implication. Future Oncol. 16:1751–1766. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gavande NS, VanderVere-Carozza PS, Hinshaw
HD, Jalal SI, Sears CR, Pawelczak KS and Turchi JJ: DNA repair
targeted therapy: The past or future of cancer treatment? Pharmacol
Ther. 160:65–83. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Blackford AN and Jackson SP: ATM, ATR, and
DNA-PK: The trinity at the heart of the DNA damage response. Mol
Cell. 66:801–817. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jette N and Lees-Miller SP: The
DNA-dependent protein kinase: A multifunctional protein kinase with
roles in DNA double strand break repair and mitosis. Prog Biophys
Mol Biol. 117:194–205. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Medová M, Medo M, Hovhannisyan L,
Muñoz-Maldonado C, Aebersold DM and Zimmer Y: DNA-PK in human
malignant disorders: Mechanisms and implications for
pharmacological interventions. Pharmacol Ther.
215(107617)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sak A, Stuschke M, Wurm R, Schroeder G,
Sinn B, Wolf G and Budach V: Selective inactivation of
DNA-dependent protein kinase with antisense oligodeoxynucleotides:
Consequences for the rejoining of radiation-induced DNA
double-strand breaks and radiosensitivity of human cancer cell
lines. Cancer Res. 62:6621–6624. 2002.PubMed/NCBI
|
|
24
|
Yanai M, Makino H, Ping B, Takeda K,
Tanaka N, Sakamoto T, Yamaguchi K, Kodani M, Yamasaki A, Igishi T
and Shimizu E: DNA-PK inhibition by NU7441 enhances
chemosensitivity to topoisomerase inhibitor in non-small cell lung
carcinoma cells by blocking DNA damage repair. Yonago Acta Med.
60:9–15. 2017.PubMed/NCBI
|
|
25
|
Azad A, Jackson S, Cullinane C, Natoli A,
Neilsen PM, Callen DF, Maira SM, Hackl W, McArthur GA and Solomon
B: Inhibition of DNA-dependent protein kinase induces accelerated
senescence in irradiated human cancer cells. Mol Cancer Res.
9:1696–1707. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hirsch FR, McElhinny A, Stanforth D,
Ranger-Moore J, Jansson M, Kulangara K, Richardson W, Towne P,
Hanks D, Vennapusa B, et al: PD-L1 immunohistochemistry assays for
lung cancer: Results from phase 1 of the blueprint PD-L1 IHC assay
comparison project. J Thorac Oncol. 12:208–222. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
The Cancer Genome Atlas Program-National
Cancer Institute. https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga.
Accessed February 20, 2021.
|
|
28
|
Tsao MS, Kerr KM, Kockx M, Beasley MB,
Borczuk AC, Botling J, Bubendorf L, Chirieac L, Chen G, Chou TY, et
al: PD-L1 immunohistochemistry comparability study in real-life
clinical samples: Results of blueprint phase 2 project. J Thor
Oncol. 13:1302–1311. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kintsler S, Cassataro MA, Drosch M,
Holenya P, Knuechel R and Braunschweig T: Expression of programmed
death ligand (PD-L1) in different tumors. Comparison of several
current available antibody clones and antibody profiling. Ann Diagn
Pathol. 41:24–37. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Rittmeyer A, Barlesi F, Waterkamp D, Park
K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols
MC, et al: Atezolizumab versus docetaxel in patients with
previously treated non-small-cell lung cancer (OAK): A phase 3,
open-label, multicentre randomised controlled trial. Lancet.
389:255–265. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
D'Incecco A, Andreozzi M, Ludovini V,
Rossi E, Capodanno A, Landi L, Tibaldi C, Minuti G, Salvini J,
Coppi E, et al: PD-1 and PD-L1 expression in molecularly selected
non-small-cell lung cancer patients. Br J Cancer. 112:95–102.
2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Conforti F, Pala L, Bagnardi V, De Pas T,
Martinetti M, Viale G, Gelber RD and Goldhirsch A: Cancer
immunotherapy efficacy and patients' sex: A systematic review and
meta-analysis. Lancet Oncol. 19:737–746. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang S, Cowley LA and Liu XS: Sex
differences in cancer immunotherapy efficacy, biomarkers, and
therapeutic strategy. Molecules. 24(3214)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Oort J and Scheper RJ: Histopathology of
acute and chronic inflammation. Agents Actions. Suppl:25–30.
1977.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Qin W, Hu L, Zhang X, Jiang S, Li J, Zhang
Z and Wang X: The diverse function of PD-1/PD-L pathway beyond
cancer. Front Immunol. 10(2298)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Jubel JM, Barbati ZR, Burger C, Wirtz DC
and Schildberg FA: The role of PD-1 in acute and chronic infection.
Front Immunol. 11(487)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhang M, Li G and Wang Y and Wang Y, Zhao
S, Haihong P, Zhao H and Wang Y: PD-L1 expression in lung cancer
and its correlation with driver mutations: A meta-analysis. Sci
Rep. 7(10255)2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Calles A, Liao X, Sholl LM, Rodig SJ,
Freeman GJ, Butaney M, Lydon C, Dahlberg SE, Hodi FS, Oxnard GR, et
al: Expression of PD-1 and its ligands, PD-L1 and PD-L2, in smokers
and never smokers with KRAS-mutant lung cancer. J Thor Oncol.
10:1726–1735. 2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Pawelczyk K, Piotrowska A, Ciesielska U,
Jablonska K, Gletzel-Plucinska N, Grzegrzolka J, Podhorska-Okolow
M, Dziegiel P and Nowinska K: Role of PD-L1 expression in non-small
cell lung cancer and their prognostic significance according to
clinicopathological factors and diagnostic markers. Int J Mol Sci.
20(824)2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
McLaughlin J, Han G, Schalper KA,
Carvajal-Hausdorf D, Pelekanou V, Rehman J, Velcheti V, Herbst R,
LoRusso P and Rimm DL: Quantitative assessment of the heterogeneity
of PD-L1 expression in non-small-cell lung cancer. JAMA Oncol.
2:46–54. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Mohiuddin IS and Kang MH: DNA-PK as an
emerging therapeutic target in cancer. Front Oncol.
9(635)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Goodwin JF and Knudsen KE: Beyond DNA
repair: DNA-PK function in cancer. Cancer Discov. 4:1126–1139.
2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Li L, Zhu T, Gao YF, Zheng W, Wang CJ,
Xiao L, Huang MS, Yin JY, Zhou HH and Liu ZQ: Targeting DNA damage
response in the radio(Chemo)therapy of non-small cell lung cancer.
Int J Mol Sci. 17(839)2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kase M, Vardja M, Lipping A, Asser T and
Jaal J: Impact of PARP-1 and DNA-PK expression on survival in
patients with glioblastoma multiforme. Radiother Oncol.
101:127–131. 2011.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Xing J, Wu X, Vaporciyan AA, Spitz MR and
Gu J: Prognostic significance of ataxia-telangiectasia mutated,
DNA-dependent protein kinase catalytic subunit, and Ku
heterodimeric regulatory complex 86-kD subunit expression in
patients with nonsmall cell lung cancer. Cancer. 112:2756–2764.
2008.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Hu S, Qu Y, Xu X, Xu Q, Geng J and Xu J:
Nuclear survivin and its relationship to DNA damage repair genes in
non-small cell lung cancer investigated using tissue array. PLoS
One. 8(e74161)2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Osoegawa A, Hiraishi H, Hashimoto T,
Takumi Y, Abe M, Takeuchi H, Miyawaki M, Okamoto T and Sugio K: The
positive relationship between γH2AX and PD-L1 expression in lung
squamous cell carcinoma. In Vivo. 32:171–177. 2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Sato H, Niimi A, Yasuhara T, Permata TBM,
Hagiwara Y, Isono M, Nuryadi E, Sekine R, Oike T, Kakoti S, et al:
DNA double-strand break repair pathway regulates PD-L1 expression
in cancer cells. Nat Commun. 8(1751)2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Damia G: Targeting DNA-PK in cancer. Mutat
Res. 821(111692)2020.PubMed/NCBI View Article : Google Scholar
|