Introduction
Low-grade endometrial stromal sarcoma (LG-ESS) is a
rare tumor accounting for less than 1% of uterine malignancies
(1,2). Total hysterectomy and bilateral
adnexectomy with/without lymphadenectomy are recommended for
treatment of LG-ESS, and hormone therapy is also recommended as a
treatment option for patients with advanced or recurrent disease
(3). Patients with stage I-II
disease have a relatively favorable prognosis (2,3).
This tumor is most often seen in women in their 40 to 50s, but
fertility-sparing surgery and ovarian preservation may be
considered in younger patients. However, reports on
fertility-sparing treatment for LG-ESS are very limited and no such
treatment has been established. Herein, we report a case of LG-ESS
in a woman in her 20s who was diagnosed with the disease after
endometrial polyp-like lesions were removed. After tumor resection,
she underwent total hysterectomy and ovarian preservation, followed
by high-dose progesterone therapy. No recurrence has been detected
to date. We also reviewed some important reports on
fertility-sparing management of LG-ESS in the literature.
Case report
The patient was a 26-year-old, unmarried,
nulligravid woman with no particular medical or family history. She
visited a local hospital with a chief complaint of atypical genital
bleeding, and was referred to our hospital after an intrauterine
mass measuring 2 cm was pointed out. At the time of the first visit
to our department, the abdomen was soft, and speculum examination
showed no clear abnormalities. The uterus was the size of a hen egg
on pelvic examination, and the bilateral adnexa were not palpated.
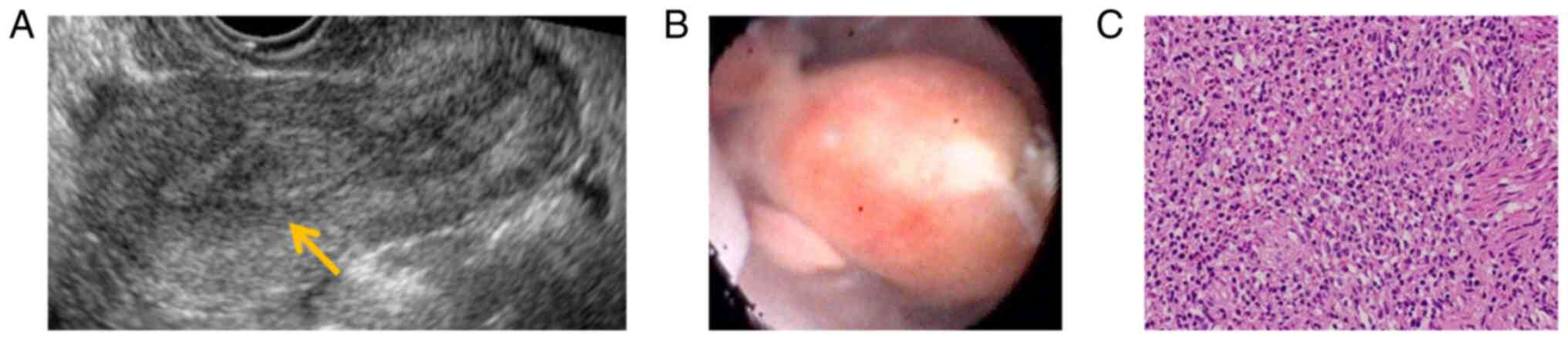
The endometrium was as thickened as 15 mm on transvaginal
ultrasonography, but there were no abnormal findings in the
myometrium (Fig. 1A). Hysteroscopy
revealed a 1.5-cm polyp-like mass in the intrauterine cavity.
Endometrial cytology test was negative. A blood test showed anemia
with a hemoglobin level of 9.6 g/dl, but the tumor markers were
within the normal ranges [CA125: 16.9 U/ml (reference value: ≤31.4
U/ml); CA19-9: 7.3 U/ml (reference value: ≤37 U/ml)]. Based on
these findings, endometrial polyps were suspected, and
hysteroscopic polypectomy was performed. Pathological examination
of the resected tissue was compatible with endometrial polyps with
hemorrhagic granulation tissue-like tissue. It was decided that the
patient should be followed up because no malignant findings were
observed. Two months after hysteroscopic surgery, however, she was
referred again by the local hospital with chief complaints of
atypical genital bleeding and recurrence of intrauterine lesions,
and hysteroscopy revealed a new smooth polyp-like mass 1 cm in
diameter in the intrauterine cavity (Fig. 1B). Because recurrence of
endometrial polyps was suspected, hysteroscopic polypectomy was
performed once again. Pathological examination of the resected
tissue showed overgrowth of endometrial stromal cells, and
neoplastic changes were not ruled out. Accordingly, LG-ESS was
included in the differential diagnosis, although clear atypical
cells were not observed (Fig. 1C).
The patient was closely examined for tumor invasion and distant
metastasis because the possibility of a malignant tumor was not
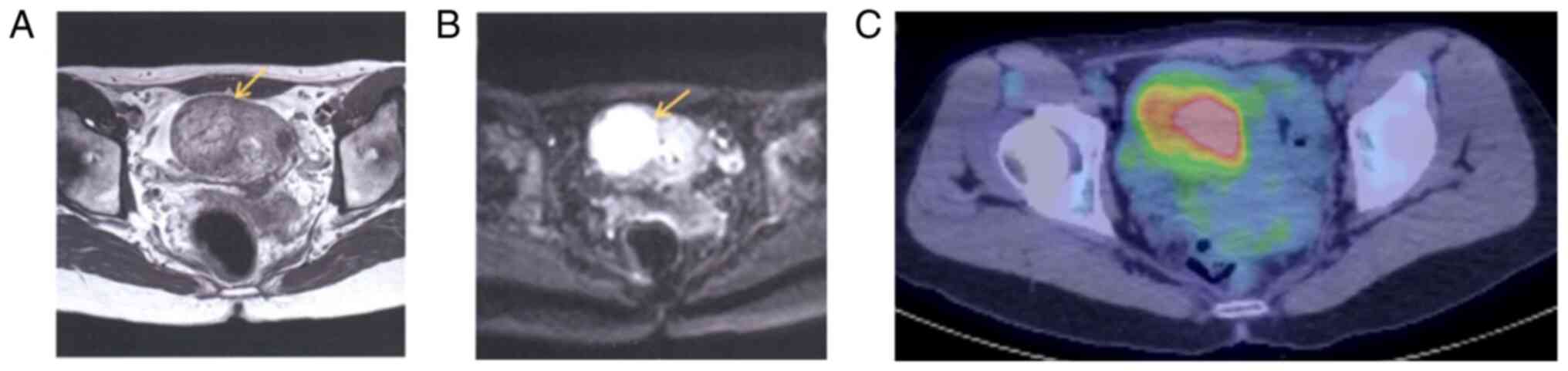
precluded. T2-weighted and diffusion-weighted magnetic resonance
imaging (MRI) revealed a 4.5-cm high-intensity area on the right
side of the uterus (Fig. 2A and
B), and positron emission
tomography/computed tomography (PET/CT) showed an accumulation of
fluorodeoxyglucose at the same site with an SUVmax of 4.9 (Fig. 2C). There were no findings
suggestive of metastasis. Surgical treatment was proposed based on
these examination results, but the patient strongly wished to
preserve fertility. It was therefore decided to perform enucleation
of the uterine mass through laparotomy, which we expected would
also assist in confirming the diagnosis. Intraoperative findings
indicated that the mass, which had an indistinct margin, protruded
into the intrauterine cavity in the form of a polyp and deeply
invaded the myometrium as well. Cytology of peritoneal lavage was
negative. A 54-g mass 5 cm in size was surgically removed. The
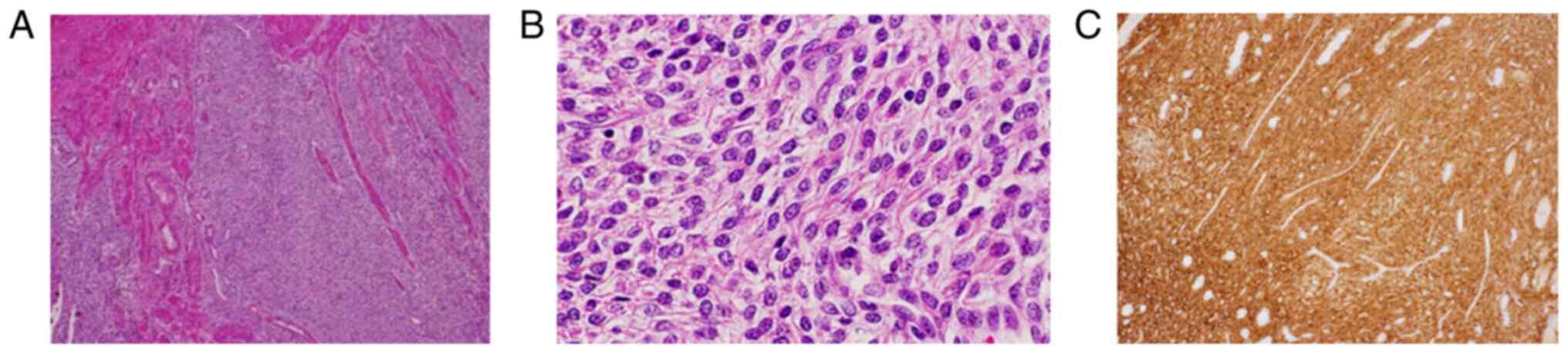
pathological findings of the excised mass showed atypical cells
similar to endometrial stromal cells proliferating and invading the
myometrium without distinct borders, and immunostaining was
positive for CD10, estrogen receptor (ER), and progesterone
receptor (PR). A diagnosis of LG-ESS was made (Fig. 3A-C). Because the surgical margin
was positive, the patient was further examined for residual disease
and metastasis in order to determine the subsequent treatment
strategy. No residual tumor was evident in the uterus on MRI, and
contrast-enhanced CT also showed no lymph node metastasis or
distant metastasis. Based on these examination results, we made a
diagnosis of stage IB LG-ESS. In light of the fact that the patient
was as young as 26 years old and she was an unmarried, nulligravid
woman, the possibility of fertility preservation and the attendant
recurrence risk were fully explained, including that future
possibility of a surrogate pregnancy or uterus transplantation
remains even if she makes a choice of hysterectomy After that, she
strongly desired hysterectomy and ovarian preservation, and total
abdominal hysterectomy and bilateral salpingectomy were performed,
with both ovaries being preserved. The pathological diagnosis of
the excised uterus was LG-ESS, pT1b v(+), ly(+), and the resection
margin was negative. Generally, follow-up observation without
adjuvant therapy may be considered for patients with stage I LG-ESS
who have undergone complete removal of the lesions. In the present
case, however, high-dose progesterone therapy [medroxyprogesterone
acetate (MPA), 600 mg/day] was given from 1 month after surgery for
1 year in consideration of recurrence risk because the ovaries were
preserved. Two years have elapsed since the surgery, and no
recurrence has been observed to date.
Discussion
In the present case, the diagnosis of LG-ESS was
made after the patient underwent surgery to excise endometrial
polyp-like lesions twice. Because she was as young as 26 years old,
fertility-sparing surgery including ovarian and uterine
preservation was considered. The choice of operative procedure was
not straightforward, but the patient first underwent tumor
resection after due informed consent. She then underwent two-stage
total hysterectomy, with the ovaries being preserved. Considering
the risk of postoperative recurrence, we provided high-dose oral
progesterone therapy. Two years have passed since the surgery, and
no recurrence has been observed to date.
LG-ESS is common in women in their 40 and 50s, and
patients with LG-ESS present with symptoms such as atypical genital
bleeding, heavy menstrual bleeding, enlarged uterus, and lower
abdominal pain (4). In general, a
yellowish-white polyp-like mass is formed and often grows to fill
the intrauterine cavity, but it may also invade the myometrium and
vasculature and extend to the ovaries, pelvis, abdominal cavity,
and retroperitoneal lymph nodes (5,6).
These characteristics are congruent with the fact that our patient
had atypical genital bleeding as the initial symptom and that
endometrial polyp-like lesions first appeared and then detected as
a tumor invading the myometrium. According to previous reports, it
is often difficult to distinguish LG-ESS from endometrial polyps or
uterine fibroids preoperatively, and LG-ESS is sometimes diagnosed
only after postoperative pathological examination (5,6). In
the present case, polyp-like lesions were confined to the
intrauterine cavity when they were found, and there were no lesions
in the myometrium on ultrasonography, so we did not perform MRI at
this timing. The pathological findings at the time of the first
polypectomy were not clearly malignant due to lack of cellular
atypia. However, the polyp-like lesions recurred soon afterwards
and grew further into the myometrium. It is therefore inferred that
the initial polyp-like mass may have been an incipient lesion of
LG-ESS. We speculate that the tumor was initially confined to the
polypoid lesion within the uterine cavity at the time of the first
hysteroscopic resection, but it had progressed rapidly and invaded
into the myometrium at the second hysteroscopic resection; thus, it
might have been better to examine the tumor extension using MRI
before the second hysteroscopic polypectomy and to evaluate the
safety and adequacy of the hysteroscopic resection.
Standard treatment for LG-ESS is hysterectomy and
bilateral adnexectomy (3).
Lymphadenectomy or lymph node biopsy is also recommended because
9-33% of this disease has pelvic node metastasis (7,8),
although its impact on survival is not clear. In our patient, it
might have been better to have lymphadenectomy to decide the
disease stage correctly, but we did not perform it because there
was no suspicious finding of lymph node metastasis on PET/CT.
By contrast, management of LG-ESS in young patients
who wish to preserve their fertility has not yet been established,
with only a few reports available on the outcome of
fertility-sparing treatment of LG-ESS (9-12)
as summarized in Table I. Xie
et al (9) studied 17
younger patients (aged 15-37 years) with stage I LG-ESS (6 stage IA
patients and 11 stage IB patients) in whom
laparoscopic/hysteroscopic or open tumor resection was performed
with successful fertility preservation. In their study, 12 of the
17 patients received postoperative hormonal therapy. Recurrence of
the disease was seen in 10 of the 17 patients (59%), but subsequent
treatment resulted in a favorable outcome, with no mortality being
reported in any patient. Furthermore, five of eight patients who
wished to become pregnant succeeded in giving birth. Similarly,
Laurelli et al (10)
reported that six stage IA LG-ESS patients aged 18 to 40 years who
had polypoid or submucosal tumors in the intrauterine cavity
underwent hysteroscopic tumor resection, and five of them were
treated with hormonal therapy with megestrol acetate (MA) for 1 to
2 years. All of these patients survived without recurrence, and
three patients became pregnant. Jin et al (11) also reported that five LG-ESS
patients aged 28 to 37 years who wished to preserve their fertility
underwent local tumor resection and adjuvant hormonal therapy.
Although one patient had recurrent disease and subsequently
underwent total hysterectomy, all five patients were alive, and
three of them became pregnant and gave birth. They recommend that
hormonal therapy should be given for 6 months after
fertility-sparing surgery. By contrast, a retrospective analysis of
153 LG-ESS patients by Bai et al (12) showed that the recurrence rate was
as high as 79% (15 of 19 patients) in patients who underwent tumor
enucleation alone at the initial surgery, and that uterine
preservation with tumor enucleation alone, ovarian preservation,
and positive surgical margins were the three independent prognostic
factors for recurrence. However, ovarian preservation and tumor
enucleation did not significantly affect the overall survival.
Based on these results, Bai et al (12) suggested that tumor enucleation for
LG-ESS should be performed after adequate informed consent only in
patients with a strong desire for fertility preservation, and total
hysterectomy should be recommended after the completion of
pregnancy and delivery.
 | Table ISummary of the reports on
fertility-sparing treatment for young patients with low-grade
endometrial stromal sarcoma. |
Table I
Summary of the reports on
fertility-sparing treatment for young patients with low-grade
endometrial stromal sarcoma.
| Author, year | Case no. | Age, years | Stage | Surgical resection
procedure | Adjuvant hormone
therapy | Recurrence | Pregnancy | Final clinical
outcome | (Refs.) |
|---|
| Xie et al,
2017 | 17 | 15-37 | IA:6 IB:11 | Laparotomy:5
Laparoscopy:7 Hysteroscopy:5 | MPA:3 MA:5 GnRHa:2
GnRHa/LNG:2 None:5 | 10 | 5 | NED:17 | (9) |
| Laurelli et
al, 2015 | 6 | 18-40 | IA:6 | Hysteroscopy:6 | MA:5 None:1 | 0 | 3 | NED:6 | (10) |
| Jin et al,
2015 | 5 | 28-37 | IA:3 IB:2 | Laparoscopy:5 | MA:4 GnRHa:1 | 1 | 3 | NED:5 | (11) |
| Bai et al,
2014 | 19 | Not described | Not described | Not described in
detail | Not described in
detail | 15 | 5 | NED:18 DOD:1 | (12) |
Since positive resection margins can be a prognostic
factor for recurrence (12), it is
of importance that fertility-sparing surgery should be performed
for polypoid lesions in the intrauterine cavity, and for cases with
the presence of intramuscular involvement, the surrounding normal
myometrium should also be excised. In our case, the patient
strongly desired to preserve fertility, and tumor enucleation was
performed accordingly. Later on, however, it was decided to perform
two-stage total hysterectomy after careful consideration of the
high risk of recurrence due to positive resection margins, although
the ovaries were preserved according to her strong wish. Ovarian
preservation has been reported to be associated with an increased
recurrence rate in premenopausal women since the growth and
development of LG-ESS is hormone-dependent (13-15).
However, there are no definitive reports that ovarian preservation
shortens overall survival (16),
and the rights and wrongs of ovarian preservation should be
thoroughly discussed with the patient.
Being highly sensitive to hormonal therapy, tumor
cells in LG-ESS frequently express ER and PR (5,6) and
in most cases express aromatase as well (17). They are also characterized by
diffuse CD10 positivity on immunostaining (5,6). In
keeping with the previous reports, ER, PR, and CD10 were strongly
positive in our patient. MA is most commonly used in adjuvant
hormonal therapy following fertility-sparing surgery (9-11),
and other treatments, such as MPA (9), levonorgestrel-releasing intrauterine
device (LNG-IUD) (9) and
gonadotropin-releasing hormone analogues (GnRHa) (9), have been reported in several cases.
In our case, MPA at a daily oral dose of 600 mg with low-dose
aspirin, which is widely used as a fertility-sparing therapy in
young women with endometrial carcinoma in Japan (18), was administered for 1 year, and no
recurrence has been observed after completion of hormonal therapy.
Careful follow-up over a long period is warranted.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HM, NO and KI conceived and designed this case
report. HM, NO and KI wrote the initial draft of the report. KN, TN
and NI collected the clinical data. YM, SY and SM analyzed the data
from images and pathological examination. NO and KI confirm the
authenticity of all the raw data. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Written informed consent for surgery and tissue
collection was obtained from the patient.
Patient consent for publication
Written informed consent for publication of the
present report was obtained from the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hosh M, Antar S, Nazzal A, Warda M,
Gibreel A and Refky B: Uterine sarcoma: Analysis of 13,089 cases
based on surveillance, epidemiology and end results database. Int J
Gynecol Cancer. 26:1098–1104. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Brooks SE, Zhan M, Cote T and Baquet CR:
Surveillance, epidemiology, and end results analysis of 2677 cases
of uterine sarcoma 1989-1999. Gynecol Oncol. 93:204–208.
2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Amant F, Coosemans A, Debiec-Rychter M,
Timmerman D and Vergote I: Clinical management of uterine sarcomas.
Lancet Oncol. 10:1188–1198. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Thiel FC and Halmen S: Low-grade
endometrial stromal sarcoma-a review. Oncol Res Treat. 41:687–692.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chang KL, Crabtree GS, Lim-Tan SK, Kempson
RL and Hendrickson MR: Primary uterine endometrial stromal
neoplasms. A clinicopathologic study of 117 cases. Am J Surg
Pathol. 14:415–438. 1990.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hoang L, Chiang S and Lee CH: Endometrial
stromal sarcomas and related neoplasms: New developments and
diagnostic considerations. Pathology. 50:162–177. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Leath CA III, Huh WK, Hyde J Jr, Cohn DE,
Resnick KE, Taylor NP, Powell MA, Mutch DG, Bradley WH, Geller MA,
et al: A multi-institutional review of outcomes of endometrial
stromal sarcoma. Gynecol Oncol. 105:630–634. 2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Riopel J, Plante M, Renaud MC, Roy M and
Têtu B: Lymph node metastasis in low-grade endometrial stromal
sarcoma. Gynecol Oncol. 96:402–406. 2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Xie W, Cao D, Yang J, Jiang X, Shen K, Pan
L, Huang H, Lang J, You Y and Chen J: Fertility-sparing surgery for
patients with low-grade endometrial stromal sarcoma. Oncotarget.
8:10602–10608. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Laurelli G, Falcone F, Scaffa C, Messalli
EM, Del Giudice M, Losito S and Greggi S: Fertility-sparing
management of low-grade endometrial stromal sarcoma: Analysis of an
institutional series and review of the literature. Eur J Obstet
Gynecol Reprod Biol. 195:61–66. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jin Y, Li Y, Deng CY, Tian QJ, Chen H and
Pan LY: Fertility-sparing treatment of low-grade endometrial
stromal sarcoma. Int J Clin Exp Med. 8:5818–5821. 2015.PubMed/NCBI
|
|
12
|
Bai H, Yang J, Cao D, Huang H, Xiang Y, Wu
M, Cui Q, Chen J, Lang J and Shen K: Ovary and uterus-sparing
procedures for low-grade endometrial stromal sarcoma: A
retrospective study of 153 cases. Gynecol Oncol. 132:654–660.
2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Amant F, De Knijf A, Van Calster B, Leunen
K, Neven P, Berteloot P, Vergote I, Van Huffel S and Moerman P:
Clinical study investigating the role of lymphadenectomy, surgical
castration and adjuvant hormonal treatment in endometrial stromal
sarcoma. Br J Cancer. 97:1194–1199. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Berchuck A, Rubin SC, Hoskins WJ, Saigo
PE, Pierce VK and Lewis JL Jr: Treatment of endometrial stromal
tumors. Gynecol Oncol. 36:60–65. 1990.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li N, Wu LY, Zhang HT, An JS, Li XG and Ma
SK: Treatment options in stage I endometrial stromal sarcoma: A
retrospective analysis of 53 cases. Gynecol Oncol. 108:306–311.
2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shah JP, Bryant CS, Kumar S, Ali-Fehmi R,
Malone JM Jr and Morris RT: Lymphadenectomy and ovarian
preservation in low-grade endometrial stromal sarcoma. Obstet
Gynecol. 112:1102–1108. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Reich O and Regauer S: Aromatase
expression in low-grade endometrial stromal sarcomas: An
immunohistochemical study. Mod Pathol. 17:104–108. 2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ushijima K, Yahata H, Yoshikawa H, Konishi
I, Yasugi T, Saito T, Nakanishi T, Sasaki H, Saji F, Iwasaka T, et
al: Multicenter phase II study of fertility-sparing treatment with
medroxyprogesterone acetate for endometrial carcinoma and atypical
hyperplasia in young women. J Clin Oncol. 25:2798–1803.
2007.PubMed/NCBI View Article : Google Scholar
|