Introduction
Precancerous lesions of the bile duct system were
classified by the WHO in 2010 as biliary intraepithelial neoplasia
(BilIN) and intraductal papillary neoplasm of the bile duct (IPNB)
(1). Intracholecystic papillary
neoplasm of the gallbladder (ICPN) is a type of IPNB that occurs at
the gallbladder (2). Adsay et
al (3) reported that ICPN
shows the following characteristics: Intramucosal, preinvasive
neoplastic or dysplastic mass formation that is exophytic
(papillary or polypoid), ≥1.0 cm in size, and compact and distinct
from the neighbouring mucosa (3).
This concept is relatively new, and therefore, there are few
reports regarding ICPN.
Menetrier's disease was first reported as a disease,
which showed extensive gastric mucosal proliferation with
hypoproteinaemia in 1888 by Menetrier (4). Menetrier's disease is a rare disease
characterized by giant hypertrophy of the gastric folds that cause
protein-losing gastroenteropathy (PLG) (5,6).
Patients with Menetrier's disease often show symptoms such as
diarrhoea, abdominal pain, nausea, postprandial fullness and weight
loss (7,8). Menetrier's disease is also known as a
possible risk factor for gastric cancer, with the incidence of
gastric cancer reaching 10% in patients with Menetrier's disease
(7). Although Menetrier's disease
is associated with malignancy, few malignancies other than that of
the stomach has been reported (9,10),
the relationship between Menetrier's disease and malignancies other
than those of the stomach are unclear. Herein, a case of a resected
ICPN complicated with Menetrier's disease that showed PLG is
described.
Case report
A 69-year-old man presented to Hokkaido Social Work
Association Obihiro Hospital with gallbladder tumours diagnosed by
ultrasonography at a previous hospital. He was diagnosed with PLG
due to Menetrier's disease at a previous hospital 2 years ago, and
had visited the previous hospital to receive intravenous albumin
every week. In addition, gallbladder tumours were incidentally
found during the follow-up for Menetrier's disease. The patient had
no history of abdominal surgery. Regarding Menetrier's disease,
fundic gland hyperplasia was found in biopsies from the gastric
mucosa. Helicobacter pylori (H. pylori) analysis came
back negative. There were no abnormalities in the results of the
colonoscopy or capsule endoscopy of the small intestine. He
suffered from diarrhoea, and exhibited iron deficiency (15 µg/dl)
and hypoalbuminemia (2.6 g/dl) at diagnosis of Menetrier's disease.
His body weight was 52.9 kg at diagnosis of Menetrier's
disease.
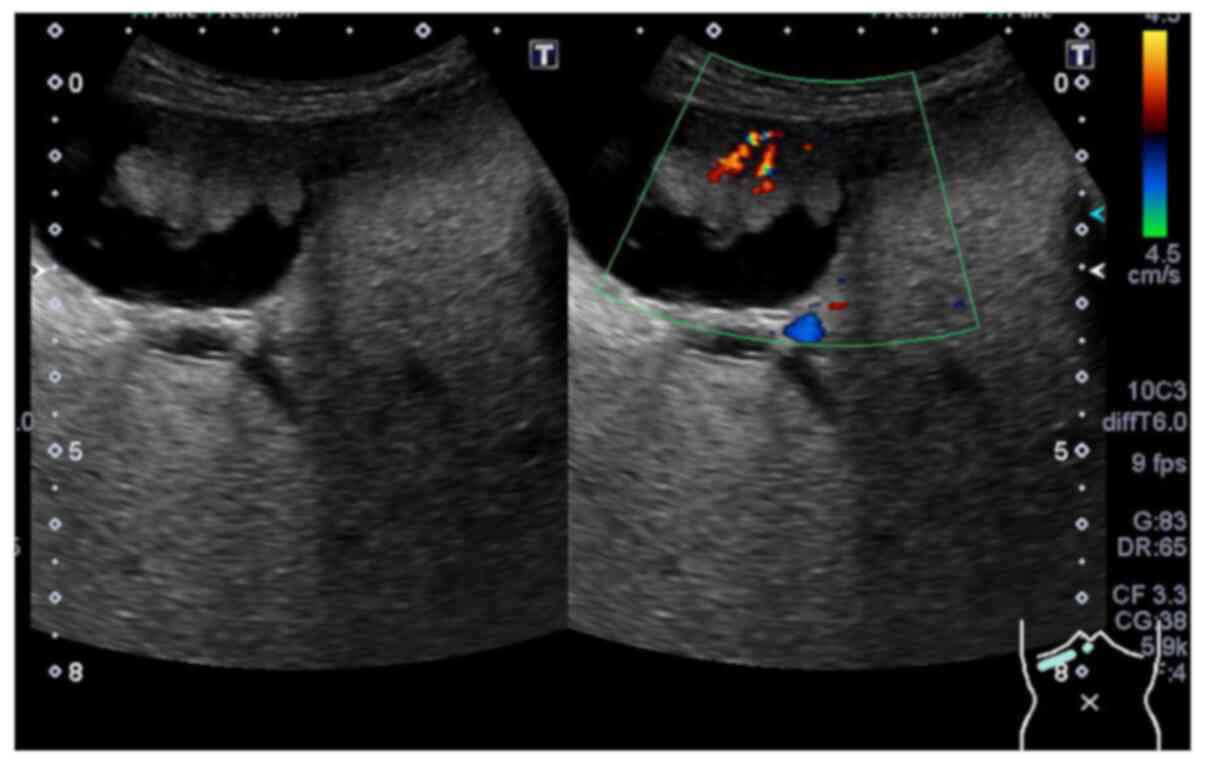
Ultrasonography showed a cauliflower homogenous
tumour that was 2.7 cm in maximum diameter with a blood supply at
the fundus of the gallbladder (Fig.
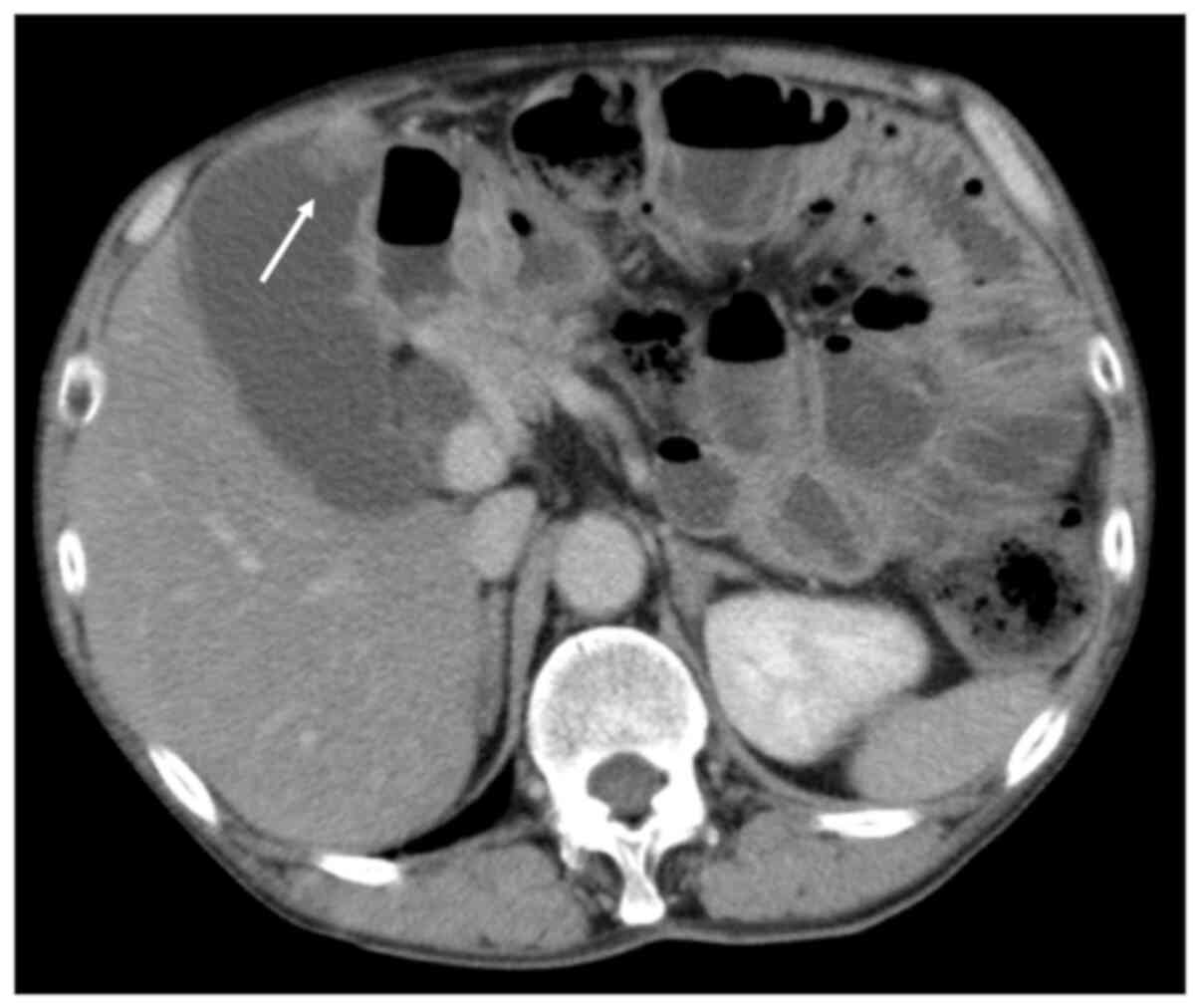
1). Abdominal contrast-enhanced computed tomography (CT) showed
an irregular mass with a contrast effect at the fundus of the
gallbladder on the free abdominal cavity side. Abdominal CT also
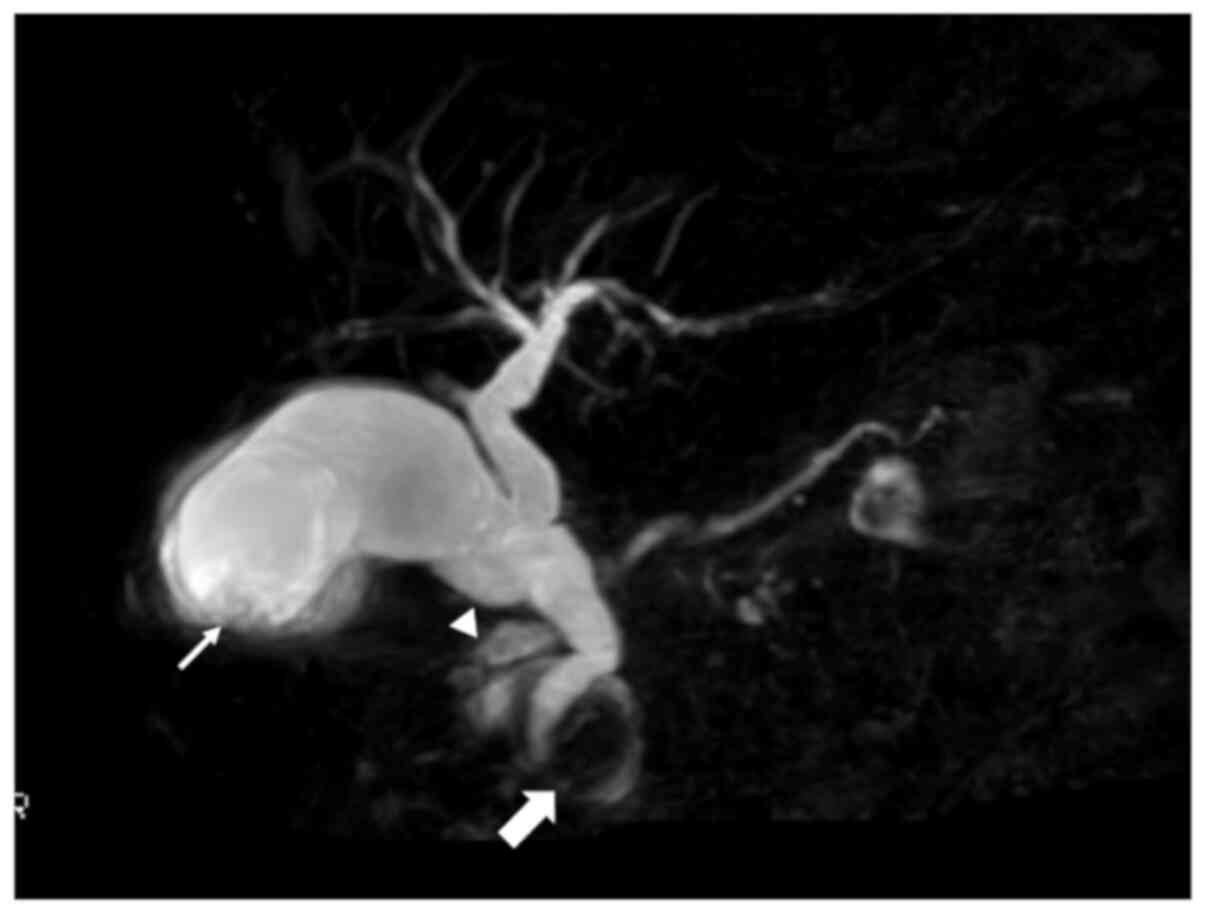
revealed an oedematous and dilated small intestine (Fig. 2). Magnetic resonance
cholangiopancreatography (MRCP) showed an intracholecystic
papillary torose lesion at the fundus and a dilated common bile
duct with a paraduodenal diverticulum. MRCP also revealed
pancreatic divisum with a dilated Santorini duct and indistinct
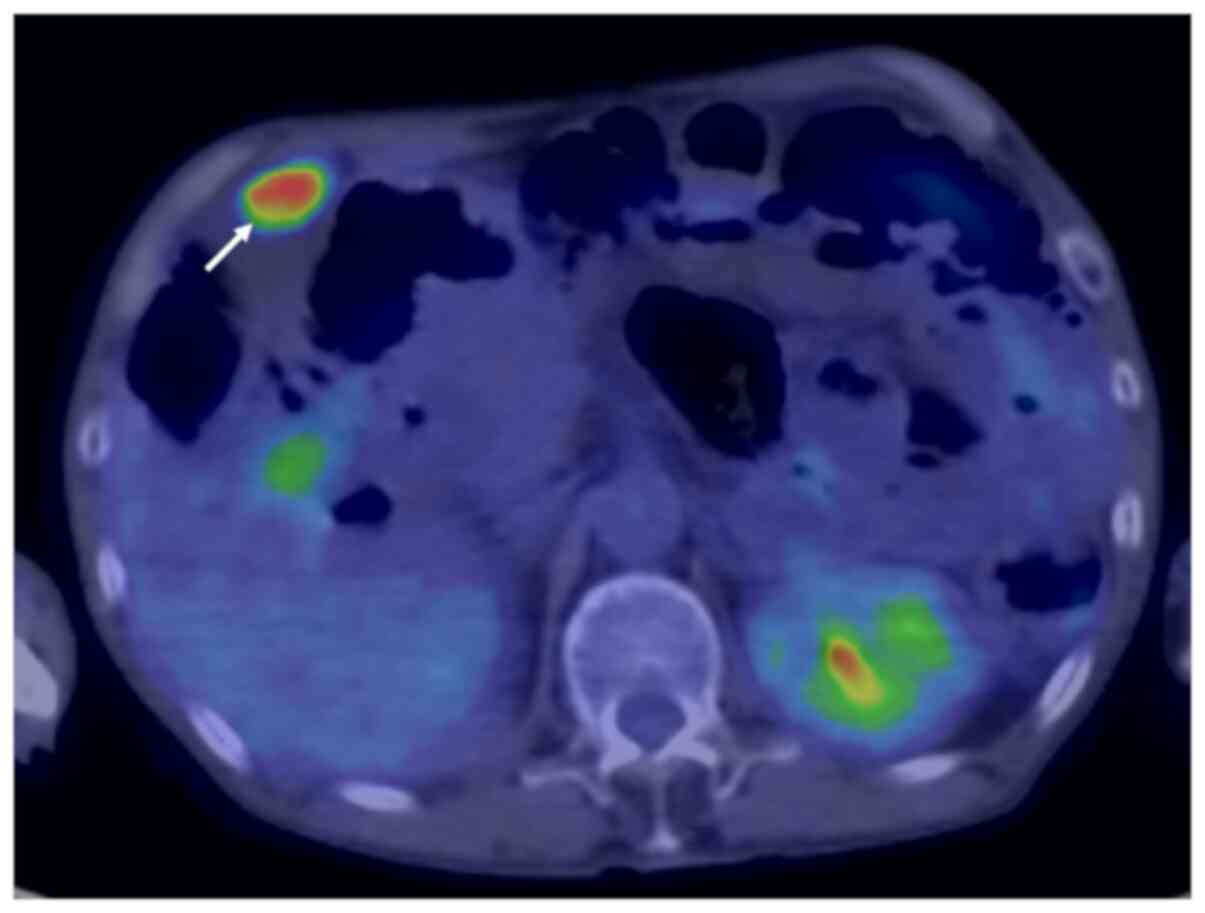
Wirsung duct (Fig. 3). Positron
emission tomography-CT showed a tumour with a SUV of 8.28 at the
fundus of the gallbladder (Fig.
4). Serum levels of carcinoembryonic antigen, carbohydrate
antigen 19-9 and carbohydrate antigen 125 were within the normal
ranges (2.1 mg/ml, <2.0 U/ml and 8.5 U/ml, respectively).
Moreover, low serum albumin (serum albumin, 3.1 g/dl) and anaemia
(haemoglobin, 8.4 g/dl) were observed (Table I). Serum iron was 14 µg/dl. Body
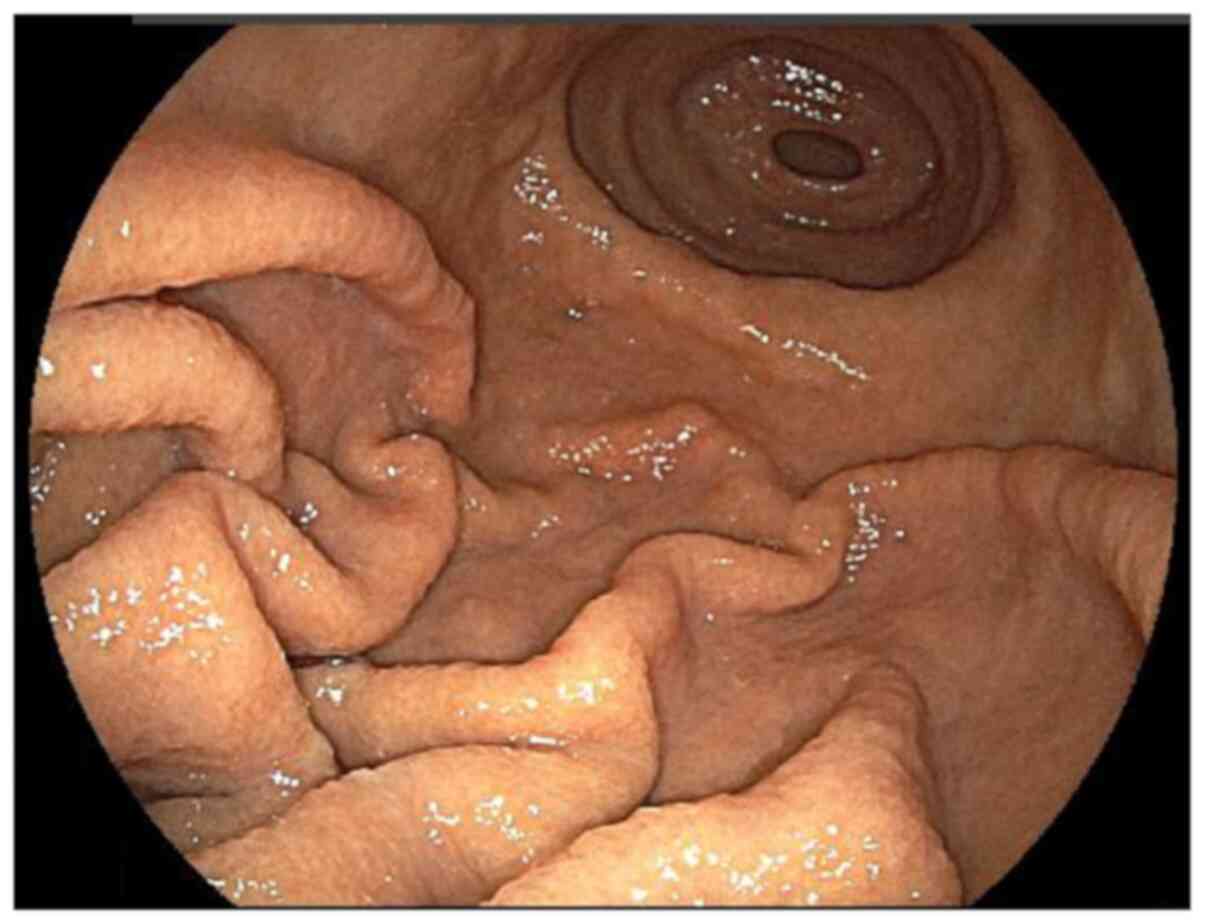
weight was 47.6 kg. Gastroscopy showed swelling, and thick gastric
folds were observed in the greater curvature of the body of the
stomach (Fig. 5).
 | Table ILaboratory data. |
Table I
Laboratory data.
| Data | Value |
|---|
| Blood counts | |
|
White blood
cell | 7,060 µl |
|
Red blood
cell |
3.53x106/µl |
|
Haemoglobin | 8.4 g/dl |
|
Haematocrit | 28.9% |
|
Platelet |
43.8x104 |
| Clotting
parameter | |
|
Prothrombin
time | 13.1 sec |
|
Activated
partial prothrombin time | 28 sec |
| Biochemical
parameters | |
|
Na+ | 139 mmol/l |
|
K+ | 4.4 mmol/l |
|
Cl- | 107 mmol/l |
|
Ca+ | 7.8 mg/dl |
|
Total
protein | 6.2 g/dl |
|
Albumin | 3.1 g/dl |
|
Total
bilirubin | 0.1 mg/dl |
|
Aspartate
aminotransferase | 32 U/I |
|
Alanine
aminotransferase | 25 U/I |
|
Alkaline
phosphatase | 567 U/I |
|
γ-glutamyltransferase | 10 U/I |
|
Amylase | 158 U/I |
|
Blood urea
nitrogen | 19.5 mg/dl |
|
Creatinine | 0.46 mg/dl |
|
C-reactive
protein | 0.15 mg/dl |
| Tumour marker | |
|
Carcinoembryonic
antigen | 2.1 ng/dl |
|
Carbohydrate
antigen 19-9 | <2.0 U/ml |
|
Carbohydrate
antigen 125 | 8.5 U/ml |
A diagnosis of gallbladder tumours was reached, but
it was not possible to rule out gallbladder cancer preoperatively.
Therefore, a cholecystectomy with resection of the gallbladder bed
by laparotomy was planned; thereafter, an intraoperative frozen
section for gallbladder tumours to judge whether to perform lymph
node dissection was planned.
During laparotomy, the tumour was located at the
fundus of the gallbladder on the free abdominal cavity side. The
serosa of the gallbladder was smooth. No hepatic invasion or
peritoneal dissemination was found. Cholecystectomy and resection
of the gallbladder bed were performed. Based on the assessment of
the intraoperative frozen section, the tumour was diagnosed as
adenocarcinoma in adenoma, which was categorized as carcinoma in
situ. Therefore, a lymph node dissection was not performed.
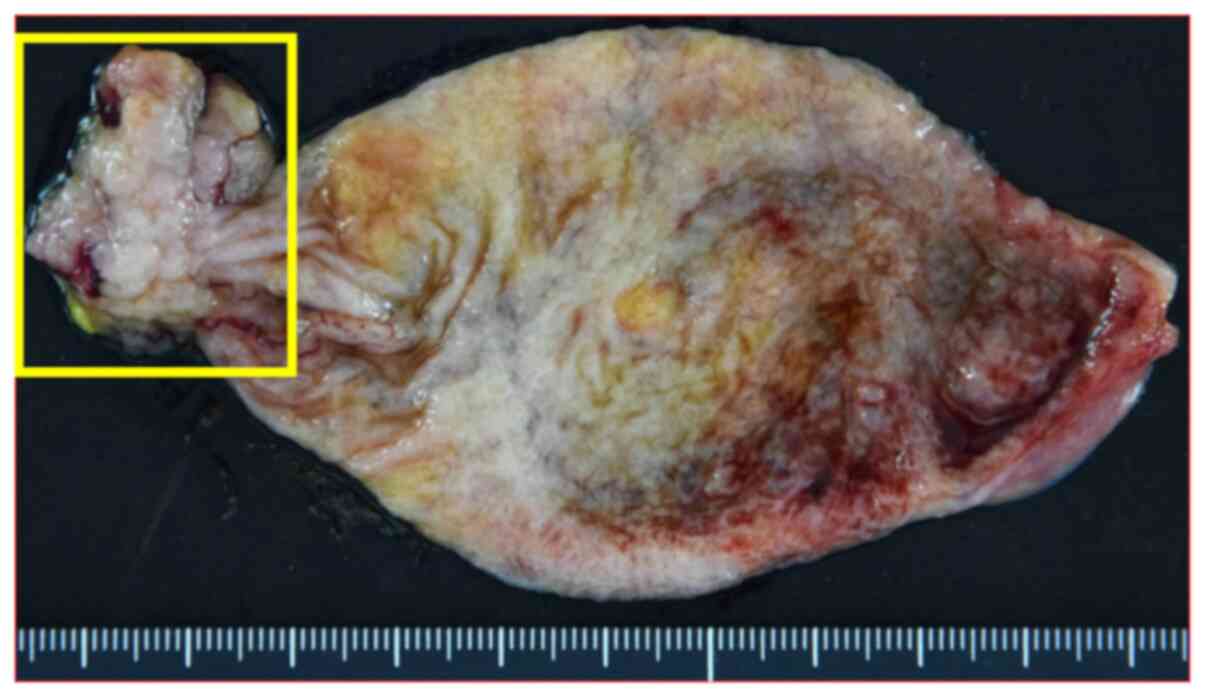
Macroscopic findings of the resected specimen are
shown in Fig. 6. The tumour,
visible as a small, fused grain that was 2.7x2.0 cm, was located at
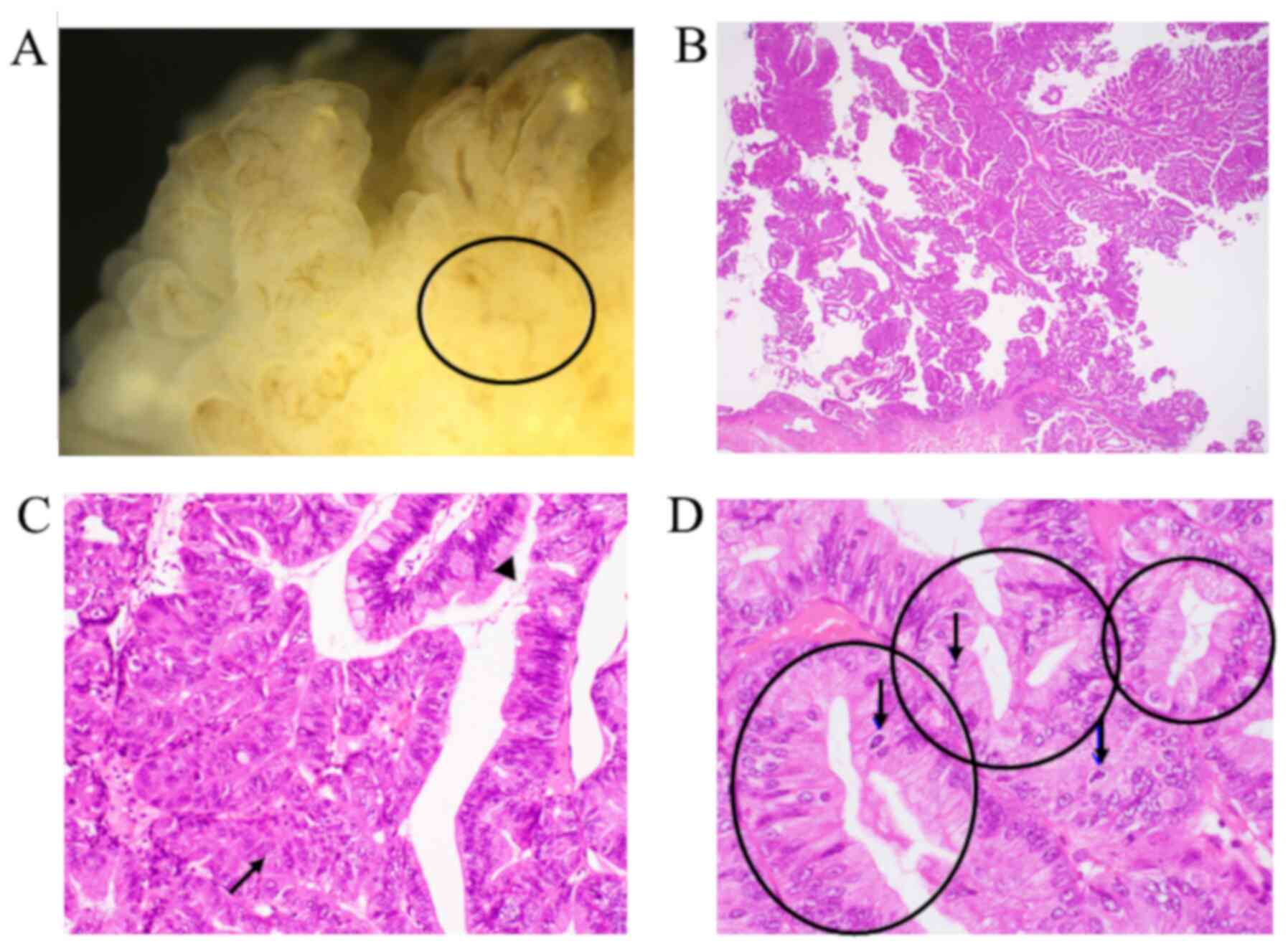
the fundus of the gallbladder. Microscopic examination revealed
that most of the tumour showed BilIN 2-3, which preserved polarity.
Based on these findings, it was diagnosed as carcinoma in situ
(Fig. 7). No vascular invasion or
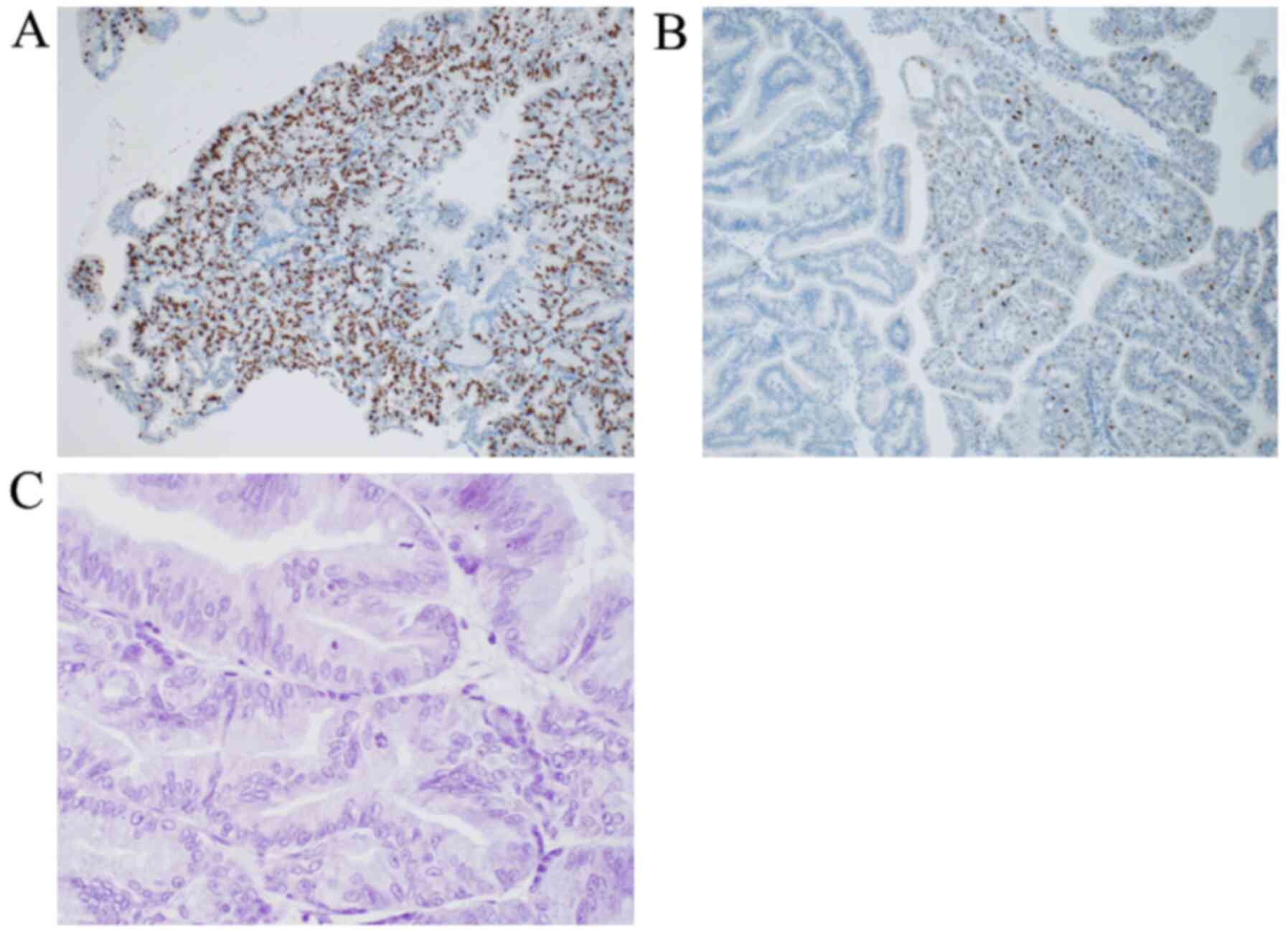
nerve invasion was found. Immunohistochemical staining yielded the
following results: p16 was 1%, S100P was negative, the E3
ubiquitin-protein ligase MIB1 index was 80% (Fig. 8A), the p53 index was 20% (Fig. 8B) and epidermal growth factor
receptor (EGFR) was negative (Fig.
8C). The tumour was classed as ICPN, and mild chronic
inflammation was found at the gallbladder.
Although the patient developed paralytic ileus, he
was discharged on postoperative day 14 because of improvement via
conservative treatment, and he has been followed up without tumour
recurrence for 12 months. However, there was no improvement or
worsening of PLG. Body weight was 48.0 kg. Although serum albumin
was 2.6 g/dl, he did not receive intravenous albumin after
surgery.
This case report was approved by the institutional
review board at the Hokkaido Social Work Association Obihiro
Hospital (2020-17). Informed consent was obtained from the patient
for the publication of his clinical data and images.
Discussion
IPNB in the bile duct epithelium is the counterpart
of intraductal papillary mucinous neoplasm in the pancreatic duct
epithelium (11,12). Similarly, the corresponding
gallbladder lesion is defined as ICPN (2). Detailed reports of ICPN are limited,
due to the relatively recent classification of this condition.
Argon et al (13) reported
that the frequency of ICPN was 45 out of 7,334 cholecystectomies,
or 0.6%. ICPN with invasive carcinoma components accounts for
approximately 55% of cases, and the proportion of cases of
cholelithiasis accompanying ICPNs has been found to be 20-22%
(3,13). In general, few cases of ICPN
diagnosed preoperatively, such as the present case, have been
reported (14). Previous case
reports used imaging modalities to show papillary or papillonodular
tumours within the gallbladder (14,15).
However, it is very difficult to diagnose ICPN with carcinoma
preoperatively.
Recently, Kang et al (16) compared ICPN to conventional
adenocarcinoma of the gallbladder. According to their report,
patients with ICPN seemed to exhibit a greater macro- and
microscopic curative resection rate, lower preoperative serum
carcinoembryonic antigen and carbohydrate antigen 19-9 levels,
improved differentiation grade, lower regional lymph node
metastasis rates and lower rates of distant metastases. In
addition, Adsay et al (3)
reported that ICPN tended to be detected at an earlier stage than
conventional gallbladder carcinoma, and they showed that the
proportion of patients with T1 ICPN and that of patients with
conventional gallbladder carcinoma were 32 and 9%, respectively. In
the present case, T0 stage and both carcinoembryonic antigen and
carbohydrate antigen 19-9 were within the normal ranges, with no
metastases. Although reports regarding the prognoses of ICPN are
limited, the 5-year OS rate of ICPN has been investigated and found
to be 63-73% (15,16). The proportion of patients without
invasive carcinoma is reported to be 71-78%, and 60-61% of such
patients have invasive carcinoma (3,13).
Conventional gallbladder cancer exhibits 5-year OS rates of 26-43%
(16,17). In a previous study, ICPN seemed to
yield a better prognosis than conventional gallbladder cancer, but
the prognoses and local and systemic recurrence rates were similar
after T-stage matching (16).
Therefore, patients with advanced ICPN should receive adjuvant
systemic chemotherapy. In this case, a cholecystectomy with
resection of the gallbladder bed was performed, and the tumour was
diagnosed as adenocarcinoma in adenoma, which was categorized as
carcinoma in situ based on intraoperative frozen sections.
Therefore, a lymph node dissection or perform adjuvant therapy were
not performed.
While Menetrier's disease is known as a rare disease
characterized by giant hypertrophy of the gastric folds that causes
PLG (5,6), to date, no definite diagnostic
criteria have been established. Although the exact mechanism of the
development of Menetrier's disease is not yet clear, it has been
hypothesized that this disease is associated with overexpression of
transforming growth factor-α (TGF-α) in the gastric mucosa,
resulting in gastric wall thickening and the suppression of gastric
acid (18,19). TGF-α combines with EGFR and plays a
role in cell proliferation. These mechanisms are similar to those
underlying the development of malignant tumours. TGF-α and EGFR
facilitate cell proliferation, invasion, metastases and
angiogenesis in malignant tumours (20,21).
The cause of activation or overexpression of TGF-α and EGFR in
Menetrier's disease is unclear; it has been proposed that H.
pylori in adults and cytomegalovirus in children are related to
this activation or overexpression (22,23).
While patients with Menetrier's disease are a population that are
at high risk for gastric malignancy (7), the possible correlation between
Menetrier's disease and a malignancy other than a malignancy of the
stomach is still unknown. Sato et al (24) reported a case of Menetrier's
disease seemingly caused by hilar cholangiocarcinoma (24). Analysis of their case showed that
H. pylori was negative, and PLG significantly improved after
resection of hilar cholangiocarcinoma. In addition, cancer cells
were positive for both TGF-α and EGFR. In the present case,
although H. pylori was negative, the cause of Menetrier's
disease was not considered due to ICPN, as the discovery of ICPN
was 2 years after the onset of Menetrier's disease. Additionally,
tumour cells were negative for EGFR, and the patient did not show
significant improvement in PLG after surgery. The present case may
represent a pure coincidence of Menetrier's disease and IPCN, both
of which are rare diseases. However, it has been reported that the
rate of EGFR expression is significantly lower in early cancer than
in advanced cancer (25). Because
the present case presented with carcinoma in situ, the stage may
have been too early for the expression of EGFR to be detectable.
Therefore, it was not possible to rule out a carcinogenesis related
to the Menetrier's disease. Data on additional cases of Menetrier's
disease combined with malignancy not involving the stomach are thus
required.
Regarding postsurgical complications, evidence is
lacking as to whether patients with Menetrier's disease have a
higher risk or incidence of postsurgical complications.
Furthermore, certain patients with PLG show postoperative
complications after surgery due to low protein levels and
nutritional status. In addition, certain patients with PLG present
ileus (26,27) or non-occlusive mesenteric ischemia
(28). The patient in the present
case showed ileus after cholecystectomy and improvement after
conservative treatment. Therefore, it may be necessary to monitor
for the onset of ileus after surgery in patients with PLG.
Limitations of the present study to be acknowledged
are: Protein content in the bile sample was not evaluated; this
should be taken into consideration to monitor protein loss.
Additionally, TGF-α staining of the resected specimen could not be
performed due to a lack of available facilities to perform such
experiments.
In conclusion, a case of resected ICPN complicated
with Menetrier's disease in a patient who showed PLG is described
in the present report. Such cases are extremely rare, however,
patients with Menetrier's disease may need to be screened for
malignancies.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
SS wrote the manuscript. SS, TH and KH performed the
surgical procedure. KK and HA managed the perioperative course. IM
and CM performed the pathological diagnosis. All authors have read
and approved the final version of the manuscript. SS, TH, and KH
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
This case report was approved by the institutional
review board at the Hokkaido Social Work Association Obihiro
Hospital (2020-17).
Patient consent for publication
Informed consent was obtained from the patient for
the publication of his clinical data and images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nakanuma Y, Curabo MP, Franceschi S, Gores
G, Paradis V, Sripa B, Tsui WMS and Wee A: Intrahepatic
cholangiocarcinoma. In: WHO Classification of Tumours of the
Digestive System. Bosman FT, Carneiro F, Hruban RH and Theise ND
(eds). 4th edition. IARC Press, Lyon, pp217-p224, 2010.
|
|
2
|
Albores-Saavedra J, Adsay NV, Crawford JM,
Klimstra DS, Klöppel G, Sripa B, Tsui WMS and Paradis V: Carcinoma
of the gallbladder and extrahepatic bile ducts. WHO Classification
of Tumours of the Digestive System: World Health Organization of
Tumours. Bosman FT, Carneiro F, Hruban RH and Theise ND (eds). 4th
edition. IARC Press, Lyon, pp266-p273, 2010.
|
|
3
|
Adsay V, Jang KT, Roa JC, Dursun N, Ohike
N, Bagci P, Basturk O, Bandyopadhyay S, Cheng JD, Sarmiento JM, et
al: Intracholecystic papillary-tubular neoplasms (ICPN) of the
gallbladder (neoplastic polyps, adenomas, and papillary neoplasms
that are ≥1.0 cm): Clinicopathologic and immunohistochemical
analysis of 123 cases. Am J Surg Pathol. 36:1279–1301.
2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Menetrier P: Des polyadenomes gastriques
et deleurs rapports avec le cancer de l'estomac. Arch Physiol Norm
Pathol. 1:32–55, 236-262. 1888.
|
|
5
|
Jarnum S and Jensen KB: Plasma protein
turnover (albumin, transferrin, IgG, IgM) in Ménétrier's disease
(giant hypertrophic gastritis): Evidence of non-selective protein
loss. Gut. 13:128–137. 1972.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wolfsen HC, Carpenter HA and Talley NJ:
Menetrier's disease: A form of hypertrophic gastropathy or
gastritis? Gastroenterology. 104:1310–1319. 1993.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Almazar AE, Penfield JD, Saito YA and
Talley NJ: Survival times of patients with Menetrier's disease and
risk of gastric cancer. Clin Gastroenterol Hepatol. 19:707–712.
2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kamal MU, Tariq H, Mehak V, Azam S, Kumar
K, Niazi M and Dev A: A rare etiology of abnormally large gastric
folds: Menetrier's disease. Case Rep Gastrointest Med.
2019(7927083)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pryczynicz A, Bandurski R,
Guzińska-Ustymowicz K, Niewiarowska K, Kemona A and Kędra B:
Ménétrier's disease, a premalignant condition, with coexisting
advanced gastric cancer: A case report and review of the
literature. Oncol Lett. 8:441–445. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kuo AH, Martinez N and Rosenkranz L: A
concurrent case of Menetrier's disease and signet ring carcinoma.
ACG Case Rep J. 3(e176)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zen Y, Sasaki M, Fujii T, Chen TC, Chen
MF, Yeh TS, Jan YY, Huang SF, Nimura Y and Nakanuma Y: Different
expression patterns of mucin core proteins and cytokeratins during
intrahepatic cholangiocarcinogenesis from biliary intraepithelial
neoplasia and intraductal papillary neoplasm of the bile duct - an
immunohistochemical study of 110 cases of hepatolithiasis. J
Hepatol. 44:350–358. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rocha FG, Lee H, Katabi N, DeMatteo RP,
Fong Y, D'Angelica MI, Allen PJ, Klimstra DS and Jarnagin WR:
Intraductal papillary neoplasm of the bile duct: A biliary
equivalent to intraductal papillary mucinous neoplasm of the
pancreas? Hepatology. 56:1352–1360. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Argon A, Barbet FY and Nart D: The
relationship between intracholecystic papillary-tubular neoplasms
and invasive carcinoma of the gallbladder. Int J Surg Pathol.
24:504–511. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hara A, Kamata K, Takenaka M, Chikugo T
and Kudo M: Intracystic papillary neoplasm preoperatively diagnosed
by high-quality cytology derived from endoscopic nasogallbladder
drainage. Gastrointest Endosc. 89:1257–1259. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Akita M, Fujikura K, Ajiki T, Fukumoto T,
Otani K, Hirose T, Tominaga M, Itoh T and Zen Y: Intracholecystic
papillary neoplasms are distinct from papillary gallbladder
cancers: A clinicopathologic and exome-sequencing study. Am J Surg
Pathol. 43:783–791. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kang JS, Lee KB, Choi YJ, Byun Y, Han Y,
Kim H, Kwon W and Jang JY: A comparison of outcomes in patients
with intracholecystic papillary neoplasms or conventional
adenocarcinomas of the gallbladder. Available from: https://doi.org/10.1016/j.hpb.2020.09.011.
|
|
17
|
Creasy JM, Goldman DA, Gonen M, Dudeja V,
O'Reilly EM, Abou-Alfa GK, Cercek A, Harding JJ, Balachandran VP,
Drebin JA, et al: Evolution of surgical management of gallbladder
carcinoma and impact on outcome: Results from two decades at a
single-institution. HPB (Oxford). 21:1541–1551. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Dempsey PJ, Goldenring JR, Soroka CJ,
Modlin IM, McClure RW, Lind CD, Ahlquist DA, Pittelkow MR, Lee DC,
Sandgren EP, et al: Possible role of transforming growth factor
alpha in the pathogenesis of Ménétrier's disease: Supportive
evidence form humans and transgenic mice. Gastroenterology.
103:1950–1963. 1992.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Nomura S, Settle SH, Leys CM, Means AL,
Peek RM Jr, Leach SD, Wright CV, Coffey RJ and Goldenring JR:
Evidence for repatterning of the gastric fundic epithelium
associated with Ménétrier's disease and TGFalpha overexpression.
Gastroenterology. 128:1292–1305. 2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kidd M, Schimmack S, Lawrence B, Alaimo D
and Modlin IM: EGFR/TGFalpha and TGFbeta/CTGF signaling in
neuroendocrine neoplasia: Theoretical therapeutic targets.
Neuroendocrinology. 97:35–44. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ciardiello F and Tortora G: Epidermal
growth factor receptor (EGFR) as a target in cancer therapy:
Understanding the role of receptor expression and other molecular
determinants that could influence the response to anti-EGFR drugs.
Eur J Cancer. 39:1348–1354. 2003.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Badov D, Lambert JR, Finlay M and Balazs
ND: Helicobacter pylori as a pathogenic factor in
Ménétrier's disease. Am J Gastroenterol. 93:1976–1979.
1998.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Megged O and Schlesinger Y:
Cytomegalovirus-associated protein-losing gastropathy in childhood.
Eur J Pediatr. 167:1217–1220. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sato N, Nakahara K, Morita R, Suetani K,
Michikawa Y, Nakano H, Koizumi S, Otsubo T, Fujino T and Itoh F: A
case of Ménétrier's disease seemingly caused by hilar
cholangiocarcinoma. Nihon Shokakibyo Gakkai Zasshi. 113:975–982.
2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Takemura K, Obara T, Okano S, Yokota K,
Ura H, Saitoh Y, Koike Y, Okamura K and Namiki M:
Immunohistochemical study on the expression of epidermal growth
factor receptor (EGFR) in human colorectal carcinomas. Nihon
Shokakibyo Gakkai Zasshi. 88:1177–1183. 1991.PubMed/NCBI
|
|
26
|
Schaad U, Zimmermann A, Gaze H, Kaiser G,
Vésy J and Hadorn B: Protein-losing enteropathy due to segmental
erosive and ulcerative intestinal disease cured by limited
resection of the bowel. Helv Paediatr Acta. 33:289–297.
1978.PubMed/NCBI
|
|
27
|
Lenzhofer R, Lindner M, Moser A, Berger J,
Schuschnigg C and Thurner J: Acute jejunal ileus in intestinal
lymphangiectasia. Clin Investig. 71:568–571. 1993.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Shima T, Ozeki M, Kinoshita T, Honda K,
Inoue H and Morita S: Protein-losing enteropathy secondary to
nonocclusive mesenteric ischemia: A case report. Medicine
(Baltimore). 97(e13403)2018.PubMed/NCBI View Article : Google Scholar
|