Introduction
Gastric cancer is a highly heterogeneous disease.
According to Laurén's classification, gastric cancer is categorized
as intestinal type and diffuse type (1). Intestinal tumor cells exhibit adhesion
and have tubular formation. On the other hand, diffuse type
exhibits single cells or poorly cohesive cells infiltrating the
gastric wall, such as signet ring cell carcinoma (Sig) and
non-solid poorly differentiated adenocarcinoma (Por2) according to
the Japanese Classification, and they are included in the poorly
cohesive carcinoma subtype in the World Health Organization (WHO)
classification of 2010 (2,3). Sig and Por2 tumors tend to infiltrate
diffusely and preferentially develop peritoneal metastases,
resulting poor clinical outcomes (4). Gastric carcinomas often consist of a
mixture of histological patterns (5). The percentage of mixed tissue is
reported to be 21% (5). In the
Japanese Classification of Gastric Cancer, the predominant
histologic type is used even if mixed with undifferentiated
components, so it may be defined as differentiated type (2). Sig and Por2 often coexist with other
histologies, but their clinical significance is unclear.
Furthermore, the clinical difference between Sig and Por2 in
advanced gastric cancer remains unclear.
Patients show various sensitivities to chemotherapy;
therefore, tailoring anti-cancer drugs on an individual basis for
the treatment of gastric cancer is important. S-1-based
chemotherapy is a standard postoperative adjuvant therapy for
patients with stage II or III gastric cancer in Asia (6). S-1, an oral fluoropyrimidine
derivative, is known to be a pivotal agent for the treatment of
patients with gastric cancer in Japan. The usefulness of S-1 alone
or S-1 combined with cisplatin or docetaxel has been reported for
peritoneal metastasis (7).
Currently, the management of patients with gastric cancer is
dependent on the clinical and pathological TNM stage. As a
consequence, treatment guidelines have not yet been tailored by
histology. Histological type could be a surrogate marker of disease
biology.
The aim of this study was to retrospectively
investigate the relationship of the presence of diffuse type in
primary tumor and clinicopathological background with prognosis,
including recurrence after postoperative adjuvant chemotherapy, in
patients with advanced stage II and III gastric cancer.
Patients and methods
Clinical data
A retrospective analysis of the gastric cancer
database of the Department of Surgical Oncology, Osaka City
University Graduate School of Medicine, was performed.
Clinicopathological data of 968 patients with gastric carcinoma who
underwent curative resection (i.e., R0 resection) without
preoperative chemotherapy between 2007 and 2016 were examined.
Patients with postoperative death within 30 days or incomplete
follow-up were excluded.
The histological type was determined basically
according to the 15th edition of the Japanese Gastric Cancer
classification. According to it, the tissue-type is decided based
on the quantitatively predominant tissue-type. In this study,
intestinal predominant type was defined as the histological type in
which papillary adenocarcinoma (pap), well-differentiated tubular
adenocarcinoma (tub1), moderately differentiated adenocarcinoma
(tub2), or solid-type poorly differentiated adenocarcinoma (por1)
was quantitatively dominant, and diffuse predominant type was
defined as the histological type in which Por2 and Sig were
dominant. Furthermore, diffuse mixed intestinal type was defined as
present when Por2 or Sig were mixed, even though the intestinal
type was dominant. In this study, the intestinal type refers to a
tumor that does not contain Sig or Por2 at all, and the diffuse
type refers to both diffuse predominant and diffuse mixed
intestinal types.
Adjuvant chemotherapy consisting of S-1 was
basically administered orally twice daily for the first 4 weeks of
a 6-week cycle. The dose of S-1 administered per day was based on
the patient's body surface area as follows: <1.25 m2,
80 mg; 1.25-1.50 m2, 100 mg; and >1.5 m2,
120 mg. Treatment of both groups was continued until one of the
following occurred: disease progression, administration difficulty
due to adverse effects, or decision to stop treatment at the
discretion of the treating physician.
Statistical analysis
Kruskal-Wallis test with Bonferroni post hoc test,
chi-square test and Fisher's exact test were used to assess the
associations between histological types and clinicopathological
features using SPSS ver.26 software (SPSS Japan). Overall survival
(OS) and disease-free survival (DFS) curves were drawn using the
Kaplan-Meier method. The day of surgery was used as the starting
point for the measurement of OS. The log-rank test and Renyi test
were used to assess the significance of differences in survival.
Prognostic factors were analyzed using the cox proportional hazards
model using the JMP software program (SAS Institute, Inc.). Renyi
test was performed using R version 3.6.3 (R Core Team, 2020).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Differences in background
characteristics between diffuse type and intestinal type
The cohort in this study consisted of 218 (22%)
diffuse predominant type and 750 (78%) intestinal predominant type
cases. Overall, 89 of 750 intestinal predominant type had mixed
diffuse type histology, which was defined as a diffuse mixed
intestinal type (Table I). Finally,
661 cases of intestinal type and 307 cases of diffuse type were
compared and examined (Table I).
Diffuse type was more common in young women and type 4 gastric
cancer than intestinal type, while early gastric cancer was
abundant in the diffuse type, and 64% had pathological stage I.
Patients with diffuse type had peritoneal recurrence more
frequently than patients with intestinal type, whereas intestinal
type had more hepatic recurrence caused by venous infiltration than
diffuse type. A similar tendency was recognized in the comparison
of diffuse mixed intestinal type and diffuse predominant type
(Table I). In other words, diffuse
mixed-intestinal type should be treated as diffuse type.
 | Table IDifference in clinicopathological
characteristics between diffuse type (n=307) and intestinal
predominant type (n=750). |
Table I
Difference in clinicopathological
characteristics between diffuse type (n=307) and intestinal
predominant type (n=750).
| Characteristics | Intestinal type
(n=661) | Diffuse mixed
intestinal type (n=89) | Diffuse predominant
type (n=218) | P-value (intestinal
vs. diffuse mixed and predominant) | P-value (Diffuse
mixed vs. predominant) |
|---|
| Median age,
years | 70 | 65 | 64 | 0.003a | 0.632a |
| Sex, n | | | | | |
|
Male | 175 | 57 | 121 |
<0.001b | 0.661b |
|
Female | 486 | 32 | 97 | | |
| Macroscopic type,
n | | | | | |
|
Type 0 | 357 | 48 | 112 |
<0.001c | 0.113c |
|
Type
1,2,3 | 296 | 38 | 87 | | |
|
Type 4 | 8 | 3 | 19 | | |
| Surgery, n | | | | | |
|
Distal
gastrectomy | 471 | 70 | 158 | 0.210c | 0.397c |
|
Proximal
gastrectomy | 10 | 0 | 1 | | |
|
Total
gastrectomy | 180 | 19 | 59 | | |
| pT, n | | | | | |
|
1/2 | 476 | 54 | 107 |
<0.001b | 0.049b |
|
3/4 | 185 | 35 | 111 | | |
| pN, n | | | | | |
|
0/1 | 532 | 64 | 167 | 0.416b | 0.308b |
|
2/3 | 129 | 25 | 51 | | |
| pStage, n | | | | | |
|
I | 419 | 46 | 115 | 0.019b | 0.939b |
|
II/III | 242 | 43 | 103 | | |
| Lymphatic invasion,
n | | | | | |
|
Negative | 368 | 51 | 118 | 0.503b | 0.711b |
|
Positive | 293 | 38 | 100 | | |
| Venous invasion,
n | | | | | |
|
Negative | 546 | 73 | 201 | 0.006b | 0.017b |
|
Positive | 115 | 16 | 17 | | |
| Recurrence, n | | | | | |
|
Peritoneum | 30 | 11 | 38 | 0.001c | 0.177c |
|
Liver | 32 | 2 | 2 | 0.006c | 0.330c |
|
Lymph
nodes | 30 | 3 | 9 | 0.519c | 0.523c |
|
Other | 18 | 3 | 7 | 0.449c | 0.593c |
Clinical relevance of Sig compared to
Por2
Table II shows a
comparison of the background characteristics between Sig and Por2
in the diffuse type. Of the 307 patients with diffuse type, Sig was
present in 189, Por2 in 177, and both Sig and Por2 in 59. Patients
with Sig predominance without Por2 had more early cancers, less
lymphatic invasion, and less recurrence, whereas Por2 cases had
more cases of pT3 or more, positive for lymph node metastasis, and
pathological stage II/III than Sig cases. The background of the
cases including both of Sig and Por2 was similar to that of Por2
cases, and peritoneal recurrence occurred at a high rate.
 | Table IIComparison of patient background
characteristics between Sig and Por2 groups. |
Table II
Comparison of patient background
characteristics between Sig and Por2 groups.
|
Characteristics | Sig (n=130) | Por2 (n=118) | P-value (Sig vs.
Por2) | Sig with Por2
(n=59) | P-value (Sig vs.
Sig with Por2) | P-value (Por2 vs.
Sig with Por2) |
|---|
| Mean age ± SD,
years | 62±12 | 63±12 | 0.520a | 64±13 | 0.173a | 0.561a |
| Sex, n | | | | | | |
|
Male | 67 | 77 | 0.169b | 34 | 0.529b | 0.328b |
|
Female | 63 | 41 | | 25 | | |
| Macroscopic type,
n | | | | | | |
|
Type 0 | 93 | 43 |
<0.001b | 24 |
<0.001b | 0.574b |
|
Type
1-3 | 35 | 61 | | 27 | | |
|
Type 4 | 2 | 14 | | 8 | | |
| pT, n | | | | | | |
|
1/2 | 108 | 47 |
<0.001b | 26 |
<0.001b | 0.669b |
|
3/4 | 22 | 71 | | 33 | | |
| pN, n | | | | | | |
|
0/1 | 116 | 77 | 0.002b | 38 |
<0.001b | 0.751b |
|
2/3 | 14 | 41 | | 21 | | |
| pStage, n | | | | | | |
|
I | 101 | 39 |
<0.001b | 21 |
<0.001b | 0.831b |
|
II/III | 29 | 79 | | 38 | | |
| Lymphatic invasion,
n | | | | | | |
|
Negative | 96 | 48 |
<0.001b | 25 |
<0.001b | 0.932b |
|
Positive | 34 | 70 | | 34 | | |
| Venous invasion,
n | | | | | | |
|
Negative | 122 | 98 | 0.041b | 54 | 0.749b | 0.409b |
|
Positive | 8 | 20 | | 5 | | |
| Recurrence, n | | | | | | |
|
Peritoneum | 12 | 20 | 0.412c | 17 | 0.001c | 0.079c |
|
Liver | 0 | 3 | 0.160c | 1 | 0.312c | 1.000c |
|
Lymph
nodes | 3 | 7 | 0.224c | 2 | 0.648c | 0.720c |
|
other | 2 | 7 | 0.049c | 1 | 1.000c | 0.272c |
Of the 189 cases of Sig, 79 were cases in which Sig
was histologically dominant, and 110 cases in which it was
histologically non-predominant (Table
III). A total of 139 (78%) had histological predominance of
Por2. There was no clinically significant difference between
Por2-dominant and Por2-non predominant type. Sig-dominant tumors
were more frequent in early-stage cancers, and peritoneal
dissemination was more frequent in patients with Sig-non
predominant cancers.
 | Table IIIImpact of histological occupancy in
patients with diffuse type. |
Table III
Impact of histological occupancy in
patients with diffuse type.
|
Characteristics | Sig predominant
(n=79) | Sig non-predominant
(n=110) | P-value | Por2 predominant
(n=139) | Por2
non-predominant (n=38) | P-value |
|---|
| Mean age ± SD,
years | 60±13 | 64±12 | 0.138a | 63±13 | 66±11 | 0.891a |
| Sex, n | | | | | | |
|
Male | 35 | 66 | 0.039b | 86 | 25 | 0.709b |
|
Female | 44 | 44 | | 53 | 13 | |
| Macroscopic type,
n | | | | | | |
|
Type 0 | 58 | 59 | 0.077b | 54 | 13 | 0.700b |
|
Type
1-3 | 19 | 43 | | 68 | 22 | |
|
Type 4 | 2 | 10 | | 17 | 3 | |
| pT, n | | | | | | |
|
1/2 | 67 | 67 | 0.001b | 60 | 13 | 0.091b |
|
3/4 | 12 | 43 | | 79 | 25 | |
| pN, n | | | | | | |
|
0/1 | 72 | 82 | 0.002b | 95 | 20 | 0.131b |
|
2/3 | 7 | 28 | | 44 | 18 | |
| pStage, n | | | | | | |
|
I | 64 | 58 | 0.001b | 51 | 9 | 0.310b |
|
II/III | 15 | 52 | | 88 | 29 | |
| Lymphatic invasion,
n | | | | | | |
|
Negative | 59 | 62 | 0.018b | 59 | 14 | 0.461b |
|
Positive | 20 | 48 | | 80 | 24 | |
| Venous invasion,
n | | | | | | |
|
Negative | 74 | 102 | 0.841b | 127 | 25 | 0.001b |
|
Positive | 5 | 8 | | 12 | 13 | |
| Recurrence, n | | | | | | |
|
Peritoneum | 6 | 23 | 0.014c | 32 | 5 | 0.260c |
|
Liver | 0 | 1 | 1.000c | 2 | 2 | 0.202c |
|
Lymph
nodes | 2 | 3 | 1.000c | 7 | 2 | 1.000c |
|
Other | 1 | 2 | 1.000c | 6 | 2 | 0.681c |
Peritoneal metastasis has a large effect on the
prognosis, which accounts for most recurrences in the diffuse type.
Therefore, factors associated with peritoneal recurrence were
investigated using a Cox proportional hazards model (Table IV). On multivariate analysis using
factors found to be related on univariate analysis, total
gastrectomy and coexistence of Sig were independent risk factors
associated with peritoneal metastases after curative surgery.
 | Table IVRisk factors for peritoneal
recurrence. |
Table IV
Risk factors for peritoneal
recurrence.
| | Univariate
analysis | Multivariate
analysis |
|---|
|
Characteristics | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age | 0.61
(0.07-4.27) | 0.6312 | | |
| Macroscopic
type | | | | |
|
Type 4 vs.
type 0 | 5.60
(1.24-30.78) | 0.025 | 2.19
(0.23-50.1) | 0.710 |
| Surgery | | | | |
|
Total
gastrectomy vs. distal gastrectomy | 4.18
(1.95-9.60) | 0.0002 | 3.70
(1.59-9.16) | 0.002 |
| Histology | | | | |
|
Por2 vs.
non-Por2 | 3.27
(1.58-6.92) | 0.0015 | 2.23
(0.90-5.58) | 0.081 |
|
Sig vs.
non-Sig | 4.23
(1.92-9.26) | 0.0004 | 3.34
(1.37-8.16) | 0.009 |
| pT category | | | | |
|
pT4a vs.
pT3 | 2.39
(1.10-5.57) | 0.0272 | 1.61
(0.63-4.27) | 0.135 |
| pN category | | | | |
|
pN3 vs.
pN1 | 2.65
(0.92-8.82) | 0.0713 | | |
| Lymphatic
invasion | | | | |
|
Positive vs.
negative | 1.38
(0.47-4.60) | 0.569 | | |
| Venous
invasion | | | | |
|
Positive vs.
negative | 0.39
(0.06-2.85) | 0.728 | | |
Impact of Sig on OS and relapse-free
survival after S-1 adjuvant chemotherapy
The median length of follow-up was 36 months.
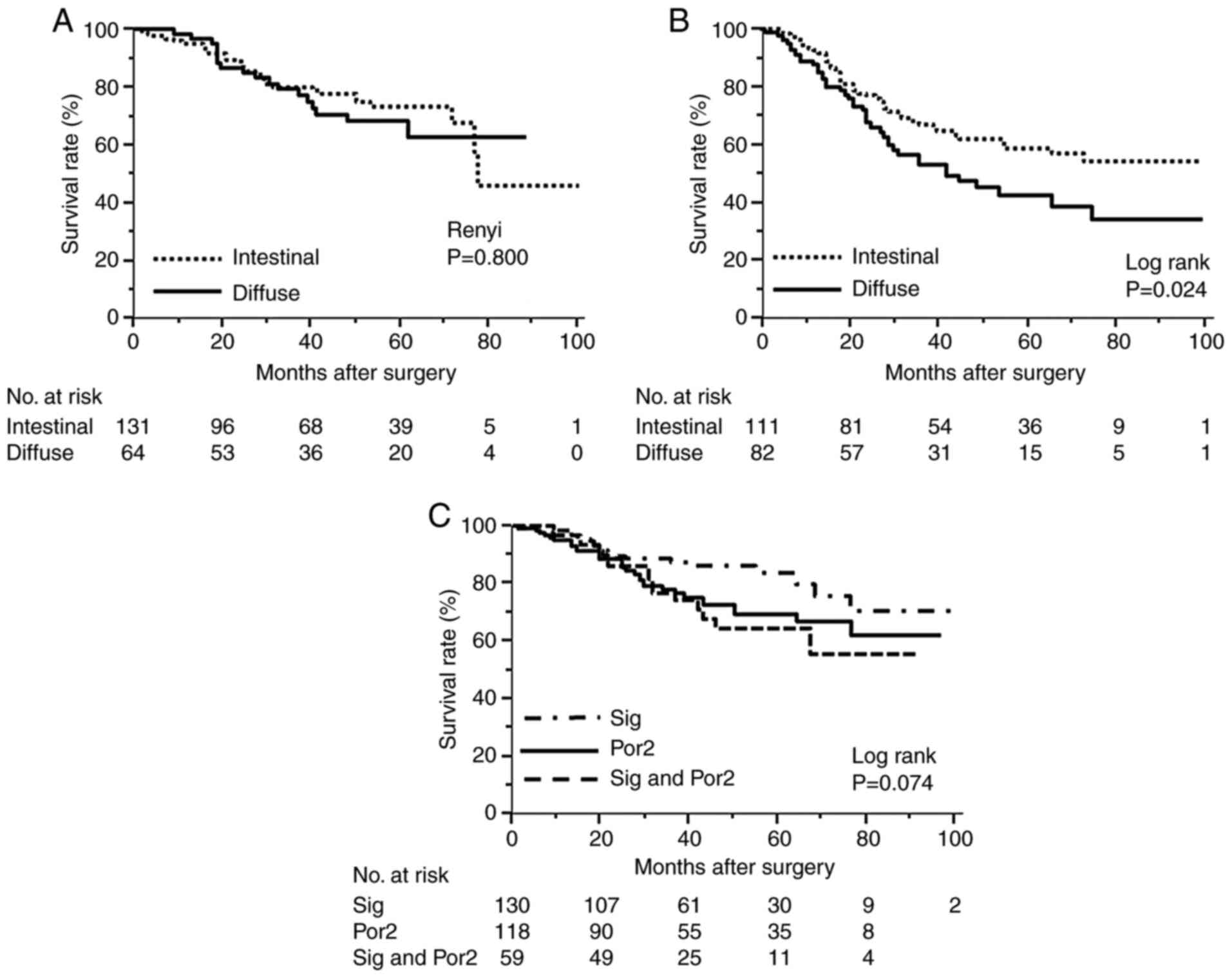
Kaplan-Meier curves according to pathological stage are shown in
Fig. 1. OS analysis by pathological
stage showed that there was no difference between diffuse type and
intestinal type in stage II (Fig.
1A). However, patients with diffuse type had a significantly
worse prognosis in pathological stage III (5-year OS 42%) compared
to intestinal type (5-year OS 59%) (Fig. 1B). Recurrence for peritoneal
dissemination was seen in 14 (24%) of 24 mixed Sig patients and 16
(36%) of 45 mixed Por2 patients. The difference in prognosis
between patients with Sig and Por2 and those with both is shown in
Fig. 1C. 5-year survival for
patients with Sig alone was slightly better (79%), but not
statistically significant.
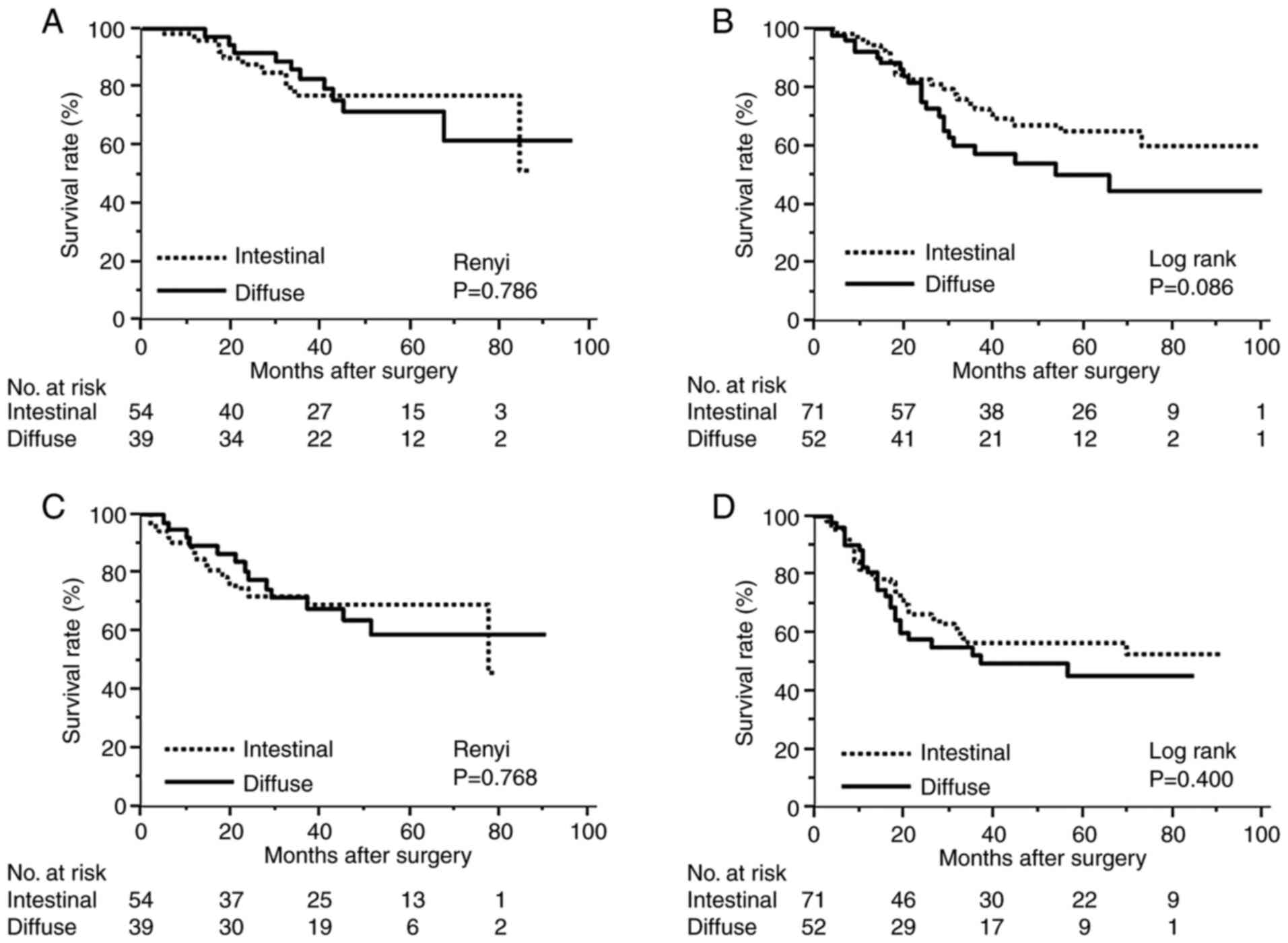
Of the cases with pathological stage II or III, the
proportion of patients who received postoperative adjuvant
chemotherapy was 61% in Sig, 67% in Por2, and 49% in intestinal
type. Although there was no significant difference among all
patients who received adjuvant chemotherapy with S-1, the 5-year
survival rate was marginally lower for the diffuse type than for
the intestinal type in stage III (Fig.
2A and B). Regarding
recurrence-free survival (RFS), there were few survival differences
(Fig. 2C and D).
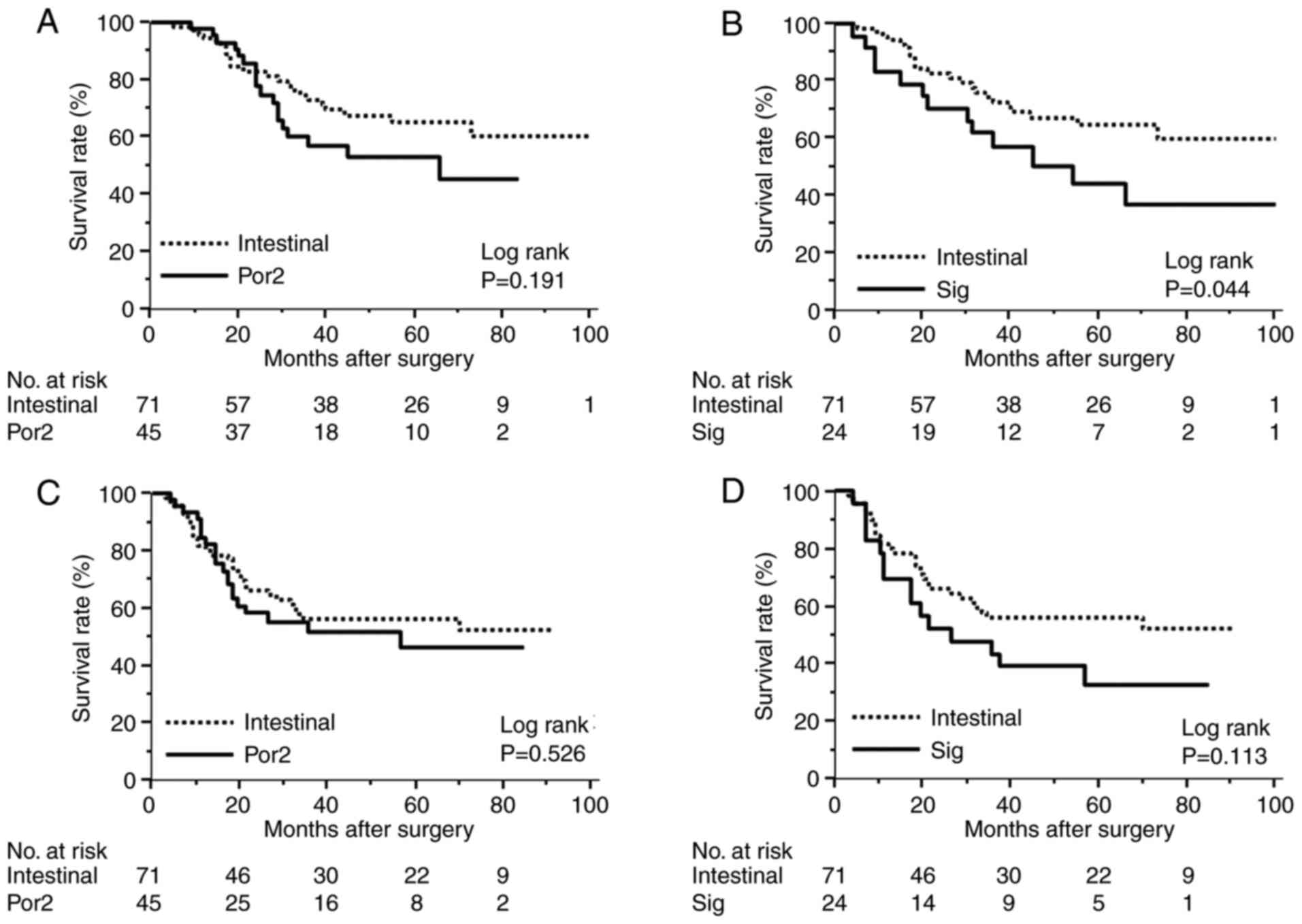
Comparing Por2 and Sig in pathological stage III,
the prognosis of patients with Sig was significantly worse than
that of patients with intestinal type (Fig. 3A and B). There was little effect on RFS in
patients with Por2, but patients with Sig had significantly worse
RFS (Fig. 3C and D). In other words, patients with Sig are
more likely to experience an early relapse while taking S-1.
Discussion
In this study, the prognosis of patients with
diffuse mixed type gastric cancer was worse than that of patients
with intestinal type in pathological stage III. Moreover, in stage
III patients who received postoperative adjuvant chemotherapy with
S-1, patients with Sig had a significantly worse prognosis than
patients with non-solid type poorly gastric carcinoma.
The diffuse type was more common in women and
younger patients, and it was associated with type 4 cancer and
peritoneal metastases as the site of initial recurrence after
surgery. A meta-analysis of 73 studies showed that patients with
diffuse type had the worst prognosis (8). They found that the risk of death was
increased by 23% regardless of race, stage, and chemotherapy.
Microsatellite instability (MSI) of four genomic subtypes
classified by the TCGA study of gastric cancer was mainly present
in intestinal distal cancer, whereas chromosomal instability was
seen in diffuse type cancers (9).
In the molecular classification of the Asian Cancer Research Group
(ACRG), diffuse type corresponds to microsatellite stable and the
epithelial-to-mesenchymal transition (MSS/EMT) phenotype (10). The MSS/EMT was often observed in
stage III/IV advanced gastric cancer and had the worst prognosis
due to frequent peritoneal metastases. The EMT was also observed in
younger patients and corresponded to Laurén's diffuse type
(11). Thus, diffuse gastric cancer
cells appear to possess the capacity for epithelial-mesenchymal
transition, which promotes peritoneal metastasis (12). Mixed type was seen in 15% of
patients, and they showed a metastatic, as well as a prognostic,
pattern similar to predominant Sig and Por2 tumors. Chen et
al examined 3071 patients with gastric cancer and divided them
into three groups according to the Lauren classification:
intestinal type 46%, diffuse type 32%, and mixed type 21%. They
demonstrated that the clinical appearance and outcome of mixed type
in the Lauren classification were similar to those of diffuse type
gastric cancer (5).
In the diffuse type, the two histotypes of Sig and
Por2 differ in their clinical and molecular features to the point
of representing distinct entities (13). Poorly differentiated carcinoma cells
have the potential to convert into the EMT phenotype. On the other
hand, Sig is also common in early-stage cancers, and the overall
prognostic impact of the presence or absence of Sig is equivocal
(14-16).
Pure Sig is usually present in the intramucosal layer, whereas its
morphology is often lost during tumor growth and transformation
into poorly cohesive carcinoma (17). Sig can easily transform into poorly
cohesive carcinoma in invasive areas and is most frequent in
advanced gastric cancer (18).
Piessen et al demonstrated that Sig often developed
peritoneal metastasis and lymph node invasion and would often fail
R0 resection, and Sig was associated with a worse prognosis than
non-Sig in a group matched-controlled study (19). Possible reasons for a poor prognosis
are unsuspected peritoneal carcinomatosis and lymph node
involvement, which are frequent. We previously reported that Signet
ring cells themselves have the capacity to produce immune
suppressive enzymes, which increased metastasis (20). Therefore, Sig in advanced gastric
cancer is associated with a poorer prognosis than the poorly
differentiated type.
The predictive effect of each histological subtype
on the efficacy of chemotherapy has not been definitively
elucidated. A decrease in the objective response rate was found in
the presence of a diffuse component of advanced gastric cancer. The
Laurén diffuse type of gastric cancer is frequently highly
infiltrative and resistant to chemotherapy (21). Yoon et al demonstrated that
RhoA activity plays a critical role in maintaining cancer stem cell
phenotype, and direct RhoA inhibition was effective with
chemotherapy (22). The survival
rate was better in intestinal type than in diffuse type with
regimens containing docetaxel. Subgroup analysis of JCOG9912
indicated that S-1 was more effective than 5-FU alone in the
treatment of diffuse type (23).
S-1 combined with docetaxel therapy was superior to S-1 monotherapy
in patients with diffuse type in the START trial (24). The outcomes in the present study
suggest that more intensive adjuvant chemotherapy is required for
stage III diffuse type. In addition, in the examination according
to histological type, the prognosis was poor in cases in which Sig
was histologically mixed. In other words, considering the
above-mentioned tendency of Sig to cause peritoneal dissemination,
it appears that stronger adjuvant chemotherapy is necessary for
cases with Sig in Stage III. It has recently been reported that S-1
plus docetaxel improved efficacy in patients with stage III gastric
cancer (7). If effective
intraperitoneal treatment is developed, it should be directed at
patients with a high risk of peritoneal recurrence. More effective
adjuvant therapy is needed, perhaps with immunotherapy or new
target agents.
The limitations of this study are that it was a
retrospective study in a single institution. However, the subjects
of this study were accumulated over a period of approximately 12
years, indicating an adequate investigation. Furthermore, the
impact of histology on second-line chemotherapy, which could affect
OS, was not examined.
In conclusion, Sig, when observed in advanced cancer
tissue, is associated with a high rate of peritoneal recurrence
even after radical resection. The present results suggest that
early recurrence during postoperative adjuvant chemotherapy should
be considered in patients with stage III gastric cancer showing
histological coexistence of Sig.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed in the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HT acquired, analyzed and interpreted the data,
confirmed the authenticity of the data and drafted the manuscript.
MY made substantial contributions to the conception and design of
the study, interpreted the data, confirmed the authenticity of the
data and revised the manuscript critically. TI reviewed and oversaw
the data on the statistics section of the Renyi test. TTa, TTo and
KM acquired and analyzed the data. KH and MO contributed to the
conception and design of the study, and critically revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This investigation was carried out according to the
Declaration of Helsinki. All experimental procedures after 2013
were approved by the Osaka City University ethics committee
(approval no. 4423), and all patients provided written informed
consent for collection and analysis of the specimens. On the other
hand, because the other experimental procedures up to 2013 were
performed with only all-inclusive consent, it was considered an
observational study with an opt-out form.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lauren P: The two histological main types
of gastric carcinoma: Diffuse and so-called intestinal-type
carcinoma. An attempt at a histo-clinical classification. Acta
Pathol Microbiol Scand. 64:31–49. 1965.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Japanese Gastric Cancer Association.
Japanese classification of gastric carcinoma: 3rd English edition.
Gastric Cancer. 14:101–112. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Nagtegaal ID, Odze RD, Klimstra D, Paradis
V, Rugge M, Schirmacher P, Washington KM, Carneiro F and Cree IA:
WHO Classification of Tumours Editorial Board. The 2019 WHO
classification of tumours of the digestive system. Histopathology.
76:182–188. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lee JH, Chang KK, Yoon C, Tang LH, Strong
VE and Yoon SS: Lauren histologic type is the most important factor
associated with pattern of recurrence following resection of
gastric adenocarcinoma. Ann Surg. 267:105–113. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chen YC, Fang WL, Wang RF, Liu CA, Yang
MH, Lo SS, Wu CW, Li AFY, Shyr YM and Huang KH: Clinicopathological
variation of lauren classification in gastric cancer. Pathol Oncol
Res. 22:197–202. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sasako M, Sakuramoto S, Katai H, Kinoshita
T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T and
Ohashi Y: Five-year outcomes of a randomized phase III trial
comparing adjuvant chemotherapy with S-1 versus surgery alone in
stage II or III gastric cancer. J Clin Oncol. 29:4387–4393.
2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yoshida K, Kodera Y, Kochi M, Ichikawa W,
Kakeji Y, Sano T, Nagao N, Takahashi M, Takagane A, Watanabe T, et
al: Addition of docetaxel to oral fluoropyrimidine improves
efficacy in patients with stage III gastric cancer: Interim
analysis of JACCRO GC-07, a randomized controlled trial. J Clin
Oncol. 37:1296–1304. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Petrelli F, Berenato R, Turati L, Mennitto
A, Steccanella F, Caporale M, Dallera P, de Braud F, Pezzica E, Di
Bartolomeo M, et al: Prognostic value of diffuse versus intestinal
histotype in patients with gastric cancer: A systematic review and
meta-analysis. J Gastrointest Oncol. 8:148–163. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cancer Genome Atlas Research Network.
Comprehensive molecular characterization of gastric adenocarcinoma.
Nature. 513:202–209. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cristescu R, Lee J, Nebozhyn M, Kim KM,
Ting JC, Wong SS, Liu J, Yue YG, Wang J, Yu K, et al: Molecular
analysis of gastric cancer identifies subtypes associated with
distinct clinical outcomes. Nat Med. 21:449–456. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Cislo M, Filip AA, Arnold Offerhaus GJ,
Ciseł B, Rawicz-Pruszyński K, Skierucha M and Polkowski WP:
Distinct molecular subtypes of gastric cancer: From Lauren to
molecular pathology. Oncotarget. 9:19427–19442. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zheng HC, Li XH, Hara T, Masuda S, Yang
XH, Guan YF and Takano Y: Mixed-type gastric carcinomas exhibit
more aggressive features and indicate the histogenesis of
carcinomas. Virchows Arch. 452:525–534. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Fujimoto A, Ishikawa Y, Ishii T, Yamada A,
Igarashi Y, Ohmoto Y and Kaise M: Differences between gastric
signet-ring cell carcinoma and poorly differentiated
adenocarcinoma: A comparison of histopathologic features determined
by mucin core protein and trefoil factor family peptide
immunohistochemistry. Pathol Int. 67:398–403. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pernot S, Voron T, Perkins G,
Lagorce-Pages C, Berger A and Taieb J: Signet-ring cell carcinoma
of the stomach: Impact on prognosis and specific therapeutic
challenge. World J Gastroenterol. 21:11428–11438. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li C, Kim S, Lai JF, Hyung WJ, Choi WH,
Choi SH and Noh SH: Advanced gastric carcinoma with signet ring
cell histology. Oncology. 72:64–68. 2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chon HJ, Hyung WJ, Kim C, Park S, Kim JH,
Park CH, Ahn JB, Kim H, Chung HC, Rha SY, et al: Differential
prognostic implications of gastric signet ring cell carcinoma:
stage adjusted analysis from a single high-volume center in Asia.
Ann Surg. 265:946–953. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lee SH, Jee SR, Kim JH and Seol SY:
Intramucosal gastric cancer: The rate of lymph node metastasis in
signet ring cell carcinoma is as low as that in well-differentiated
adenocarcinoma. Eur J Gastroenterol Hepatol. 27:170–174.
2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Machlowska J, Puculek M, Sitarz M,
Terlecki P, Maciejewski R and Sitarz R: State of the art for
gastric signet ring cell carcinoma: From classification, prognosis,
and genomic characteristics to specified treatments. Cancer Manag
Res. 11:2151–2161. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Piessen G, Messager M, Leteurtre E,
Jean-Pierre T and Mariette C: Signet ring cell histology is an
independent predictor of poor prognosis in gastric adenocarcinoma
regardless of tumoral clinical presentation. Ann Surg. 250:878–887.
2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yoshii M, Tanaka H, Ohira M, Muguruma K,
Iwauchi T, Lee T, Sakurai K, Kubo N, Yashiro M, Sawada T and
Hirakawa K: Expression of Forkhead box P3 in tumour cells causes
immunoregulatory function of signet ring cell carcinoma of the
stomach. Br J Cancer. 106:1668–1674. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jimenez Fonseca P, Carmona-Bayonas A,
Hernandez R, Custodio A, Cano JM, Lacalle A, Echavarria I, Macias
I, Mangas M, Visa L, et al: Lauren subtypes of advanced gastric
cancer influence survival and response to chemotherapy: Real-world
data from the AGAMENON national cancer registry. Br J Cancer.
117:775–782. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yoon C, Cho SJ, Aksoy BA, Park DJ, Schultz
N, Ryeom SW and Yoon SS: Chemotherapy resistance in diffuse-type
gastric adenocarcinoma is mediated by RhoA activation in cancer
stem-like cells. Clin Cancer Res. 22:971–983. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Takahari D, Boku N, Mizusawa J, Takashima
A, Yamada Y, Yoshino T, Yamazaki K, Koizumi W, Fukase K, Yamaguchi
K, et al: Determination of prognostic factors in Japanese patients
with advanced gastric cancer using the data from a randomized
controlled trial, Japan clinical oncology group 9912. Oncologist.
19:358–366. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Koizumi W, Kim YH, Fujii M, Kim HK,
Imamura H, Lee KH, Hara T, Chung HC, Satoh T, Cho JY, et al:
Addition of docetaxel to S-1 without platinum prolongs survival of
patients with advanced gastric cancer: A randomized study (START).
J Cancer Res Clin Oncol. 140:319–328. 2014.PubMed/NCBI View Article : Google Scholar
|