Introduction
Mammary analogue secretory carcinoma (MASC) is a
rare tumour of the head and neck region first described in 2010 by
Skálová et al (1). As of
2017, MASC has been listed in the Classification of Head and Neck
Tumours by the World Health Organization (2). MASC is a malignancy of the salivary
glands, predominantly observed in the parotid gland, with an
incidence rate of 4-4.5% among all malignant salivary gland tumours
(3-5).
However, when considering only tumours of the small salivary
glands, the incidence of MASC is markedly lower (4). MASC usually occurs at a mean age of 45
years and is slightly more common among men (6).
To diagnose MASC, microscopic examination is
necessary. On histopathological examination, the tumour typically
displays cystic, tubular and solid areas with infiltration. Most
MASCs consist of relatively monomorphic cells with a moderate
amount of vacuolated eosinophilic cytoplasm. On immunohistochemical
examination, the tumour shows immunoreactivity for S-100,
mammaglobin, MUC4 and CK7(3).
Mammaglobin and S100 protein show high sensitivity (95%), but are
not specific for MASC (6-8).
By contrast, an ETV6-neurotrophic receptor tyrosine kinase (NTRK)3
fusion gene is highly specific for MASC, at least considering
salivary gland malignancies. This translocation can be detected by
fluorescence in situ hybridization (FISH) or PCR analysis
and confirms the diagnosis of MASC (1,3,9-11).
In addition to NTRK1 and NTRK2, NTRK3 is a membrane-bound receptor
and part of the NTRK neurotrophin receptor family, which is
involved in neuronal cell differentiation and proliferation.
Mutations play an important role in carcinogenesis, such as that in
secretory breast carcinomas, medulloblastomas and MASCs (11). Approximately 300 cases of MASC have
been reported in the literature to date. Due to this limited number
of cases, no consistent data concerning treatment procedures and
outcomes have been published. The majority of MASCs are considered
as low-grade malignancies, they are treated by radical resection
and have a good prognosis (10).
According to the previously reported cases, patients with MASC have
a 5-year overall survival rate of 95% and a 5-year disease-free
survival rate of 89%, with a low incidence of nodal metastases
(12). However, cases with
high-grade transformation, lymph node and distant metastases have
rarely been described (13). The
case of a patient with MASC of the hard palate with bony
infiltration and contralateral cervical lymph node metastases is
described in the present study. This combination is extraordinarily
rare and, to the best of our knowledge, has not been described in
the literature to date (5). The aim
of the present study was to focus on treatment procedures
recommended for high-risk patients, discuss the benefits of
selective neck dissection and adjuvant chemoradiation, and discuss
novel treatment options with entrectinib, larotrectinib and other
tyrosine kinase inhibitors (TRKIs) for patients with inoperable
tumours or under palliative care.
Case report
A 44-year-old Caucasian woman initially presented at
the Department of Oral and Maxillofacial Plastic Surgery of the
University Hospital of Wuerzburg (Wuerzburg, Germany) in June 2018.
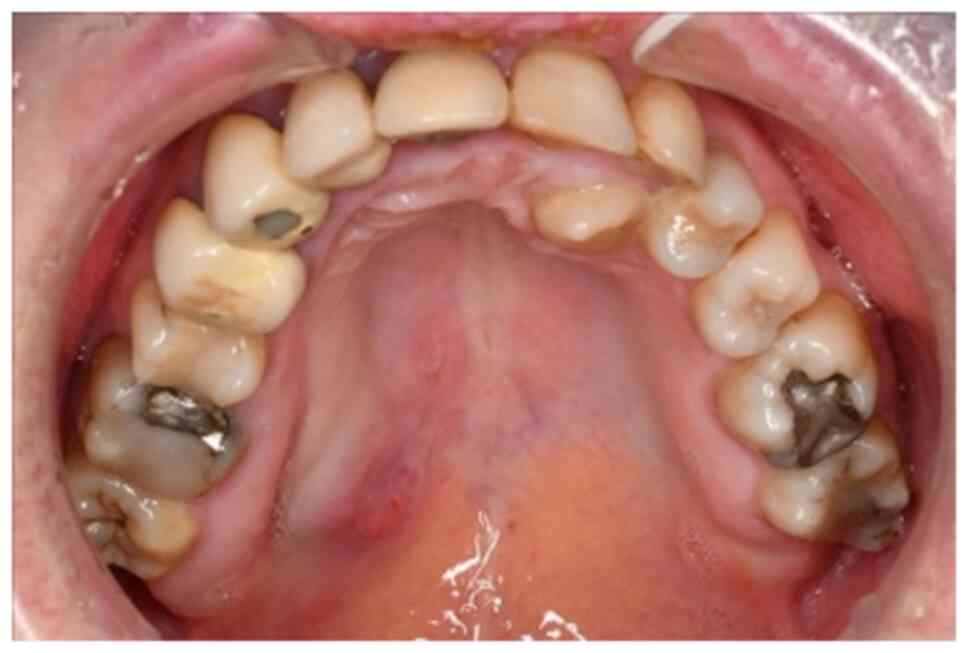
The patient reported painless swelling in the area of the right
hard palate (Fig. 1). A biopsy had
already been performed at a private practice office, and
histopathological examination revealed a carcinoma with
immunoreactivity for CK7, S100 and mammaglobin. No irregularities
and no radiotherapy of the head and neck region were noted in the
patient's history.
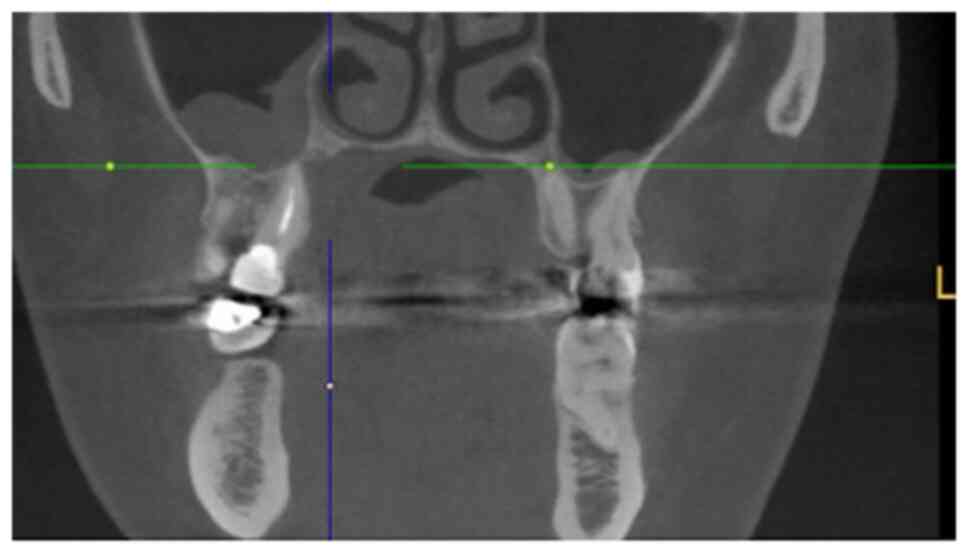
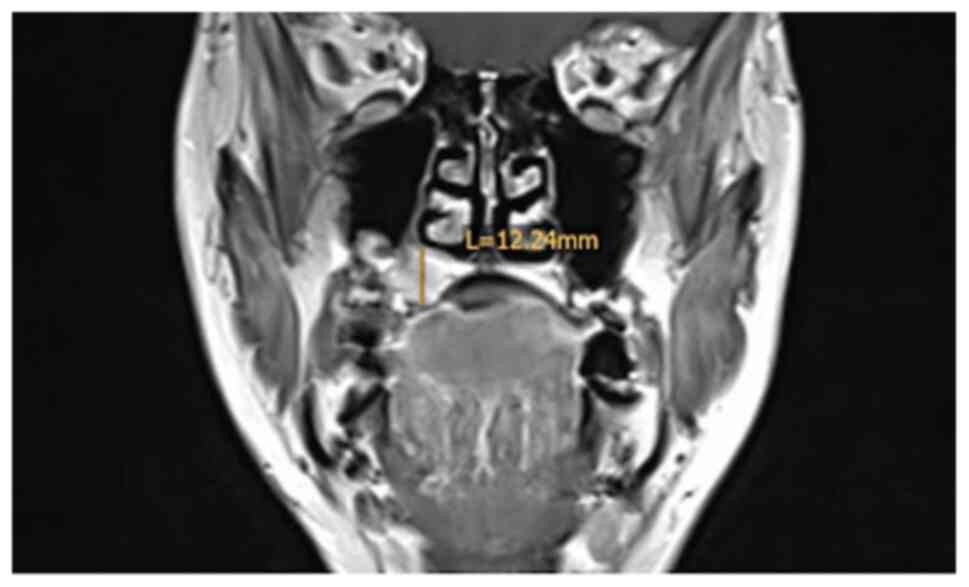
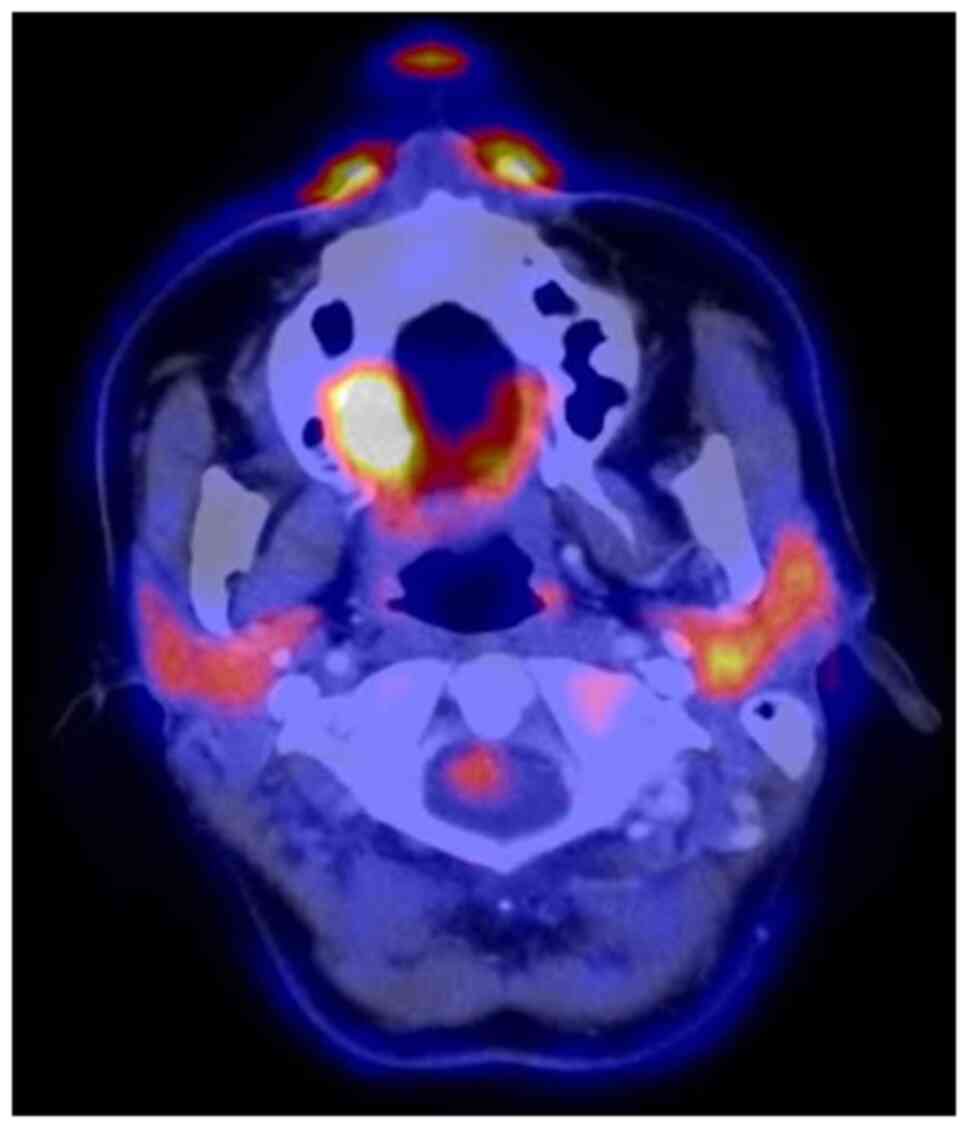
The staging procedures included clinical
examination, ultrasound (US) of the neck, MRI, cone beam CT and
[18F]-fluorodeoxyglucose-positron emission tomography/CT
(FDG-PET/CT).
The MRI examination revealed a tumour measuring
18x16x12 mm, with invasion of the right maxillary sinus and
suspected retropharyngeal and cervical lymph nodes on the left side
on FDG-PET/CT. By contrast, CT and MRI showed bilateral suspected
lymph nodes (Fig. 2, Fig. 3 and Fig.
4). A secondary suspected pharyngeal lesion at the tongue base
was also identified.
Based on the aforementioned findings, the patient
underwent local resection of the hard palate tumour, with a
clinical safety distance of 10 mm, along with bilateral radical
neck dissection (levels I-V) with temporary tracheotomy. Plastic
reconstruction of the hard palate was performed during the same
operation using a radial forearm flap. Biopsies of the suspected
lesion in the pharynx showed no signs of malignancy.
Histopathological examination of the specimen revealed a tumour
with a diameter of 13 mm, with the closest margins at 3 mm,
medullary bone invasion and two (2/37) lymph node metastases on the
contralateral side of the neck at level Va. No metastases were
detected on the ipsilateral side of the neck. Histopathological
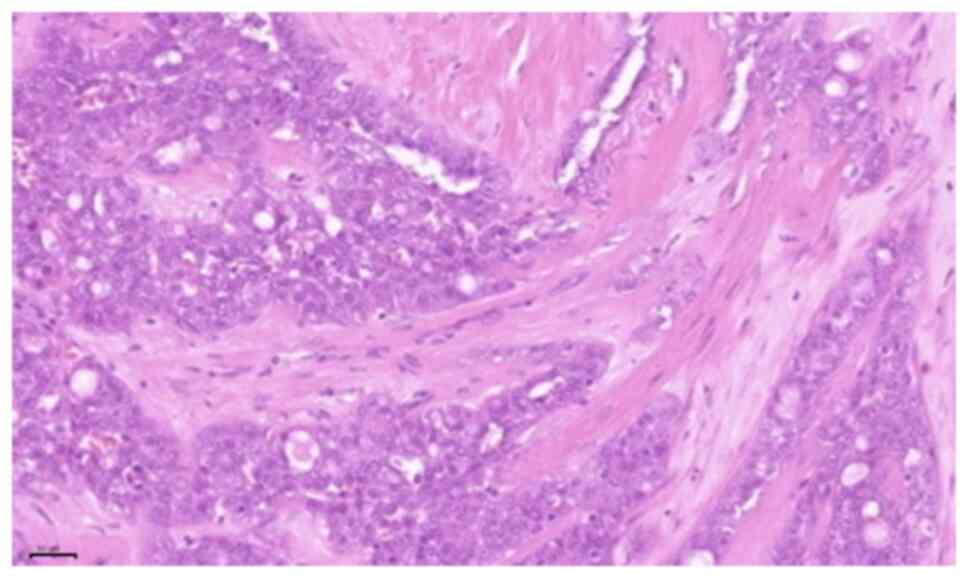
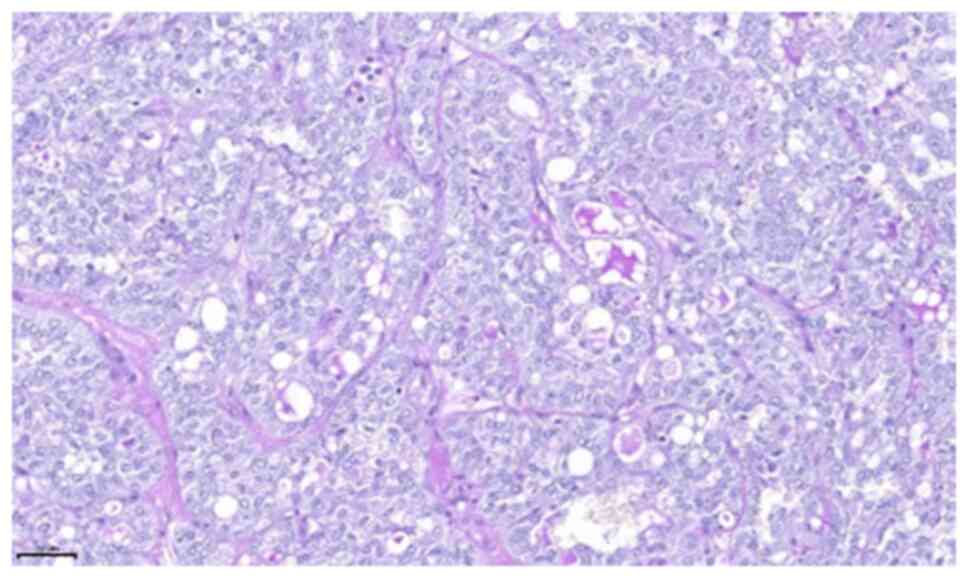
examination of the tumour revealed microcystic, tubular and solid
areas with infiltration. The tumour cells were monomorphic and
round-oval, with a moderate amount of vacuolated eosinophilic
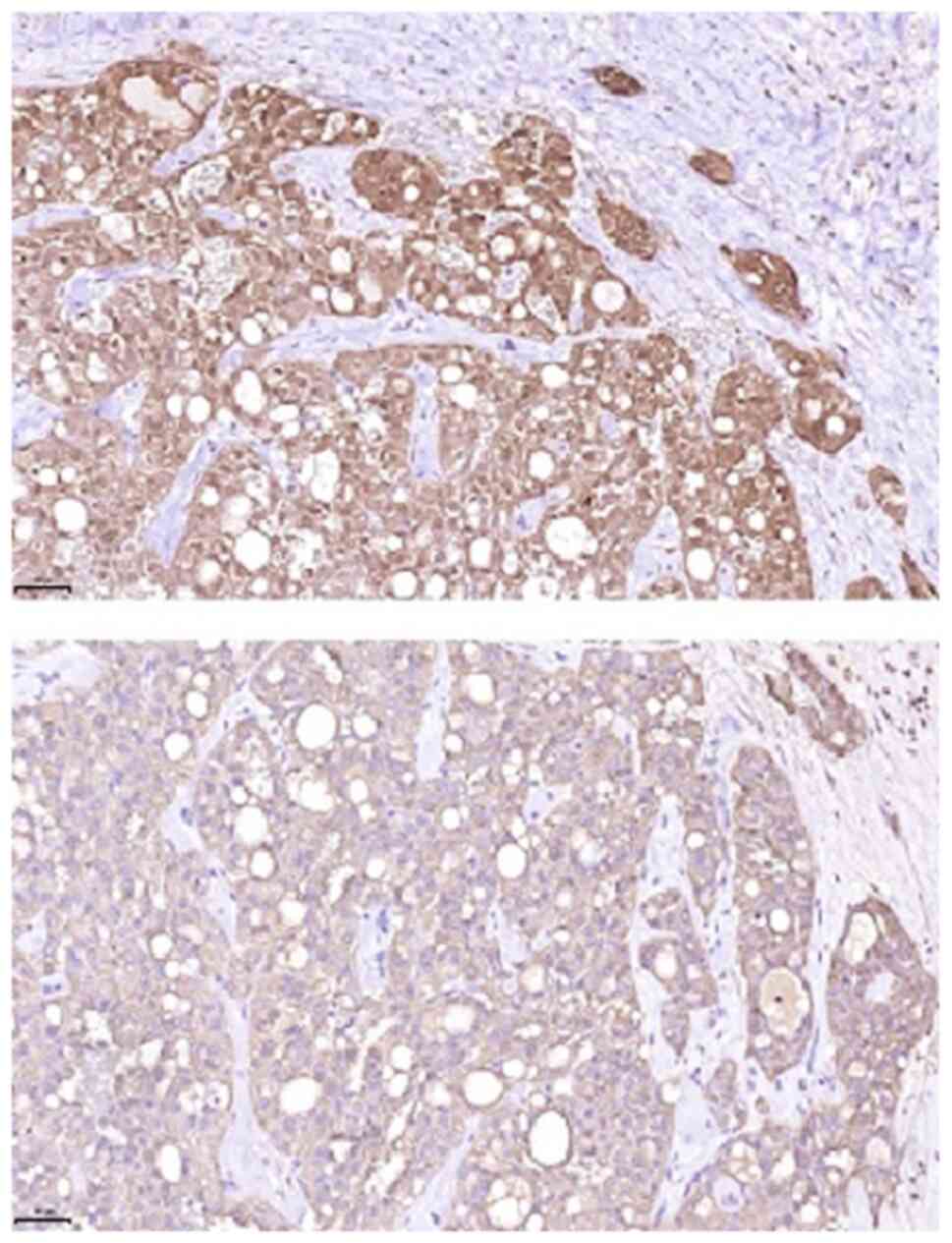
cytoplasm (Figs. 5 and 6). On immunohistochemical examination, the
tumour was positive for CK7, S100, mammaglobin, GATA3, GCDF-P15 and
pan-cytokeratin (AE1/3). P16 expression in tumour cells, partial
reactivity of SOX10 and specific reactivity for CD117 were also
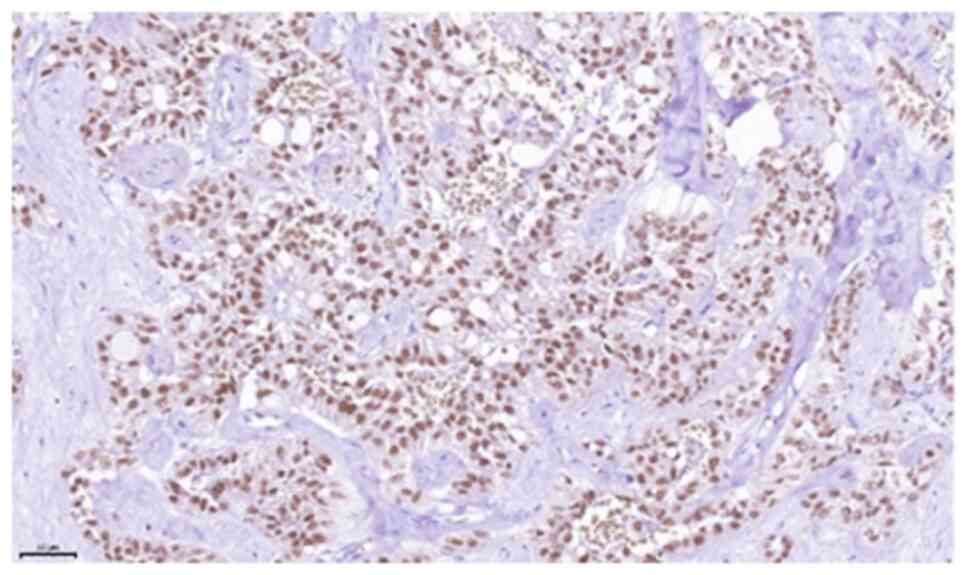
detected. The Ki-67 index was low (<5%). FISH analysis performed
using an ETV6 break-apart probe was positive. Considering the
findings of histopathological and immunohistochemical examinations,
and the FISH results with proof of a break in the ETV6-gene
(12q13), the diagnosis of MASC was confirmed (Fig. 5, Fig.
6, Fig. 7 and Fig. 8). Based on these findings, the
tumour stage was defined as follows: pT4a, pN2c (2/37), pL0, pV0
and pPn0 (8th Edition of the TNM Classification, 2017) (14). Due to the several high-risk
characteristics of this tumour, including bone invasion and
contralateral nodal metastases, adjuvant radiochemotherapy with 30
rounds of radiation (dynamic volumetric modulated arc therapy;
54.00/66.00 Gy) and cisplatin (a cumulative dose of 200 mg/m² body
surface area) was performed. A radiation dose of 54.00 Gy was
delivered to the area of lymphatic cervical drainage, and 66 Gy was
delivered to the area of the tumour in the hard palate.
The postoperative course was uneventful, and the
patient has visited our outpatient centre regularly to date (last
follow-up visit, March 2021), without signs of recurrence. The
postoperative clinical examination revealed limited abduction of
the shoulder and limited opening of the mouth, for which
physiotherapy and exercises were performed.
Discussion
MASC is a rare type of head and neck cancer that
mostly affects the major salivary glands. As a malignant tumour,
preoperative staging examinations are necessary, including clinical
examination, radiological imaging, and histological,
immunohistochemical and molecular genetic examinations.
In the case presented herein, preoperative imaging
revealed a tumour measuring 18x16x12 mm, with bony infiltration of
the right hard palate and cervical lymph node metastases. Suspected
lymph node metastases were shown on both sides of the neck on MRI
and CT scans. By contrast, FDG-PET/CT scan only showed suspected
lymph nodes on the contralateral side of the neck. There were no
signs of distant metastases.
Histological examination revealed a malignant tumour
of a minor salivary gland, and immunohistochemical examinations
confirmed the diagnosis of MASC by showing the typical
characteristics of this entity. To verify the diagnosis of MASC,
several differential diagnoses had to be excluded. In a
retrospective review, Chiosea et al (15) reported that 11 of 89 (12.4%)
patients with MASC were incorrectly diagnosed with acinic cell
carcinoma (AciCC), and 14 of 37 (37.8%) patients were incorrectly
diagnosed with adenocarcinoma (AC). In particular, MASC and AciCC
share histological similarities. Although AciCC is characterized by
basophilic cytoplasm and zymogen granules, MASC cells typically
have eosinophilic cytoplasm and no zymogen granules, which was
consistent with the histopathological findings of the present case.
However, zymogen-poor AciCCs are very difficult to differentiate
from MASCs (1,6,16).
Strong S100 protein and mammaglobin expression levels may help
differentiate between the two entities, as these findings exclude
AciCC (6,10,17).
Another important differential diagnosis of MASC is AC, which can
express S100 and mammaglobin and is typically observed in the minor
glands (6). Other differential
diagnoses, such as cystadenocarcinoma (CAC) and mucoepidermoid
carcinoma (MuC), must also be considered (6). Due to these findings in some cases,
histopathological and immunohistochemical examinations do not
ensure the accurate diagnosis of MASC. In these cases, detection of
the t(12;15)(p13;q25) translocation (ETV6-NTRK3 fusion gene) may
help differentiate between MASC and other similar tumours (3,6). The
ETV-6 gene encodes a tyrosine kinase that regulates cell
proliferation and differentiation. Considering only salivary gland
neoplasms, this translocation is highly specific for MASC (3,7). In
the case presented herein, immunohistochemical and molecular
genetic examinations confirmed the diagnosis of MASC.
As the existing literature is limited, no consistent
therapy guidelines are currently available for this tumour entity.
In the literature, the majority of the patients received treatment
based on histochemical similarities and similar growth patterns
analogous to other low-grade malignancies or AciCC, and underwent
surgery with or without adjuvant radiation or chemoradiation
(6,10,13).
In a review of the literature, primary radiotherapy or
chemoradiation were not considered as common therapeutic options
(17). Therefore, only one case
report was found concerning a patient with MASC who was treated
with primary radiotherapy. That patient had a locally advanced MASC
of the parotid gland, without nodal or distant metastases. The
patient received radiation (66 Gy) with cetuximab and achieved
stable disease. Cetuximab is an EGFR-TRKI which, along with other
TRKIs, may be useful for treating MASC (18).
For sufficient tumour resection, a clinical safety
margin of 10 mm is considered to be appropriate, and it is
obligatory in head and neck squamous cell carcinoma according to
German guidelines (19). Currently,
no valid data are available on whether selective neck dissection in
patients with a negative nodal status improves patient outcomes and
overall survival. In the present case, two nodal metastases were
identified at level Va on the contralateral side of the neck,
although MASC is normally a low-grade malignancy and nodal
metastases are rare. Chiosea et al (15) reported cervical nodal metastases in
6/34 patients (17.6%). In another study, 4/18 (22%) patients had
nodal metastases (6), which were
mostly observed in patients with a higher T stage (T3 or T4) and
submandibular gland tumours (17).
Owing to the small number of cases in those studies, a distinct
recommendation for neck dissection cannot be given. However, the
data indicate that patients may benefit from selective neck
dissection, even when imaging examinations are negative for nodal
metastases. Levels I, II, III and Va are more likely to be affected
by nodal metastases from MASC, similar to other oral malignancies
(20,21). For cases with a positive nodal
status, uni- or bilateral neck dissection of levels I-V should be
performed, and adjuvant radiation or chemoradiation is necessary
due to the more aggressive tumour behaviour (22). In cases of a low-grade malignancy
without nodal metastases, surgery alone may be sufficient (6). Based on the MRI and CT findings in the
present case, bilateral radical neck dissection was performed.
Histopathological examination of the resectate detected two (2/37)
lymph node metastases corresponding to the lesions on FDG-PET/CT on
the contralateral, but not the ipsilateral, side of the neck. Lymph
nodes on the ipsilateral side may have appeared enlarged and
suspicious on MRI and CT due to the preoperative biopsy performed
in a private practice. Of note, a biopsy should be preferably
performed after imaging examinations to prevent the detection of
false-positive lymph nodes and overtreatment (23). In the present case, nodal dissection
of the lower ipsilateral levels may have been prevented by this
approach, further demonstrating the higher specificity of
FDG-PET/CT for detecting cervical nodal metastases compared with CT
and MRI (24,25). Currently, FDG-PET/CT is not
routinely performed in patients with head and neck cancer due to
the lack of clear recommendations, limited availability and high
cost.
Due to the positive nodal status, the patient
received adjuvant chemoradiation with cisplatin and 66 Gy. It is
unclear whether chemoradiation is superior to radiation. In a study
by Sethi et al (17), only
2/86 (2.3%) of patients with MASC received adjuvant chemotherapy,
in contrast to 21/86 (24.4%) patients who received radiation
without chemotherapy. This approach is supported by a review in
2019 comparing the treatments received by 7,342 patients with
salivary gland cancer. The patients were separated into different
subgroups by histological subtype. The patients had AciCC (20.6%)
and mucoepidermoid carcinoma (36.4%), but not MASC. The treatment
differed significantly across the subgroups. Overall, 42% of the
patients with AciCC received surgery and adjuvant radiation, and
only 4% received surgery and adjuvant chemoradiation. In this
subgroup, the 2- and 5-year overall survival rates for surgery plus
radiotherapy vs. surgery plus chemoradiation were 95 and 84% vs. 81
and 63%, respectively (26). If
these data are extrapolated to MASC, the treatment for which is
similar to that for AciCC, there is no scientific evidence
supporting the benefit of adjuvant chemotherapy. Thus, adjuvant
radiation appears to be appropriate for high-risk patients with
nodal metastases.
Selective TRKI treatment may also be considered for
therapy in patients with locally advanced, metastatic or inoperable
tumours (27). For example, TRKIs
as a treatment option was reported in a clinical study of 55
patients with TRK fusion-positive cancers. MASC was identified in
12 of these patients (21.81%) and was the most frequent entity. All
the patients had advanced local tumour or metastases, or had
already received standard therapy; therefore, they were treated
with the TRKI larotrectinib. Larotrectinib at 100 mg was
administered twice per day until tumour progression, the occurrence
of adverse events, or withdrawal from the study. Of the 55
patients, 7 (13%) had a complete response, 34 (62%) had a partial
response, 7 (13%) had stable disease, 5 (9%) had progressive
disease, and 2 (4%) could not be evaluated. The overall response
rate was 80%. No difference in therapy efficiency was observed
across the subgroups. Of note, of the 12 patients with MASC, only 1
had progressive disease, while the remaining 11 patients exhibited
a partial or complete response to larotrectinib therapy.
Consistently, the European Medicines Agency approved larotrectinib
for the treatment of solid tumours with NTRK gene fusion, including
MASC. Another potential treatment option is entrectinib, which has
also been approved for the treatment of TRK-expressing tumours.
Several phase 1 and 2 studies, such as ALKA, STARTRK-1 and
STARTRK-2, are investigating the potential benefits of entrectinib
for the treatment of cancers with detected molecular alterations in
the TRK1, TRK2 and TRK3 genes (28). STARTRK-2, in particular, includes
patients with salivary gland tumours harbouring a NTRK3 gene
fusion, similar to that in MASC. In addition to entrectinib and
larotrectinib, which are two approved therapy options, other TRKIs,
such as sitravatinib, cabozantinib, belizatinib, and several more,
have been evaluated in clinical trials (29).
These data indicate a potential therapy option for
patients with relapse after standard therapy, patients with
inoperable tumours (locally advanced and/or metastatic), or those
under palliative care (30), and
underscore the significance of molecular diagnostic methods to
detect the ETV6-NTRK3 fusion gene. It may be of value to
re-evaluate whether this fusion gene is present in patients with
inoperable salivary gland tumours and those under palliative care.
This approach appears to be appropriate, as MASC is often initially
misdiagnosed as AciCC, AC, CAC or MuC; thus, molecular diagnosis
may uncover new therapy options.
Adequate follow-up must be applied postoperatively.
A mean follow-up period of 5 years with imaging examinations at
specific intervals appears to be appropriate. We suggest that the
first imaging examination be performed 6 months after surgery,
followed by imaging once per year until follow-up is completed. In
the first 2 years and in high-risk patients the intervals between
assessments should be shorter (every 3-4 months).
This case underscores the need for sufficient
staging examinations and accurate diagnosis, which are crucial for
selecting the optimal therapy procedures and may uncover different
(molecular) treatment options for patients receiving palliative
care. If a local tumour without nodal or distant metastases is
detected, surgery alone should be performed. However, when
aggressive tumour behaviour and nodal metastases are observed,
selective neck dissection should be considered, even when the nodal
status is negative. If the nodal status is positive, ipsi- or
bilateral neck dissection of levels I-V and adjuvant radiation or
chemoradiation are necessary. The benefits of chemotherapy remain
unclear. New molecular targeted therapies with TRKIs, such as
larotrectinib and entrectinib, may be promising treatment options
for patients with advanced inoperable disease and underscore the
need for molecular diagnosis in selected cases.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Clinical data and complementary examinations are
available from the corresponding author on reasonable request.
Authors' contributions
AS, RB and SH accrued all data, obtained the
patient's informed consent and drafted the manuscript. AS, RB and
AK have seen and can confirm the authenticity of the raw data. SS
provided the histopathological information and images of the
obtained resectate. All the authors have contributed to writing the
manuscript. AS, RB, JH, CL, AF and UMR prepared the final version
of the manuscript. All the authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patent consent for publication
Written informed consent was obtained from the
patient regarding the publication of the case details and any
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Skálová A, Vanecek T, Sima R, Laco J,
Weinreb I, Perez-Ordonez B, Starek I, Geierova M, Simpson RH,
Passador-Santos F, et al: Mammary analogue secretory carcinoma of
salivary glands, containing the ETV6-NTRK3 fusion gene: A hitherto
undescribed salivary gland tumor entity. Am J Surg Pathol.
34:599–608. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Seethala RR and Stenman G: Update from the
4th Edition of the World Health Organization classification of head
and neck tumours: Tumors of the salivary gland. Head Neck Pathol.
11:55–67. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Luk PP, Selinger CI, Eviston TJ, Lum T, Yu
B, O'Toole SA, Clark JR and Gupta R: Mammary analogue secretory
carcinoma: An evaluation of its clinicopathological and genetic
characteristics. Pathology. 47:659–666. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Majewska H, Skalova A, Stodulski D,
Klimková A, Steiner P, Stankiewicz C and Biernat W: Mammary
analogue secretory carcinoma of salivary glands: A new entity
associated with ETV6 gene rearrangement. Virchows Arch.
466:245–254. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Boliere C, Murphy J, Qaisi M, Manosca F
and Fung H: Mammary analogue secretory carcinoma of the palate:
Case report and review of the literature. Case Rep Dent.
2019(7416302)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Stevens TM and Parekh V: Mammary analogue
secretory carcinoma. Arch Pathol Lab Med. 140:997–1001.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shah AA, Wenig BM, LeGallo RD, Mills SE
and Stelow EB: Morphology in conjunction with immunohistochemistry
is sufficient for the diagnosis of mammary analogue secretory
carcinoma. Head Neck Pathol. 9:85–95. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Patel KR, Solomon IH, El-Mofty SK, Lewis
JS Jr and Chernock RD: Mammaglobin and S-100 immunoreactivity in
salivary gland carcinomas other than mammary analogue secretory
carcinoma. Hum Pathol. 44:2501–2508. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Khalele BA: Systematic review of mammary
analog secretory carcinoma of salivary glands at 7 years after
description. Head Neck. 39:1243–1248. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Skalova A: Mammary analogue secretory
carcinoma of salivary gland origin: An update and expanded
morphologic and immunohistochemical spectrum of recently described
entity. Head Neck Pathol 7. 1 (Suppl 1):S30–S36. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Luo Y, Kaz AM, Kanngurn S, Welsch P,
Morris SM, Wang J, Lutterbaugh JD, Markowitz SD and Grady WM: NTRK3
is a potential tumor suppressor gene commonly inactivated by
epigenetic mechanisms in colorectal cancer. PLoS Genet.
9(e1003552)2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Boon E, Valstar MH, van der Graaf WTA,
Bloemena E, Willems SM, Meeuwis CA, Slootweg PJ, Smit LA, Merkx
MAW, Takes RP, et al: Clinicopathological characteristics and
outcome of 31 patients with ETV6-NTRK3 fusion gene confirmed
(mammary analogue) secretory carcinoma of salivary glands. Oral
Oncol. 82:29–33. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Skálová A, Vanecek T, Majewska H, Laco J,
Grossmann P, Simpson RH, Hauer L, Andrle P, Hosticka L, Branžovský
J and Michal M: Mammary analogue secretory carcinoma of salivary
glands with high-grade transformation: Report of 3 cases with the
ETV6-NTRK3 gene fusion and analysis of TP53, β-catenin, EGFR, and
CCND1 genes. Am J Surg Pathol. 38:23–33. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Brierley J, Gospodarowicz M and Wittekind
C: Head and Neck Tumours, Lip and Oral Cavity. In: TNM
Classification of Malignant Tumours. 8th edition. Wiley-Blackwell,
Toronto, pp17-54, 2017.
|
|
15
|
Chiosea SI, Griffith C, Assaad A and
Seethala RR: Clinicopathological characterization of mammary
analogue secretory carcinoma of salivary glands. Histopathology.
61:387–394. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Connor A, Perez-Ordonez B, Shago M,
Skálová A and Weinreb I: Mammary analog secretory carcinoma of
salivary gland origin with the ETV6 gene rearrangement by FISH:
Expanded morphologic and immunohistochemical spectrum of a recently
described entity. Am J Surg Pathol. 36:27–34. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sethi R, Kozin E, Remenschneider A, Meier
J, VanderLaan P, Faquin W, Deschler D and Frankenthaler R: Mammary
analogue secretory carcinoma: Update on a new diagnosis of salivary
gland malignancy. Laryngoscope. 124:188–195. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tokuzen N, Goda H and Nakashiro K: Locally
advanced mammary analogue secretory carcinoma of the parotid gland.
Int J Oral Maxillofac Surg. 48:865–868. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Slootweg PJ, Hordijk GJ, Schade Y, van Es
RJ and Koole R: Treatment failure and margin status in head and
neck cancer. A critical view on the potential value of molecular
pathology. Oral Oncol. 38:500–503. 2002.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Weiss MH, Harrison LB and Isaacs RS: Use
of decision analysis in planning a management strategy for the
stage N0 neck. Arch Otolaryngol Head Neck Surg. 120:699–702.
1994.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shah JP, Candela FC and Poddar AK: The
patterns of cervical lymph node metastases from squamous carcinoma
of the oral cavity. Cancer. 66:109–113. 1990.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bier J: Radical neck dissection versus
conservative neck dissection for squamous cell carcinoma of the
oral cavity. Recent Results Cancer Res. 134:57–62. 1994.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wolff KD, Al-Nawas B, Al-Sharif U, Beck J,
Bikowski K, Bissinger O, Böhme P, Bönte-Hieronymus I, Bootz F,
Bozzato A, et al: S3-Guidline regarding diagnosis and therapy of
oral cancer. Oncology Guideline Program. AWMF, 2012 (In German).
https://www.awmf.org/uploads/tx_szleitlinien/007-100OLl_S3-Diagnostik-Therapie-Mundhoehlenkarzinom_2021-03.pdf.
Accessed March 2021.
|
|
24
|
Fukuhara R, Shinya T, Fukuma S, Ogawa N,
Masaoka Y, Tanaka T, Marunaka H, Arioka T, Hiraki T, Kaji M and
Kanazawa S: The diagnostic capacity of pre-treatment
18F-FDG PET/CT for predicting the extranodular spread of
lymph node metastases in patients with oral squamous cell
carcinoma. Acta Med Okayama. 74:123–128. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Loo SW, Geropantas K, Beadsmoore C,
Montgomery PQ, Martin WM and Roques TW: Neck dissection can be
avoided after sequential chemoradiotherapy and negative
post-treatment positron emission tomography-computed tomography in
N2 head and neck squamous cell carcinoma. Clin Oncol (R Coll
Radiol). 23:512–517. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ferrell JK, Mace JC and Clayburgh D:
Contemporary treatment patterns and outcomes of salivary gland
carcinoma: A national cancer database review. Eur Arch
Otorhinolaryngol. 276:1135–1146. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Amatu A, Sartore-Bianchi A and Siena S:
NTRK gene fusions as novel targets of cancer therapy across
multiple tumour types. ESMO Open. 1(e000023)2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Drilon A, Siena S, Ou SI, Patel M, Ahn MJ,
Lee J, Bauer TM, Farago AF, Wheler JJ, Liu SV, et al: Safety and
antitumor activity of the multitargeted Pan-TRK, ROS1, and ALK
inhibitor entrectinib: Combined results from two Phase I Trials
(ALKA-372-001 and STARTRK-1). Cancer Discov. 7:400–409.
2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Jiang T, Wang G, Liu Y, Feng L, Wang M,
Liu J, Chen Y and Ouyang L: Development of small-molecule
tropomyosin receptor kinase (TRK) inhibitors for NTRK fusion
cancers. Acta Pharm Sin B. 11:355–372. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Drilon A, Laetsch TW, Kummar S, DuBois SG,
Lassen UN, Demetri GD, Nathenson M, Doebele RC, Farago AF, Pappo
AS, et al: Efficacy of larotrectinib in TRK fusion-positive cancers
in adults and children. N Engl J Med. 378:731–739. 2018.PubMed/NCBI View Article : Google Scholar
|