Introduction
Multiple myeloma (MM) accounts for 10% of all
hematologic malignancies and approximately 1% of all cancers
(1). It affects predominantly the
elderly and consists of a malignant proliferation of plasma cells
(PC). These malignant cells are mostly found inside the bone marrow
but they can also be present in extramedullary disease (ED)
(1,2). Plasma cells are responsible for
hypersecretion of M protein, which represents defective
immunoglobulin, and lead to a well-known association of symptoms
named CRAB-hypercalcemia, renal failure, anemia and bone lytic
lesions (2,3).
Pleural effusion (PE) in multiple myeloma is not an
uncommon finding, comprising about 6-14% of MM patients (4-6).
The most common causes of MM associated PE are congestive heart
failure, renal failure with or without nephrotic syndrome,
parapneumonic effusion and amyloidosis. Other causes can be
hypoalbuminemia, pulmonary embolism, secondary neoplasm and
lymphatic obstruction with chylothorax. In less than 1% of cases,
the effusion is a direct result of MM, designated as myelomatous
pleural effusion (MPE) (7).
Several mechanisms have been proposed for the pathogenesis of
pleural effusion in MM. They mostly originate from adjacent
skeletal or parenchymal tumors such as vertebral or pulmonary
plasmacytomas, direct pleural invasion by tumor nodules
(hematogenous dissemination) and mediastinal lymph node
infiltration with lymphatic blockage. The extramedullary spread can
be triggered by an invasive procedure (surgery or catheter
insertion) or by a bone fracture (8-11).
Rodriguez et al (12) established the diagnostic criteria
for MPE: Pleural fluid electrophoresis with monoclonal protein plus
atypical plasma cells in the pleural fluid plus histological
confirmation by pleural biopsy. Eighty percent of MPEs are
associated with IgA MM (13) and
present late in the course of the disease, carrying a poor
prognosis and a life expectancy of about 4 months. There is no
distinct therapy for the MPE and in some cases, palliative
pleurodesis is the only treatment possible (14).
Here we report an MPE associated with IgG/λ MM
presenting as a septic shock and renal failure requiring
hemodialysis in which a rare diagnosis was made after excluding all
other possible etiologies in a complex intensive care patient.
Case report
The authors describe the case of a 79-year-old white
woman, with IgG/λ MM, hypertension, type 2 diabetes mellitus,
dyslipidemia and hyperuricemia. MM was diagnosed two months prior
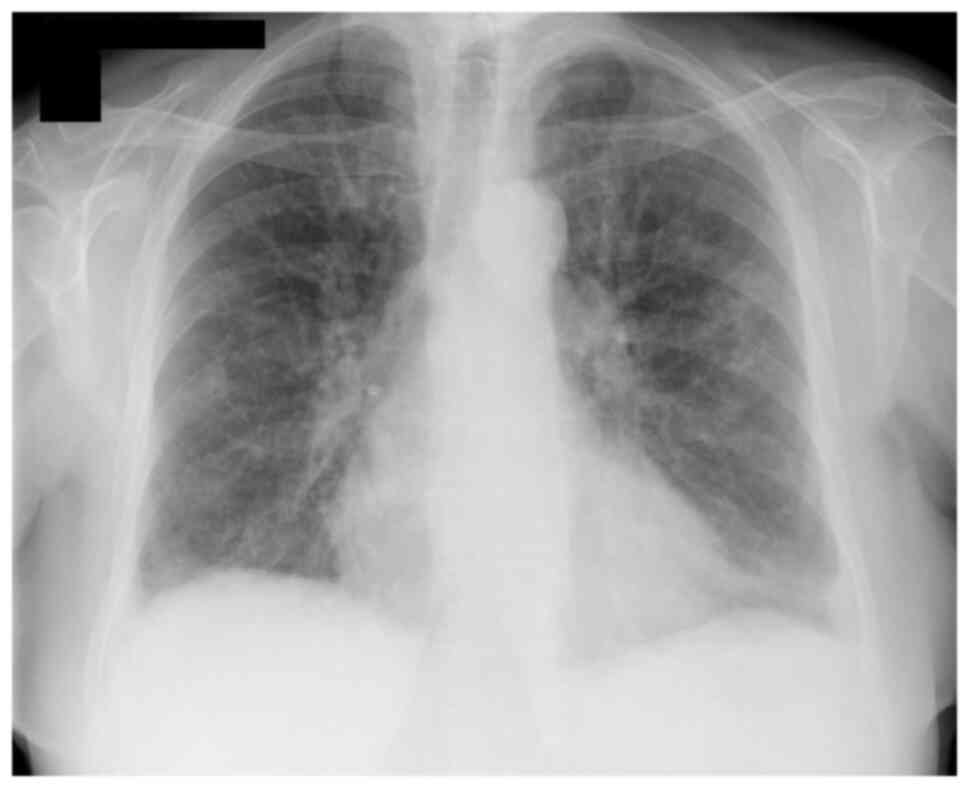
admission (Fig. 1). At diagnosis,
the patient had an increase in total serum proteins (14.77 g/dl) at
the expense of an increase in γ-globulins (8.8 g/dl; 59.40%). The
measurement of serum immunoglobulins revealed an increase in the
concentration of heavy IgG chains (10,706.0 mg/dl) and free λ light
chains (2,720 mg/dl). Immunofixation revealed the presence of an
IgG/λ monoclonal gammopathy. At that time, medullary aspirate was
performed, which was very hypocellular (10.0x103/µl),
according to flow cytometry 10% of the cells were plasmocytes with
abnormal phenotypic characteristics CD38+,
CD138+, CD19-, CD56+,
CD10-, CD20-, CD117-,
CD45-.
She presented anemia and lytic bone lesions in the
skull. She started on oral melphalan and prednisolone. She was
classified as an Eastern Cooperative Oncology Group (ECOG)
Performance Status 1.
She was admitted to the emergency department in
March 2020 with symptoms of fatigue, severe bone pain, and
diarrhea. On admission, she presented with hemodynamic instability,
oliguric, and requiring oxygen through a face mask. Physical
examination revealed a decrease in breath sounds throughout the
right hemithorax. Complete blood count revealed anemia (Hb 8.1
g/dl), leukocytosis (12.7x109/l), neutrophilia
(11.3x109/l) and thrombocytopenia (35x109/l).
Chemistry panel revealed C-reactive protein 352.4 mg/l, creatinine
3.96 mg/dl and albumin of 1.83 g/dl. Arterial blood gas analysis
showed a type 1 respiratory insufficiency and hyperlactacidemia
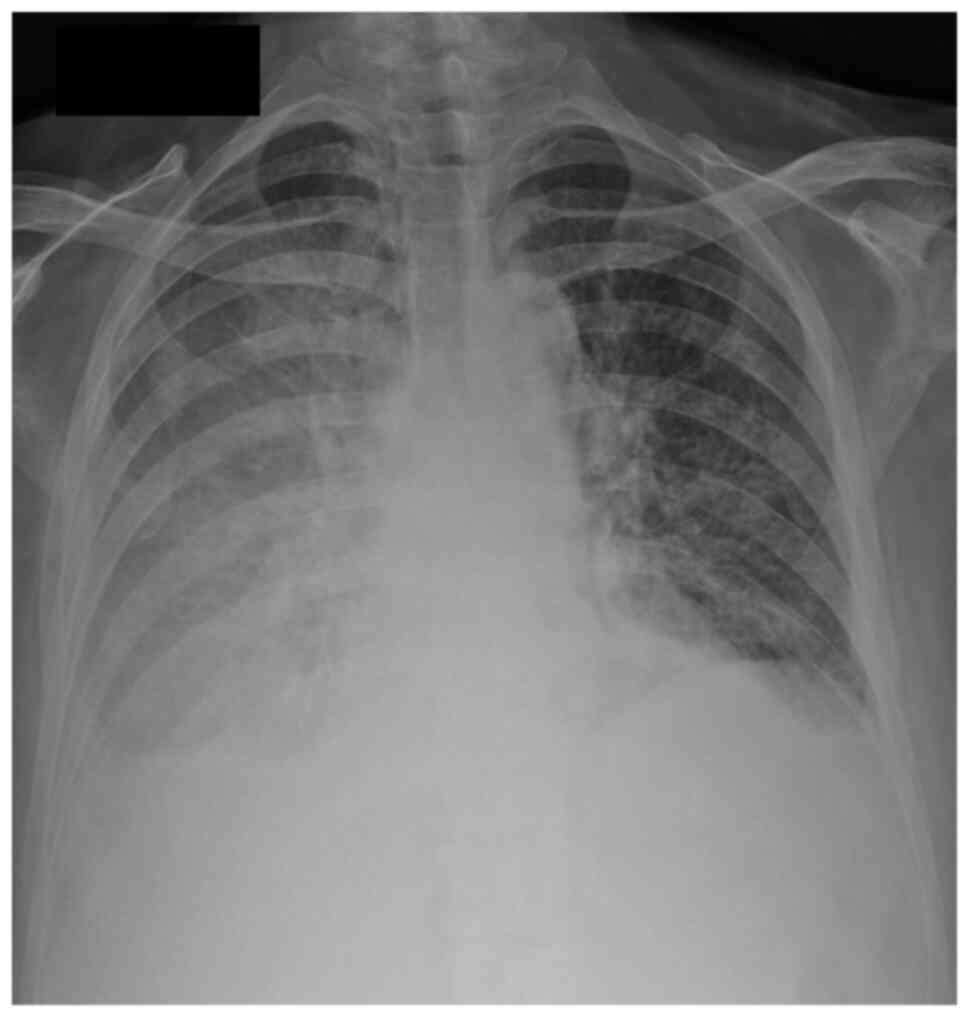
(4.9 mg/dl). The chest X-ray showed a bilateral pleural effusion
(Fig. 2). Diagnostic thoracentesis
was performed and the pleural fluid was compatible with an exudate
using Light's criteria (15).
She deteriorated and was admitted to the intensive
care unit for septic shock with multiorgan failure: renal,
hematological, neurological, cardiovascular and respiratory
failures. Blood and urine samples were withdrawn and sent for
microbiological analysis and she was started on broad-spectrum
antibiotics. Due to hypervolemia and acute renal failure, she was
put on sustained low-efficiency daily diafiltration (SLEDD) aimed
for a negative fluid balance. Despite all measures instituted, she
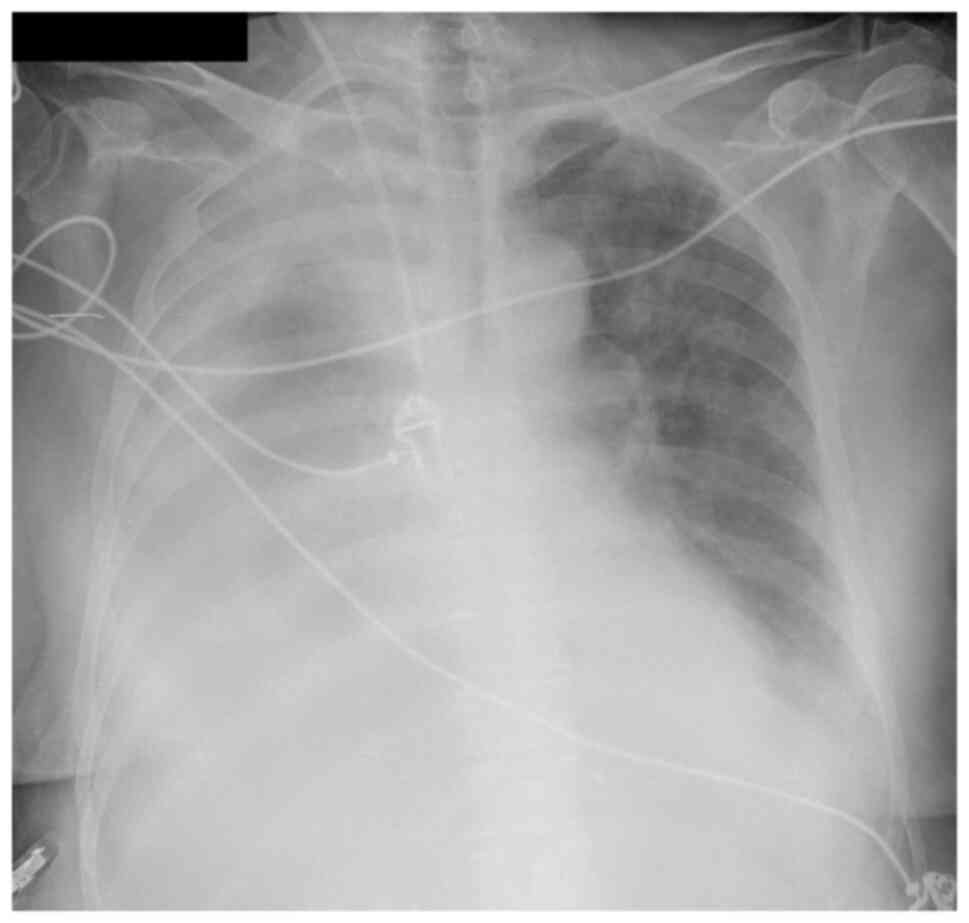
continued to deteriorate with dyspnea, tachypnea, desaturation, and
aggravated pleural effusion (Fig.
3).
The infectious etiology had been assumed as a most
likely cause for the pleural effusion. Despite diminishing
inflammatory parameters, pleural effusion enlarged causing
additional respiratory compromise. Other PE causes were also
addressed like renal failure and hypoalbuminemia. A detailed
systematic approach to investigate the etiology of this pleural
effusion was then undertaken.
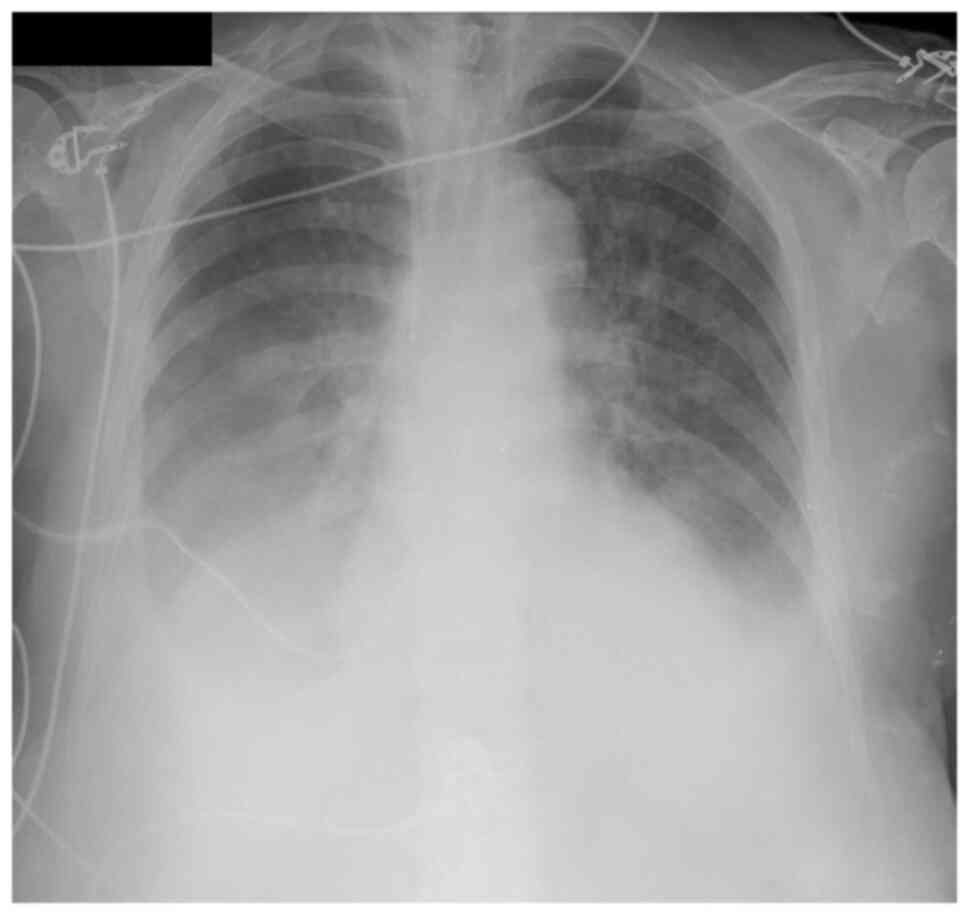
Therapeutic thoracentesis was performed and 2,650 ml
of serosanguineous fluid was removed (Fig. 4). The pleural fluid analysis showed
pH 7.9, adenosine deaminase 31.7 U/l, and glucose 88 mg/dl,
proteins 3.5 g/dl, and LDH 618U/l. The ratio between pleural and
serum LDH was indicative of exudative pleural effusion. Protein
electrophoresis of pleural fluid showed a monoclonal γ-globulin
spike (1.2 g/dl, 34,2%) (Fig.
5).
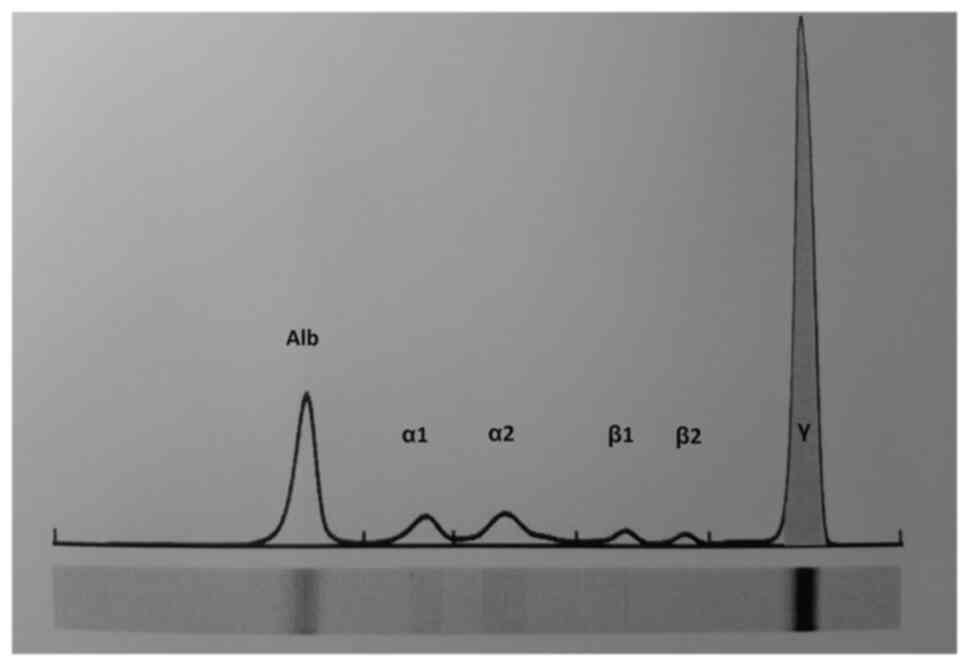 | Figure 5Pleural fluid capillary
electrophoresis showing a γ-globulin spike (Alb, 36.7%; α1, 5.7%;
α2, 10.4%; β globulina, 11.0%; γ, 34.2%; pico monoclonal, 33.4%).
Alb, albumina; α1, α1 globulinal; α2, α2 globulina; β1, β1
globulina; β2, β2 globulina; γ, γ globulina. |
Flow cytometry showed 89% of abnormal PCs positive
for CD38 (low, decreased intensity comparatively to normal PC),
CD138, CD56 and CD117, and negative for CD10, CD19, CD20 and CD45.
Normal PC phenotype, for comparison: CD45+low,
CD38+high, CD138+, CD19+,
CD20-, CD56-, CD117- (16). The pleural fluid cells were stained
with an 8-color panel of fluorochrome-conjugated monoclonal
antibodies specific for the aforementioned molecules, processed
with BD FACS™ lysing solution according to the manufacturer's
instructions (Becton Dickinson) and acquired using a BD FACSCanto™
II flow cytometer (Becton Dickinson). Data generated as FCS files
were analyzed using the Infinicyt™ software (Cytognos). FSC and SSC
were captured on a linear scale (250 channels), and SSC was
represented with a mathematical transformation to expand the values
of the lower part of the scale for a better visualization of the
populations. For fluorescence parameters, a logarithmic
amplification was used, with logical transformation, allowing for a
resolution of 262 144 channels (~5.42 decades) (Fig. 6).
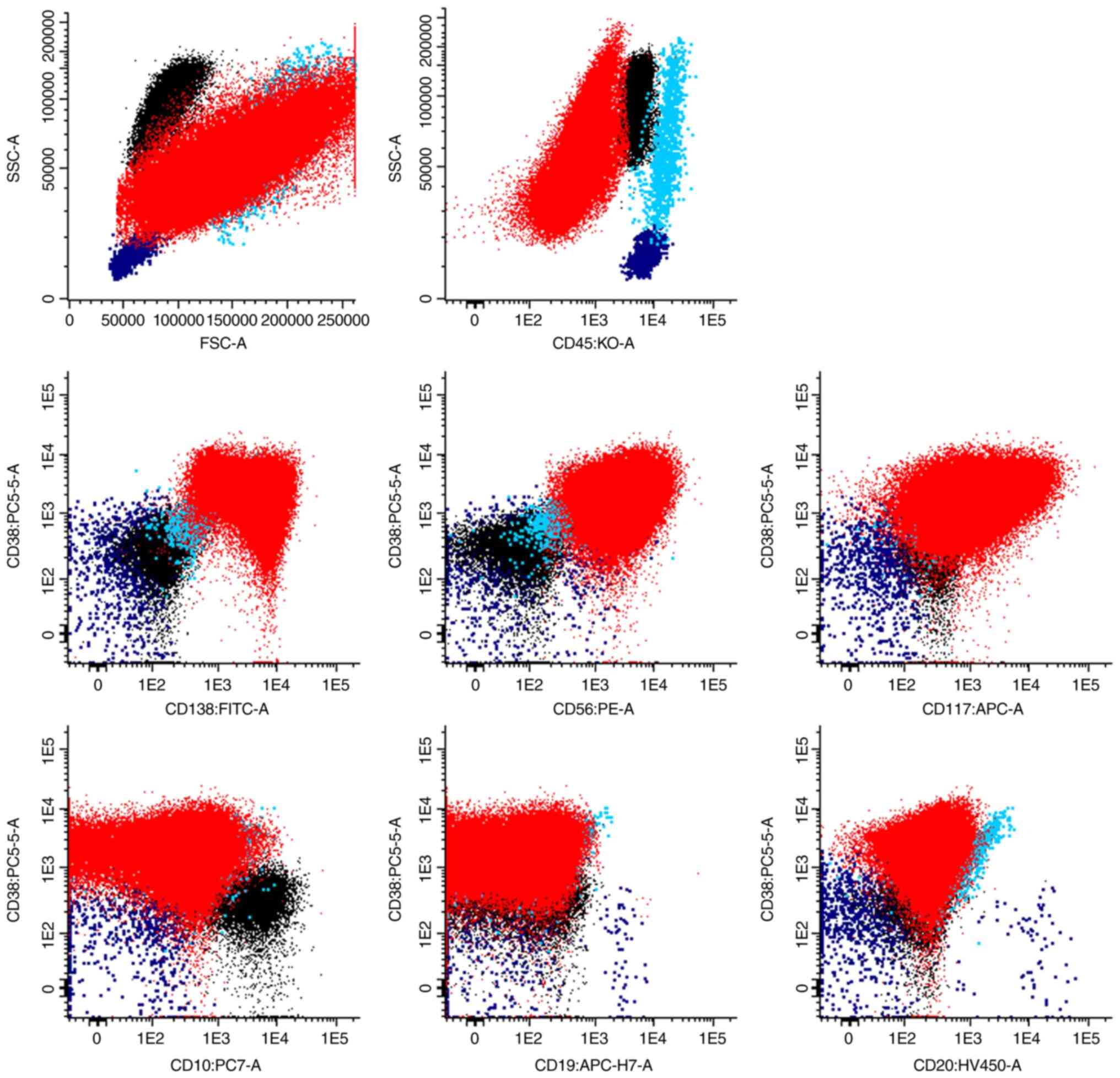 | Figure 6Flow cytometry dot plots showing 89%
of abnormal PCs in the pleural fluid (red dots), together with
neutrophils (black dots), lymphocytes (dark blue dots) and
monocytes/macrophages (blue dots). The abnormal PCs were positive
for CD38 (low, decreased intensity comparatively to normal PC),
CD138, CD56 and CD117, and negative for CD10, CD19, CD20 and CD45.
Normal PC phenotype, for comparison: CD45+low,
CD38+high, CD138+, CD19+,
CD20-, CD56-, CD117-. APC,
allophycocyanin; APC-H7, allophycocyanin-H7; FITC, fluorescein
isothiocyanate; FSC, forward scatter; KO, chrome orange; PCs,
plasma cells; PC5.5, phycoerythrin-cyanine 5.5; PC7,
phycoerythrin-cyanine 7; PE, phycoerythrin; SSC, side scatter. |
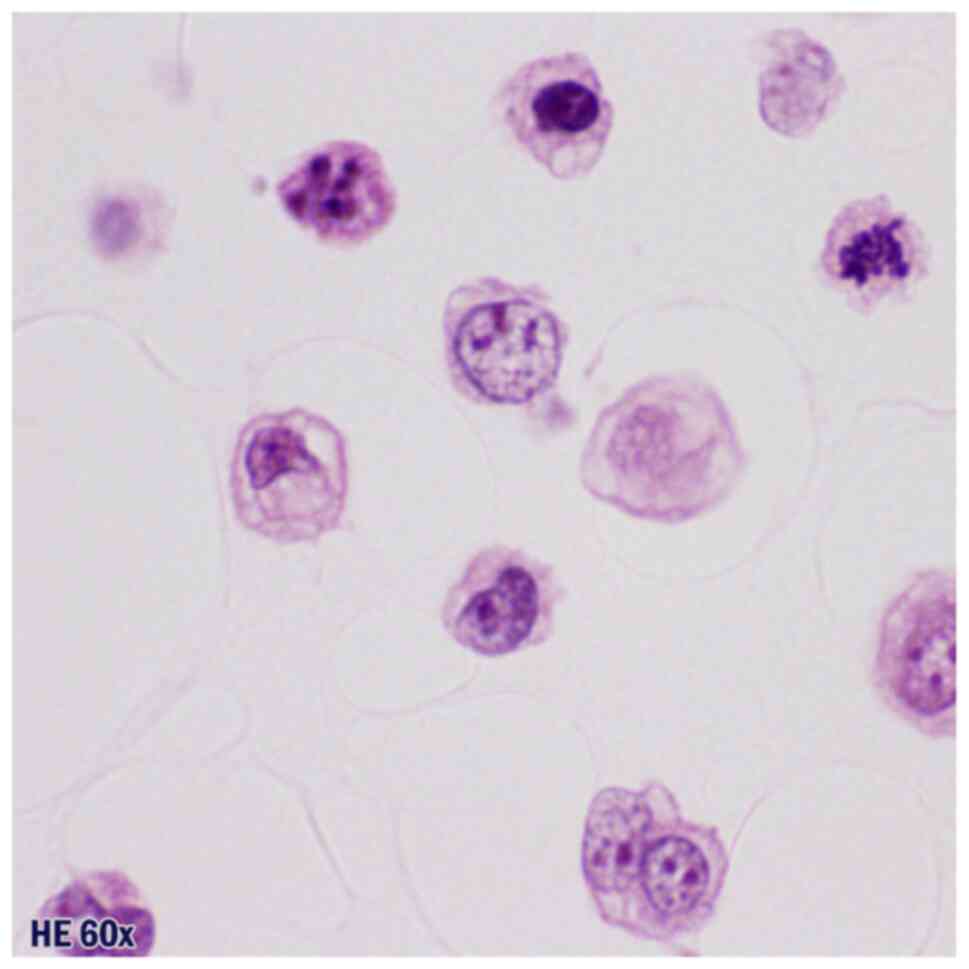
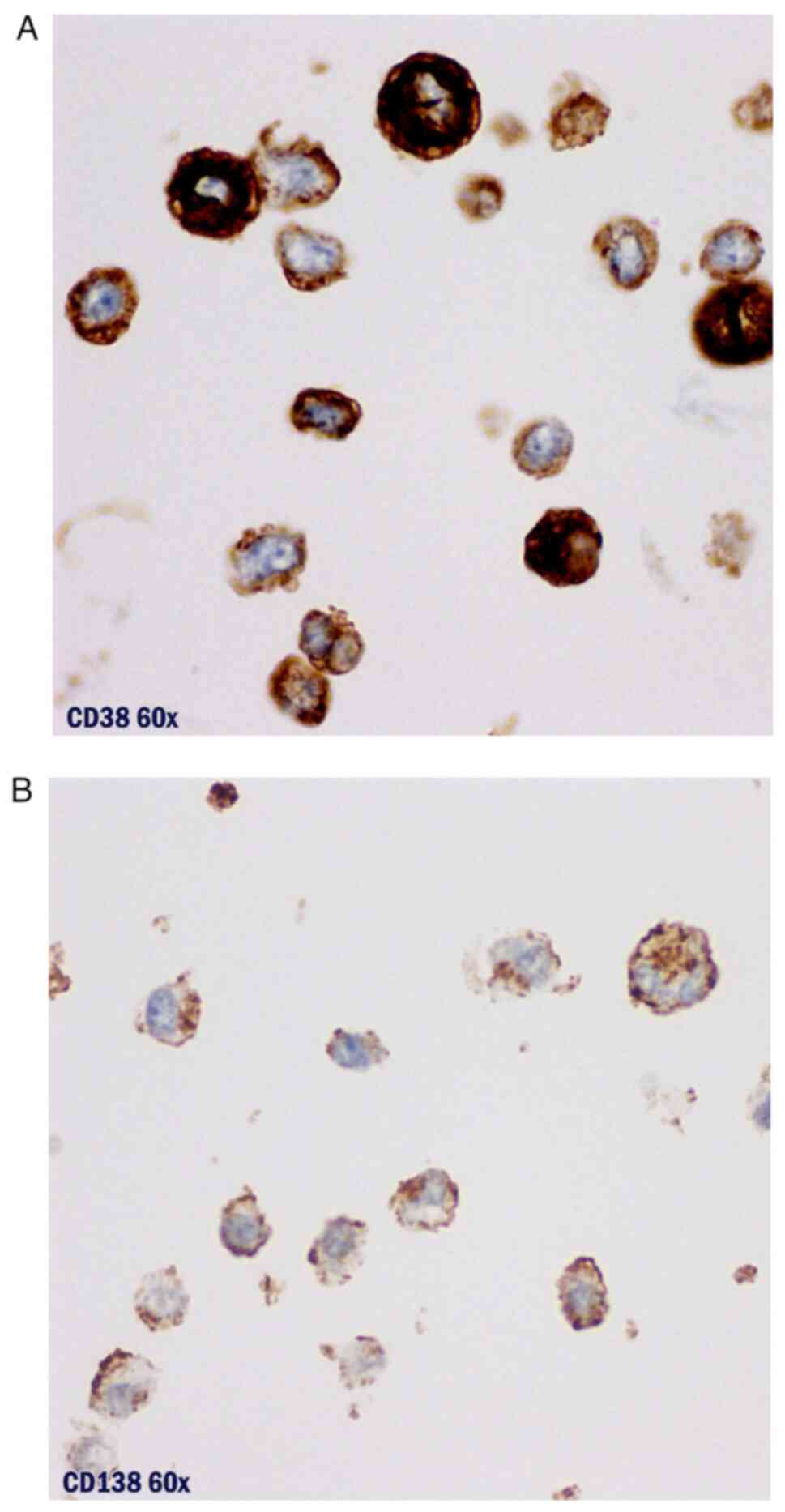
The cytological exam and immunocytochemical study
proved to be compatible with a plasma cell proliferation (Figs. 7 and 8). Bacteriological exam of the pleural
fluid revealed a multi-resistant Escherichia coli.
Histological confirmation on pleural biopsies, was deemed as
presenting with no benefit for the patient and was not performed. A
diagnosis of myelomatous pleural effusion with bacterial
overinfection was made.
Although respiratory insufficiency had improved, the
patient still degraded and the conditions to start MM induction
therapy were not met. Due to the poor prognosis of extramedullary
disease, particularly involving MPE, and the deterioration of
performance status of the patient, it was decided to withdraw
therapeutic measures and begin palliative care. The patient died 10
days after hospital admission.
Discussion
In the past few years, the incidence of EMD has
risen, probably due to the greater sensitivity of imaging tests but
also due to the increased survival of patients as a result of
advances in the therapeutic strategy of MM (17,18).
Evidence of extramedullary disease is a clear marker of poor
prognosis and the MPE is no exception. Even with aggressive
therapy, MPE has a progression-free survival and overall survival
of fewer than 4 months. The prognosis is even worst when the
mechanism involved in the progression of the disease is
hematogenous spread (17,19).
The patient had an ongoing infectious process, with
renal and cardiovascular failure combined with hypoalbuminemia, so
she had multiple factors for pleural effusion and they were much
more prevalent than MPE. There were no pulmonary or bone lesions
suggestive of plasmacytomas on chest X-ray. The initial approach to
pleural effusion was incomplete, probably due to a lack of clinical
suspicion, contributing to the delay in diagnosis.
Despite the existence of specific criteria,
diagnosing MPE remains a challenge. Pleural cytology has a
diagnosis rate for malignancy of around 60% (20). Blind pleural biopsy is a procedure
with associated risks and less attractive as a diagnostic tool,
since MPE affects the pleura irregularly. Nevertheless, pleural
fluid cytology or pleural biopsy provides the diagnosis of MPE in
approximately half of these patients (21,22).
Flow cytometry of pleural fluid is an excellent
complementary diagnostic method to the traditional strategy. It is
a fast, very sensitive and effective technique that allows not only
for plasma cell quantification but also for the identification of
phenotypically abnormal malignant plasma cells, thereby improving
the diagnosis of MPE. Its use is particularly relevant in
complementing the cytological assessment of serous fluids that lack
a predominance of plasma cells, or where these cells lack
definitive malignant morphological features (23). These characteristics add important
value to pleural fluid differential diagnosis. Thus, the authors
aim to warn of the need to review the MPE diagnostic criteria in
light of the current knowledge and available diagnostic tests.
Only a proper diagnosis will allow early
intervention. And despite the absence of aimed therapy for this
high-risk group of patients, the use of drugs, namely proteasome
inhibitors, (intravenous or intrapleural) has brought some hope in
the last few years (24). More
clinical trials are still needed in the future to better define the
correct strategy for these patients.
In conclusion, pleural effusion in patients with MM
is a heterogeneous entity that can arise from a varied number of
etiologies that require different treatments. A proper and complete
examination is essential in the diagnosis of MPE due to the
therapeutic and prognostic implications, including cytological
examination, protein electrophoresis with immunofixation, flow
cytometry and ultimately pleural biopsy.
Acknowledgements
The authors would like to thank Dr Luís Ramos Silva
(Department of Clinical Hematology, Hospital Center of São João,
4200-319 Porto, Portugal) and Professor Margarida Lima (Department
of Clinical Hematology-responsible for the cytometry and genetics
laboratories, University Hospital Center of Porto, 4099-901 Porto,
Portugal) for intellectual and technical assistance, and helping in
the writing of the manuscript.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ALR, MT and ASP contributed to the planning,
conduct, reporting, conception, design and acquisition of data, and
drafting the manuscript. JRB, CP and AP contributed to planning,
conduct, reporting, reporting, conception, design, and acquisition
of data and revision of the article. ALR, MT and ASP confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study involving a human subject was in
accordance with the Declaration of Helsinki 1975 as revised in 2000
and was approved by the Ethical Committee of University Hospital
Center of Porto, University of Porto (Porto, Portugal).
Patient consent for publication
The requirement for consent was waived and the study
was approved by the Ethical Committee of University Hospital Center
of Porto, University of Porto (Porto, Portugal).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Short K, Rajkumar S, Larson D, Buadi F,
Hayman S, Dispenzieri A, Gertz M, Kumar S, Mikhael J, Roy V, et al:
Incidence of extramedullary disease in patients with multiple
myeloma in the era of novel therapy, and the activity of
pomalidomide on extramedullary myeloma. Leukemia. 25:906–908.
2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Swerdlow SH, Campo E, Pileri SA, Harris
NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz
AD and Jaffe ES: The 2016 revision of the world health organization
classification of lymphoid neoplasms. Blood. 127:2375–2390.
2017.
|
|
3
|
Kyle RA and Rajkumar SV: Multiple myeloma.
N Engl J Med. 351:1860–73. 2004.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Byun JM, Kim KH, Choi IS, Park JH, Kim JS,
Shin DY, Koh Y, Kim I, Yoon SS and Lim HJ: Pleural effusion in
multiple myeloma: Characteristics and practice patterns. Acta
Haematol. 138:69–76. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wang Z, Xia G, Lan L, Liu F, Wang Y, Liu
B, Ding Y, Dai L and Zhang Y: Pleural effusion in multiple myeloma.
Intern Med. 55:339–345. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kintzer J, Rosenow EC III and Kyle RA:
Thoracic and pulmonary abnormalities in multiple myeloma. A review
of 958 Cases. Arch Intern Med. 138:727–730. 1978.PubMed/NCBI
|
|
7
|
Oudart JB, Maquart FX, Semouma O, Lauer M,
Arthuis-Demoulin P and Ramont L: Pleural effusion in a patient with
multiple myeloma. Clin Chem. 58:672–674. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bladé J, de Larrea CF, Rosiñol L, Cibeira
MT, Jiménez R and Powles R: Soft-tissue plasmacytomas in multiple
myeloma: Incidence, mechanisms of extramedullary spread, and
treatment approach. J Clin Oncol. 29:3805–3812. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Weinstock M and Ghobrial IM:
Extramedullary multiple myeloma. Leuk Lymphoma. 54:1135–1141.
2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
De Larrea CF, Rosiñol L, Cibeira MT,
Rozman M, Rovira M and Bladé J: Extensive soft-tissue involvement
by plasmablastic myeloma arising from displaced humeral fractures.
Eur J Haematol. 85:448–451. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Alexandrakis MG, Passam FH, Kyriakou DS
and Bouros D: Pleural effusions in hematologic malignancies. Chest.
125:1546–1555. 2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rodriguez J, Pereira A, Martínez JC, Conde
J and Pujol E: Pleural effusion in multiple myeloma. Chest.
105:622–624. 1994.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shirdel A, Attaran D, Ghobadi H and Ghiasi
T: Myelomatous pleural effusion. Tanaffos. 6:68–72. 2007.
|
|
14
|
Iqbal N, Tariq MU, Shaikh MU and Majid H:
Pleural effusion as a manifestation of multiple myeloma. BMJ Case
Rep. 12(bcr2016215433)2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Light RW: The light criteria: The
beginning and why they are useful 40 years later. Clin Chest Med.
34:21–26. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bataiile R, Jégo G, Robillard N,
Barillé-Nion D, Harousseau JL, Moreau P, Amiot M and
Pellat-Deceunynck C: The phenotype of normal, reactive and
malignant plasma cells. Identification of ‘many and multiple
myelomas’ and of new targets for myeloma therapy. Haematologica.
91:1234–1240. 2006.PubMed/NCBI
|
|
17
|
Touzeau C and Moreau P: How I treat
extramedullary myeloma. Blood. 127:971–976. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Varga C, Xie W, Laubach J, Ghobrial IM,
O'Donnell EK, Weinstock M, Paba-Prada C, Warren D, Maglio ME,
Schlossman R, et al: Development of extramedullary myeloma in the
era of novel agents: No evidence of increased risk with
lenalidomide-bortezomib combinations. Br J Haematol. 169:843–850.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Usmani SZ, Heuck C, Mitchell A, Szymonifka
J, Nair B, Hoering A, Alsayed Y, Waheed S, Haider S, Restrepo A, et
al: Extramedullary disease portends poor prognosis in multiple
myeloma and is over-represented in high-risk disease even in the
era of novel agents. Haematologica. 97:1761–1767. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Havelock T, Teoh R, Laws D and Gleeson F:
BTS Pleural Disease Guideline Group. Pleural procedures and
thoracic ultrasound: British thoracic society pleural disease
guideline 2010. Thorax. 65 (Suppl 2):ii61–ii76. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kamble R, Wilson CS, Fassas A, Desikan R,
Siegel DS, Tricot G, Anderson P, Sawyer J, Anaissie E and Barlogie
B: Malignant pleural effusion of multiple myeloma: Prognostic
factors and outcome. Leuk Lymphoma. 46:1137–1142. 2005.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kim YJ, Kim SJ, Min K, Kim HY, Kim HJ, Lee
YK and Zang DY: Multiple myeloma with myelomatous pleural effusion:
A case report and review of the literature. Acta Haematol.
120:108–111. 2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Palmer HE, Wilson CS and Bardales RH:
Cytology and flow cytometry of malignant effusions of multiple
myeloma. Diagn Cytopathol. 22:147–151. 2000.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Iannitto E, Minardi V and Tripodo C: Use
of intrapleural bortezomib in myelomatous pleural effusion. Br J
Haematol. 139:621–622. 2007.PubMed/NCBI View Article : Google Scholar
|