Introduction
Head and neck cancer is the sixth most common cancer
worldwide, accounting for ~6% of all cases and an estimated 1-2% of
all cancer deaths (1,2). More than 90% of head and neck cancers
are squamous cell carcinomas (SCC). In patients with recurrent or
metastatic head and neck squamous cell carcinoma (HNSCC),
combination therapy with platinum, fluorouracil, and cetuximab as
first-line treatment significantly prolongs overall survival
(3). Nivolumab, an anti-programmed
cell death-1 (PD-1) monoclonal antibody (Ab), shows a significant
improvement in overall survival over chemotherapy in patients with
HNSCC that is refractory to platinum-based chemotherapy (4). Nivolumab also has durable clinical
benefits in patients with various malignant tumors (5-10).
Anti-PD-1 therapies such as nivolumab and
pembrolizumab cause immune-related adverse events (irAEs) including
endocrine dysfunction, neurological disorders, hepatitis,
nephritis, cardiac insufficiency, colitis, and pneumonitis. Among
them, pneumonitis is a relatively rare but potentially fatal
adverse event (11). The incidence
of pneumonitis associated with anti-PD-1 therapies is 2.7% for
all-grade and 0.8% for grade ≥3(12). Cancer immunotherapy guidelines
recommend an intermediate or high dose of corticosteroids for
anti-PD-1 therapy-induced pneumonitis (13,14).
In cases of severe pneumonitis that is refractory to
corticosteroids, several immune suppressants such as
cyclophosphamide, mycophenolate mofetil, and the anti-tumor
necrosis factor alpha (TNF-α) Ab, infliximab, are recommended.
Infliximab is effective for pneumonitis due to anti-PD-1 therapy
(15). However, the efficacy and
feasibility of repetitive administration of infliximab for
recurrent pneumonitis remain unclear. Here, we present a case in
which amelioration of recurrent nivolumab-induced severe
pneumonitis was induced by repetitive administration of
infliximab.
Case presentation
A 59-year-old female with a heavy smoking (70
pack-years) and drinking history was diagnosed with SCC of the
oropharynx (cT2N0M0) and hypopharynx (cT2N0M0) in 201x-3. She was
treated with induction chemotherapy consisting of cisplatin and
docetaxel followed by concurrent chemoradiotherapy with
tegafur/gimeracil/oteracil (S-1), resulting in a complete response.
She also had a history of definitive chemoradiotherapy consisting
of cisplatin and 5-fluorouracil for locally advanced esophageal SCC
(cT3N1M0) in 201x-1. Although she had undergone a complete
resection for local recurrence of the hypopharyngeal SCC in August
201x, metastases to the lung and mediastinal lymph nodes were
detected in November 201x and histologically confirmed as SCCs. She
was then treated with nivolumab monotherapy at a standard dose of 3
mg/kg for metastatic HNSCC. Her Eastern Cooperative Oncology Group
performance status was 1. She had no respiratory symptoms. Blood
tests and laboratory examinations showed normal values except for
anemia (hemoglobin 9.0 g/dl) and a high level of serum C-reactive
protein (CRP; 6.3 mg/dl). The antinuclear antibody test was
negative. On the 10th day of the first administration of nivolumab,
she complained of fever and a dry cough. Oseltamivir was given for
7 days following immunological diagnosis of influenza B virus
infection. However, she was admitted to our hospital because of
progressive dyspnea on the 14th day of nivolumab administration.
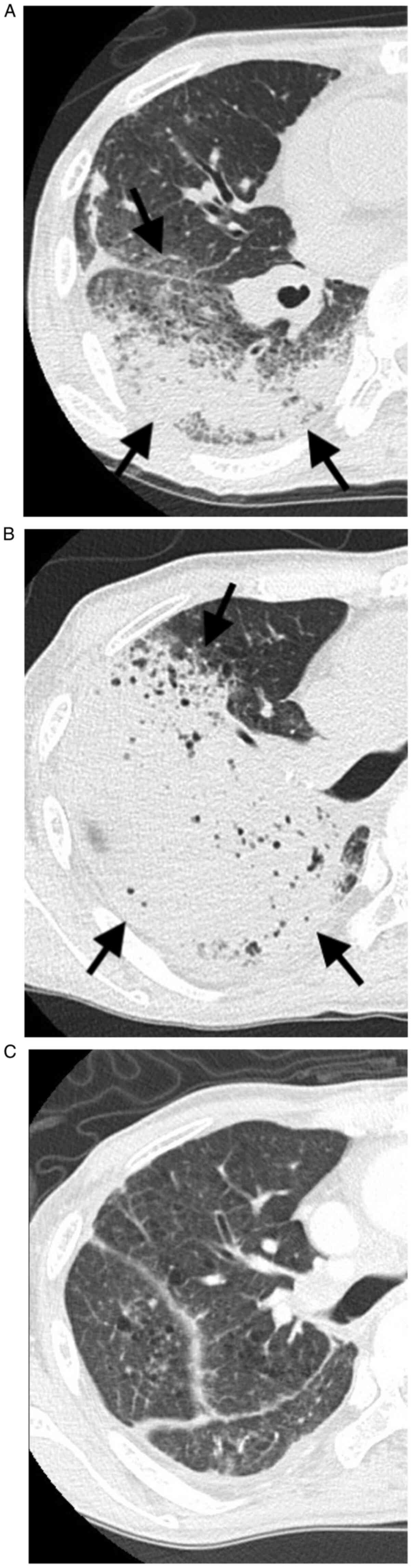
Computed tomography (CT) scans of the chest showed ground-glass
opacities in the right lung (Fig.
1A). It was difficult for us to determine whether the CT
findings were infectious pneumonia such as pulmonary suppuration or
round-glass opacities. We consulted with a pulmonologist and a
radiologist and diagnosed. Immunological tests for influenza virus
were negative. Other infectious diseases were also ruled out by
bacterial sputum/blood/urinary culture and fungal antigen tests.
Bronchoscopy and bronchoalveolar lavage revealed a high proportion
of lymphocytes: Macrophages 27.5%, neutrophils 46.4%, lymphocytes
26.1%, eosinophils 0%. These results suggested drug-induced lung
disease or viral infection. The serum concentration of Krebs von
den Lungen-6 was normal, but surfactant protein-A was elevated
(58.6 ng/ml). She was finally diagnosed with nivolumab-induced
pneumonitis. Nivolumab was then discontinued, and intravenous
high-dose methylprednisolone (1,000 mg/day for 3 days) was
administered. Prophylactic antibiotics were also started. However,
on the 17th day, dyspnea worsened to the extent that intubation was
required. CT scans showed that the ground-glass opacities in the
right lung had worsened (Fig. 1B).
Blood tests showed high concentrations of serum CRP (12.4 mg/dl)
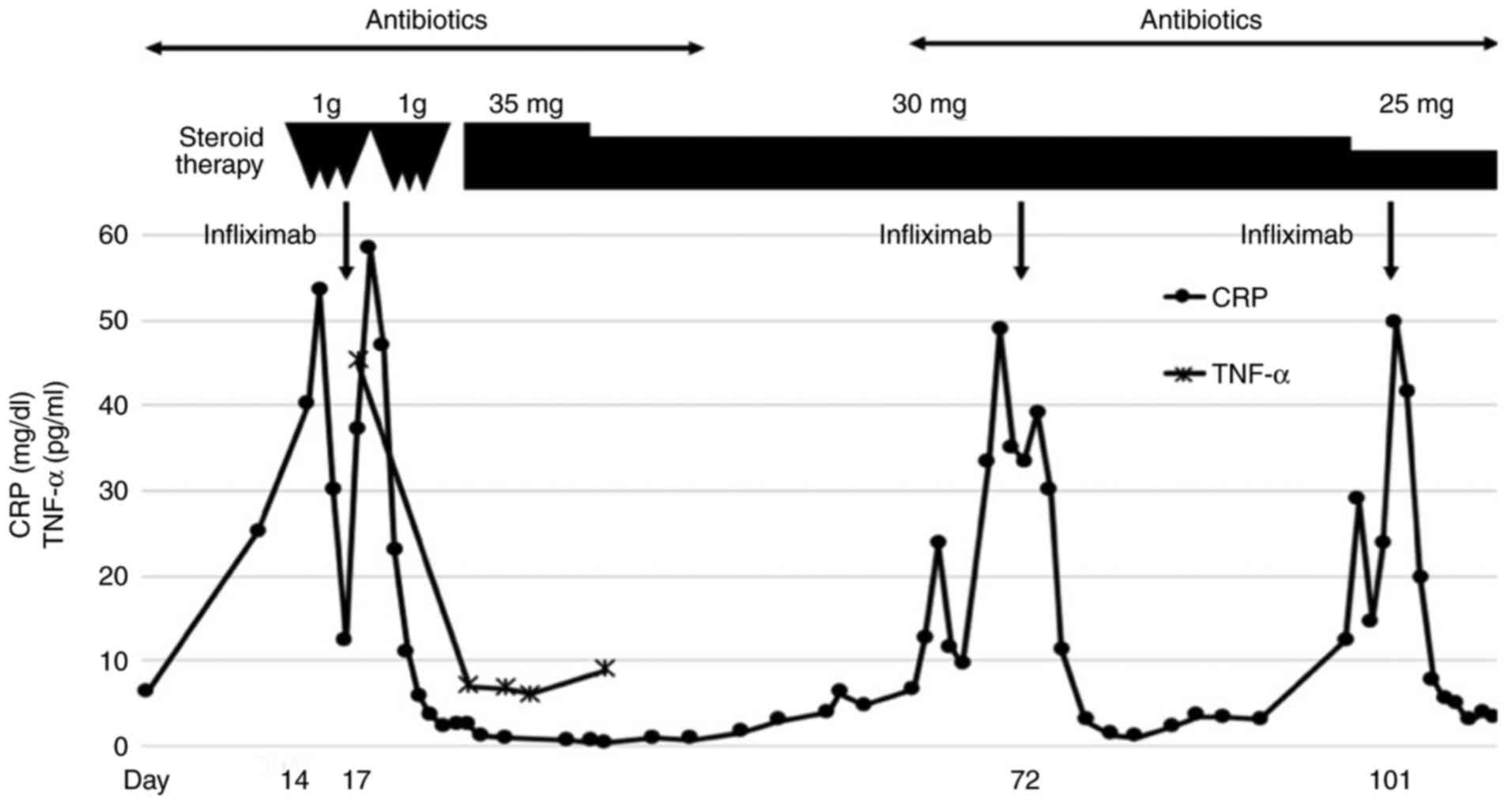
and serum TNF-α (45.2 pg/ml). She was administered a single dose of
infliximab (5 mg/kg) for nivolumab-induced pneumonitis that was
refractory to high-dose methylprednisolone. One week after
administration of infliximab, serum concentrations of CRP and TNF-α
were decreased, and CT scans showed marked regression of pulmonary
opacities (Fig. 1C). Subsequently,
the dosage of prednisolone was tapered to 30 mg daily after 35 mg
daily administration for 2 weeks (Fig.
2). On the 72nd day after nivolumab administration, the patient
complained of fever, dyspnea, diarrhea, and joint pain. Blood tests
showed that the serum level of CRP (50.0 mg/dl) was elevated again,
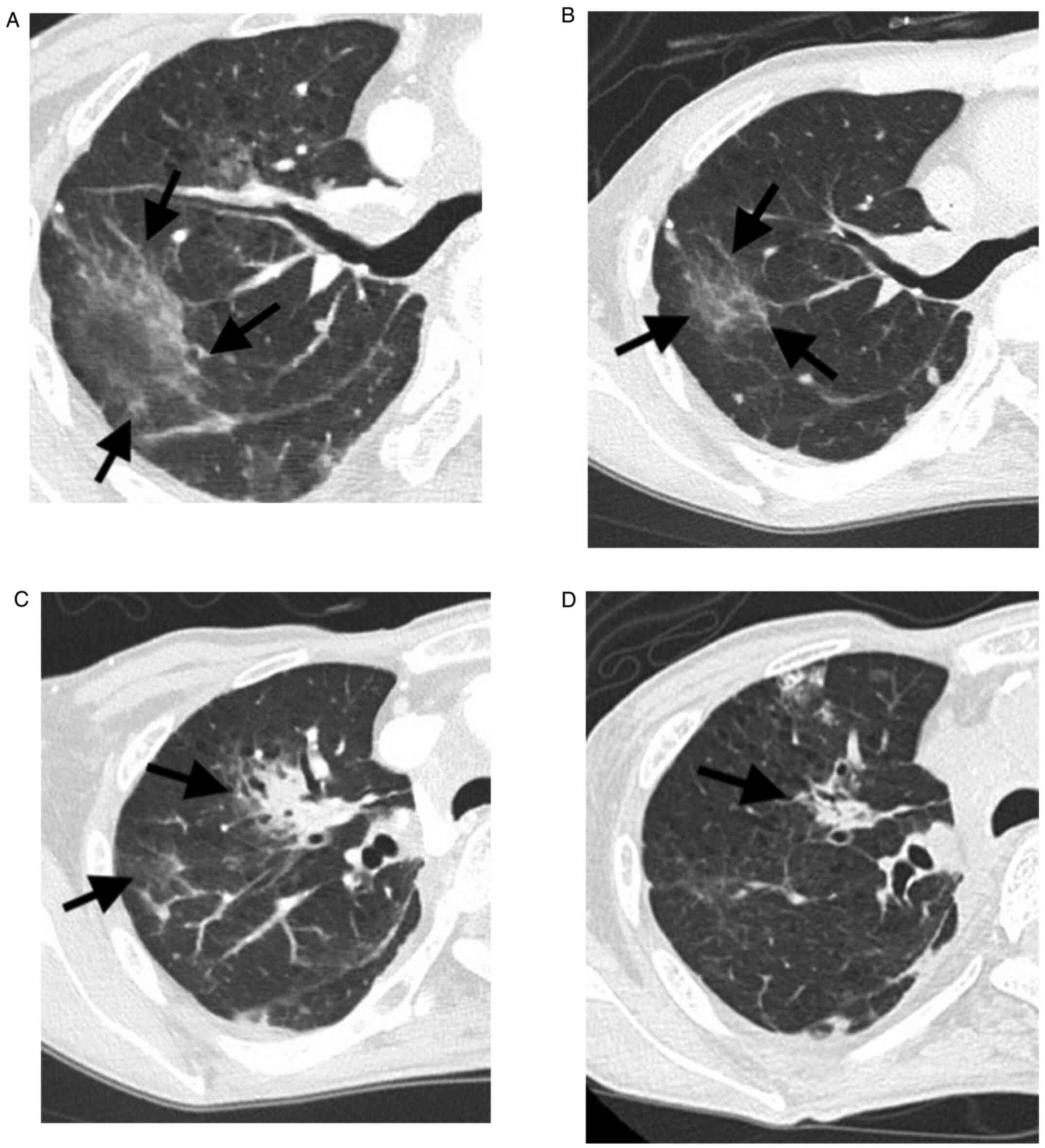
and CT scans of the chest showed that the pneumonitis had worsened
in the right lung (Fig. 3A).
Bacterial sputum/blood/urinary culture, the β-D glucan value, and
viral antibody tests including influenza virus were all negative.
She was thus diagnosed with recurrence of nivolumab-induced
pneumonitis. She was administered a single dose of infliximab again
on the 70th day because her previous pneumonitis was refractory to
high-dose methylprednisolone, and more than 8 weeks had passed
since the first dose of infliximab (Fig. 2). The second administration of
infliximab improved the pneumonitis (Fig. 3B). Although prednisolone 25 mg
daily was continued, pneumonitis relapsed on the 101st day of
nivolumab administration without any specific events; nivolumab may
have exacerbated the pneumonitis (Fig.
3C). A single dose of infliximab was again administered, and
her pneumonitis improved (Fig.
3D). Nivolumab was not re-administered due to severe irAE. We
discussed taxanes, S-1, or cetuximab for the next therapy. However,
best supportive care was performed because of the poor performance
status due to progression of the tumor. The patient died of tumor
progression the 121st day after the initial administration of
nivolumab. Informed consent was obtained from the patient for
publication of a case report.
Discussion
Immune checkpoint inhibitors (ICIs) targeting the
PD-1/PD-L1 axis and cytotoxic T lymphocyte-associated antigen-4
(CTLA-4) have demonstrated notable antitumor activity across
various tumor types. On the other hand, irAEs induced by ICIs have
become an important issue. Dermatologic, musculoskeletal,
gastrointestinal, hepatic, endocrine, and pulmonary events have
been reported (16). Among them,
pneumonitis is a serious adverse event that can have a lethal
outcome. The incidence of pneumonitis associated with anti-PD-1 Ab
is 2.7% for all-grade and 0.8% for grade ≥3(12). Huang et al reported that
nivolumab, pembrolizumab, and nivolumab plus ipilimumab therapy are
significantly higher risk factors for pneumonitis compared with
chemotherapy (17). Generally, the
time to onset of pneumonitis associated with anti-PD-1 Ab varies
from within a few weeks to months after administration (16).
Several retrospective analyses and systematic
reviews investigated the risk factors for ICI-related pneumonitis.
Pre-existing pulmonary fibrosis increases the risk of anti-PD-1
Ab-related pneumonitis in patients with non-small cell lung cancer
(18). A history of radiotherapy
of the lung was also thought to be a risk factor for ICI-related
pneumonitis (19). In the present
case, pneumonitis occurred ~2 weeks after administration of
nivolumab. Because infectious pneumonia was carefully ruled out,
the patient was diagnosed with nivolumab-induced pneumonitis.
Although the patient did not have pre-existing pulmonary fibrosis,
a history of radiotherapy for locally advanced esophageal SCC may
have affected the development of pneumonitis as reported in a
previous study (19). Furthermore,
previous influenza B virus infection may trigger or worsen
nivolumab-induced pneumonitis. Inflammatory cytokines including
TNF-α are induced early after influenza infection (20). Although these factors may be
associated with occurrence of ICI-related pneumonitis, the
mechanisms of steroid refractoriness of pneumonitis have not been
clarified.
Regarding the treatment for ICI-induced pneumonitis,
the cancer immunotherapy guidelines recommend corticosteroids for
treatment of irAEs (13,14). Immunosuppressive drugs such as
infliximab or mycophenolate mofetil are recommended for serious
irAEs that are refractory to corticosteroids (13,14).
Several studies reported that infliximab is effective for
anti-CTLA-4 Ab-induced enterocolitis that is refractory to
corticosteroids (21,22). Infliximab also improves ICI-induced
pneumonitis that is refractory to corticosteroids (15,23).
TNF-α is involved in the onset of pneumonitis associated with
autoimmune diseases (24). In the
present case, respiratory insufficiency and pulmonary opacities did
not improve with intravenous high-dose methylprednisolone but with
a single dose of infliximab. After improvement in pneumonitis with
infliximab, a maintenance dose of prednisolone was continued.
However, reactivation of pneumonitis was observed 2 months after
the first administration of infliximab, and 1 month after the
second administration of infliximab.
Multiple mechanisms may be associated with
recurrence of nivolumab-induced pneumonitis. Activation of
CD8+ T cells mediates psoriasis-like dermatitis and
hepatotoxicity caused by ICIs (25,26).
A specific T cell repertoire such as CD8 that is involved in
nivolumab-induced pneumonitis may be decreased after infliximab
administration, and then reactivation of the T cell repertoire may
induce recurrence of the pneumonitis. Alternatively, the decrease
in TNF-α by infliximab administration could induce the T cell
repertoire. Thus, serum TNF-α may play a critical role in
modulating the activity of nivolumab-induced pneumonitis.
In the present case, the serum concentration of
TNF-α clearly increased in association with exacerbation of the
pneumonitis, and it subsequently decreased in association with
amelioration of pneumonitis after infliximab therapy. These
observations suggested that TNF-α is directly involved in the
activity of nivolumab-induced pneumonitis. The dosing schedule of
infliximab, including repetitive administration of infliximab, for
steroid-refractory ICI-induced pneumonitis has not been clearly
described in the current guidelines for cancer immunotherapy
(13,14). Recently, a case was reported of
ICI-induced pneumonitis demonstrating transient improvement but
re-exacerbation 2 weeks after infliximab therapy, suggesting the
possible efficacy of repetitive administration of infliximab
(23). Immune-related colitis that
is refractory to corticosteroids is improved by repetitive
administration of infliximab when it relapses (27). Although other cytokines are
possibly related to exacerbation of ICI-induced pneumonitis such as
interleukin-1 and interleukin-6(28), the recurring nivolumab-induced
pneumonitis in the present case is strongly suggested to be mainly
dependent on TNF-α. Repetitive infliximab therapy is thought to be
effective for nivolumab-induced pneumonitis that worsens in
accordance with increased serum concentrations of TNF-α.
Steroid-refractory nivolumab-induced pneumonitis was apparently
improved with infliximab therapy. One reason for recurrence of the
pneumonitis is thought to be an increase in TNF-α caused by
reactivation of autoreactive T cells.
A limitation for this study is that TNF-α at the
second and third onset of pneumonitis could not be measured due to
insufficient blood sample volume.
In conclusion, repetitive administration of
infliximab was effective for the recurring pneumonitis. The present
case may provide valuable information to establish an appropriate
therapeutic strategy for steroid-refractory ICI-induced
pneumonitis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SU and HK made substantial contributions to the
conception and design of the study. SU, MU, HK, KS and HO made
substantial contributions to the acquisition of the data. SU and HK
drafted the manuscript. SU and HK confirm the authenticity of all
the raw data. SU, HK, MI, KT, HA, KA and EB made substantial
contributions to the analysis and interpretation of the data and
were involved in revising the manuscript critically for important
intellectual content. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of the clinical data and images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386.
2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Vermorken JB, Mesia R, Rivera F, Remenar
E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol
D, et al: Platinum-based chemotherapy plus cetuximab in head and
neck cancer. N Engl J Med. 359:1116–1127. 2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ferris RL, Blumenschein G Jr, Fayette J,
Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE,
Even C, et al: Nivolumab for recurrent squamous-cell carcinoma of
the head and neck. N Engl J Med. 375:1856–1867. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Robert C, Long GV, Brady B, Dutriaux C,
Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C,
Kalinka-Warzocha E, et al: Nivolumab in previously untreated
melanoma without BRAF mutation. N Engl J Med. 372:320–330.
2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus Docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus Docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Motzer RJ, Escudier B, McDermott DF,
George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G,
Plimack ER, et al: Nivolumab versus Everolimus in advanced
renal-cell carcinoma. N Engl J Med. 373:1803–1813. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kang YK, Boku N, Satoh T, Ryu MH, Chao Y,
Kato K, Chung HC, Chen JS, Muro K, Kang WK, et al: Nivolumab in
patients with advanced gastric or gastro-oesophageal junction
cancer refractory to, or intolerant of, at least two previous
chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet.
390:2461–2471. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Le DT, Uram JN, Wang H, Bartlett BR,
Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et
al: PD-1 blockade in tumors with mismatch-repair deficiency. N Engl
J Med. 372:2509–2520. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Nishino M, Sholl LM, Hodi FS, Hatabu H and
Ramaiya NH: Anti-PD-1-related pneumonitis during cancer
immunotherapy. N Engl J Med. 373:288–290. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nishino M, Giobbie-Hurder A, Hatabu H,
Ramaiya NH and Hodi FS: Incidence of programmed cell death 1
inhibitor-related pneumonitis in patients with advanced cancer: A
systematic review and meta-analysis. JAMA Oncol. 2:1607–1616.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Brahmer JR, Lacchetti C, Schneider BJ,
Atkins MB, Brassil K, Caterino JM, Chau I, Ernstoff MS, Gardner JM,
Ginex P, et al: Management of immune-related adverse events in
patients treated with immune checkpoint inhibitor therapy: American
society of clinical oncology clinical practice guideline. J Clin
Oncol. 36:1714–1768. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Champiat S, Lambotte O, Barreau E, Belkhir
R, Berdelou A, Carbonnel F, Cauquil C, Chanson P, Collins M,
Durrbach A, et al: Management of immune checkpoint blockade
dysimmune toxicities: A collaborative position paper. Ann Oncol.
27:559–574. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sanchez GO, Jahn K, Savic S, Zippelius A
and Läubli H: Treatment of mycophenolate-resistant immune-related
organizing pneumonia with infliximab. J Immunother Cancer.
6(85)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Postow MA, Sidlow R and Hellmann MD:
Immune-related adverse events associated with immune checkpoint
blockade. N Engl J Med. 378:158–168. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Huang Y, Fan H, Li N and Du J: Risk of
immune-related pneumonitis for PD1/PD-L1 inhibitors: Systematic
review and network meta-analysis. Cancer Med. 8:2664–2674.
2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yamaguchi T, Shimizu J, Hasegawa T, Horio
Y, Inaba Y, Yatabe Y and Hida T: Pre-existing pulmonary fibrosis is
a risk factor for anti-PD-1-related pneumonitis in patients with
non-small cell lung cancer: A retrospective analysis. Lung Cancer.
125:212–217. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Cui P, Liu Z, Wang G, Ma J, Qian Y, Zhang
F, Han C, Long Y, Li Y, Zheng X, et al: Risk factors for
pneumonitis in patients treated with anti-programmed death-1
therapy: A case-control study. Cancer Med. 7:4115–4120.
2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kido H: Influenza virus pathogenicity
regulated by host cellular proteases, cytokines and metabolites,
and its therapeutic options. Proc Jpn Acad Ser B Phys Biol Sci.
91:351–368. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Beck KE, Blansfield JA, Tran KQ, Feldman
AL, Hughes MS, Royal RE, Kammula US, Topalian SL, Sherry RM,
Kleiner D, et al: Enterocolitis in patients with cancer after
antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J
Clin Oncol. 24:2283–2289. 2006.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Johnston RL, Lutzky J, Chodhry A and
Barkin JS: Cytotoxic T-lymphocyte-associated antigen 4
antibody-induced colitis and its management with infliximab. Dig
Dis Sci. 54:2538–2540. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sawai Y, Katsuya Y, Shinozaki-Ushiku A,
Iwasaki A, Fukayama M, Watanabe K and Nagase T: Rapid temporal
improvement of pembrolizumab-induced pneumonitis using the
anti-TNF-α antibody infliximab. Drug Discov Ther. 13:164–167.
2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gosset P, Perez T, Lassalle P, Duquesnoy
B, Farre JM, Tonnel AB and Capron A: Increased TNF-alpha secretion
by alveolar macrophages from patients with rheumatoid arthritis. Am
Rev Respir Dis. 143:593–597. 1991.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tanaka R, Ichimura Y, Kubota N, Saito A,
Nakamura Y, Ishitsuka Y, Watanabe R, Fujisawa Y, Kanzaki M, Mizuno
S, et al: Activation of CD8 T cells accelerates anti-PD-1
antibody-induced psoriasis-like dermatitis through IL-6. Commun
Biol. 3(571)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zen Y and Yeh MM: Hepatotoxicity of immune
checkpoint inhibitors: A histology study of seven cases in
comparison with autoimmune hepatitis and idiosyncratic drug-induced
liver injury. Mod Pathol. 31:965–973. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Minor DR, Chin K and Kashani-Sabet M: .
Infliximab in the treatment of anti-CTLA4 antibody (ipilimumab)
induced immune-related colitis. Cancer Biother Radiopharm.
24:321–325. 2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Fishman JA, Hogan JI and Maus MV:
Inflammatory and infectious syndromes associated with cancer
immunotherapies. Clin Infect Dis. 69:909–920. 2019.PubMed/NCBI View Article : Google Scholar
|