Introduction
In Japan, the prevalence of colorectal cancer
continues to increase with the westernization of lifestyles, and
this cancer has become the number one cause of death in women
(age-adjusted mortality by site) and the third leading cause of
death in men after lung cancer and gastric cancer (1). Patients with stage I/II colorectal
cancer, who do not have lymph node metastases, have a relatively
good prognosis, but in patients with stage III, who do have lymph
node metastases, the 5-year survival rate is only 60 to 70% and the
recurrence rate is 30 to 40% (2).
Patients with stage III/N1, who have 3 or fewer lymph node
metastases, have a better prognosis than patients with stage
III/N2, and their 5-year survival rate is about 70% with
postoperative multi-combination chemotherapy (2). However, patients with stage III/N2,
who have 4 or more lymph node metastases, have a significantly
higher recurrence rate, even after the same postoperative adjuvant
chemotherapy for the same duration. This higher recurrence rate may
be due to individual responses to anticancer drugs, but these drugs
are assumed to fail to control potential metastasis or recurrence
in patients with multiple lymph node metastases. Nevertheless, in
daily clinical practice physicians see patients with stage III/N2
who often survive for 5 years or longer without metastasis or
recurrence (low-risk group for recurrence).
In Japan, 6-month adjuvant chemotherapy with a
5-FU-based regimen such as 5FU/LV, FOLFIRI, FOLFOX, and CapeOX, is
recommended for stage III colorectal cancer after radical resection
(2). More intensive postoperative
adjuvant chemotherapy may be required for patients with a poor
prognosis (high-risk group for recurrence) who experience
metastasis or recurrence early after surgery. However, to date few
studies have reported on clinical indicators that can be used to
classify patients into a high-risk group for recurrence or to
determine their prognosis. Pathologists routinely compare and
examine distinctive properties of tumor morphology. A number of
reports indicate that in particular the degree of tissue
differentiation is the most important prognostic factor, and the
World Health Organization tumor classification standardizes
morphology in the most tumor-infiltrated region from well
differentiated to poorly differentiated/others (3). However, tumors have been reported to
consist of heterogeneous cell groups with multiple different
mutations, and regions with morphologically different degrees of
differentiation are known to coexist within the same lesion
(4). Therefore, the morphology of
metastatic lesions, such as in the lung, liver, and lymph nodes,
may reflect the severity of the disease more closely than the
morphology of the resected tissue sample from the primary lesion
and may thus contribute more to prognosis.
Accordingly, the purpose of this study was to
identify new clinical indicators of prognosis in patients with
stage III/N2 colorectal cancer by comparing clinicopathological
features of primary lesions and lymph node metastases to classify
patients into a group with poor prognosis (high-risk group for
recurrence) and one with good prognosis (low-risk group for
recurrence).
Patients and methods
Ethics approval
This study was approved by the Institutional Review
Board of Tokai University School of Medicine as a cumulative
retrospective study (IRB approval no. 20R-137, Kanagawa,
Japan).
Patients
From the 668 patients with colorectal cancer who
underwent radical resection at Tokai University Hachioji Hospital,
Tokyo, Japan, during the approximately 9 years from January 2006
until December 2014, we were able to include 53 patients (7.9%)
with 4 or more lymph node metastases (N2) and 5-year survival data
(Table I). All patients had
received 6 courses of postoperative adjuvant chemotherapy with
5FU/LV or FOLFOX/CapeOX and oral adjuvant chemotherapy with Cape or
UFT/UZEL for 6 months to 1 year (5). In the event of recurrence,
margin-negative (R0) resection until no residual cancer was present
was performed as quickly as possible, and after the procedure
patients were administered 6 courses of chemotherapy with a potent
new combination that included a camptothecin-11 drug, such as
FOLFIRI/IRIS (5). The 5-year
relapse-free survival (5Y-RFS) and 5-year overall survival (5Y-OS)
rates were calculated starting from the date of radical resection
as specified in the pathology records and electronic medical
records and ending with the date when recurrence was confirmed by
ultrasonography, computed tomography, or magnetic resonance
imaging; patients who died were censored at their date of death,
and surviving patients without recurrence were censored on December
31, 2019. The cause of death was based on the ICD10 (Ver.2013)
(6). Two patients died from other
causes of death. They were both male, in the SPF group, and were in
their 60 s. Their causes of death were malignant lymphoma and
cerebral stroke. Patients who were transferred to other hospitals
during the study period, e.g., because of relocation, were followed
up by contacting these hospitals by telephone.
 | Table IClinicopathological features of 53
patients with stage III/N2 colorectal cancer. |
Table I
Clinicopathological features of 53
patients with stage III/N2 colorectal cancer.
| Variable | Total cases
(n=53) | CLP group (n=16) | SPF group (n=37) | P-value |
|---|
| Sex, n (%) | | | | |
|
Male | 30 (56.6) | 8 (50.0) | 22 (59.5) | 0.737 |
|
Female | 23 (43.4) | 8 (50.0) | 15 (40.5) | |
| Median age at
operation, years (range) | 64 (38-90) | 67 (57-85) | 62 (38-90) | 0.123 |
| Tumor location, n
(%) | | | | |
|
Colon | 42 (79.2) | 14 (87.5) | 28 (75.7) | 0.471 |
|
Rectum | 11 (20.8) | 2 (12.5) | 9 (24.3) | |
| Macroscopic
classificationa, n
(%) | | | | |
|
Type 1 | 7 (13.2) | 4 (25.0) | 3 (8.1) | 0.091 |
|
Type 2 | 41 (77.4) | 12 (75.0) | 29 (78.4) | |
|
Type 3 | 5 (9.4) | 0 (0.0) | 5 (13.5) | |
| Tumor size, n
(%) | | | | |
|
≤20 mm | 3 (5.7) | 2 (12.5) | 1 (2.7) | 0.586 |
|
21-50
mm | 34 (64.2) | 10 (62.5) | 24 (64.9) | |
|
51-100
mm | 15 (28.3) | 4 (25.0) | 11 (29.7) | |
|
>100
mm | 1 (1.9) | 0 (0.0) | 1 (2.7) | |
| Pathological T
categorya, n (%) | | | | |
|
T2 | 7 (13.2) | 3 (18.8) | 4 (10.8) | 0.620 |
|
T3 | 37 (69.8) | 12 (75.0) | 25 (67.6) | |
|
T4a | 8 (15.1) | 1 (6.3) | 7 (18.9) | |
|
T4b | 1 (1.9) | 0 (0.0) | 1 (2.7) | |
| Histological type,
n (%) | | | | |
|
Well | 14 (26.4) | 6 (37.5) | 8 (21.6) | 0.372 |
|
Mod | 37 (69.8) | 10 (62.5) | 27 (73.0) | |
|
Por | 2 (3.8) | 0 (0.0) | 2 (5.4) | |
| Lymphatic invasion,
n (%) | | | | |
|
ly0 | 1 (1.9) | 0 (0.0) | 1 (2.7) | 0.203 |
|
ly1 | 30 (56.6) | 12 (75.0) | 18 (48.6) | |
|
ly2 | 16 (30.2) | 2 (12.5) | 14 (37.8) | |
|
ly3 | 6 (11.3) | 2 (12.5) | 4 (10.8) | |
| Venous invasion, n
(%) | | | | |
|
v0 | 19 (35.8) | 8 (50.0) | 11 (29.7) | 0.127 |
|
v1 | 27 (50.9) | 8 (50.0) | 19 (51.4) | |
|
v2 | 7 (13.2) | 0 (0.0) | 7 (18.9) | |
| Pathological N
categorya, n (%) | | | | |
|
N2a (LN:
4-6) | 34 (64.2) | 10 (62.5) | 24 (64.9) | >0.999 |
|
N2b (LN:
≥7) | 19 (35.8) | 6 (37.5) | 13 (35.1) | |
Immunohistochemical staining and
assessment
Lymph node specimens were stained with hematoxylin
and eosin (H&E), and formalin-fixed paraffin-embedded tissue
was prepared for cytokeratin immunostaining. For this
immunohistochemical staining, we used an autostainer (VENTANA
BenchMark XT, Roche Diagnostics, Indianapolis, IN, USA) and AE1/3
antibody (VENTANA I-VIEW Pan-cytokeratin kit, Roche Diagnostics),
in accordance with the manufacturer's protocol.
Patterns seen after H&E and immunohistochemical
staining were classified as either circumferential localization
patterns like a cystic mass (CLP) or scatter patterns like
fireworks (SPF). In CLP, cancer cell nests are relatively uniformly
and smoothly localized around the edges of the cortex and
paracortex of metastatic lymph nodes; the associated tumor
secretions and necrotizing substances cause the lymph nodes to
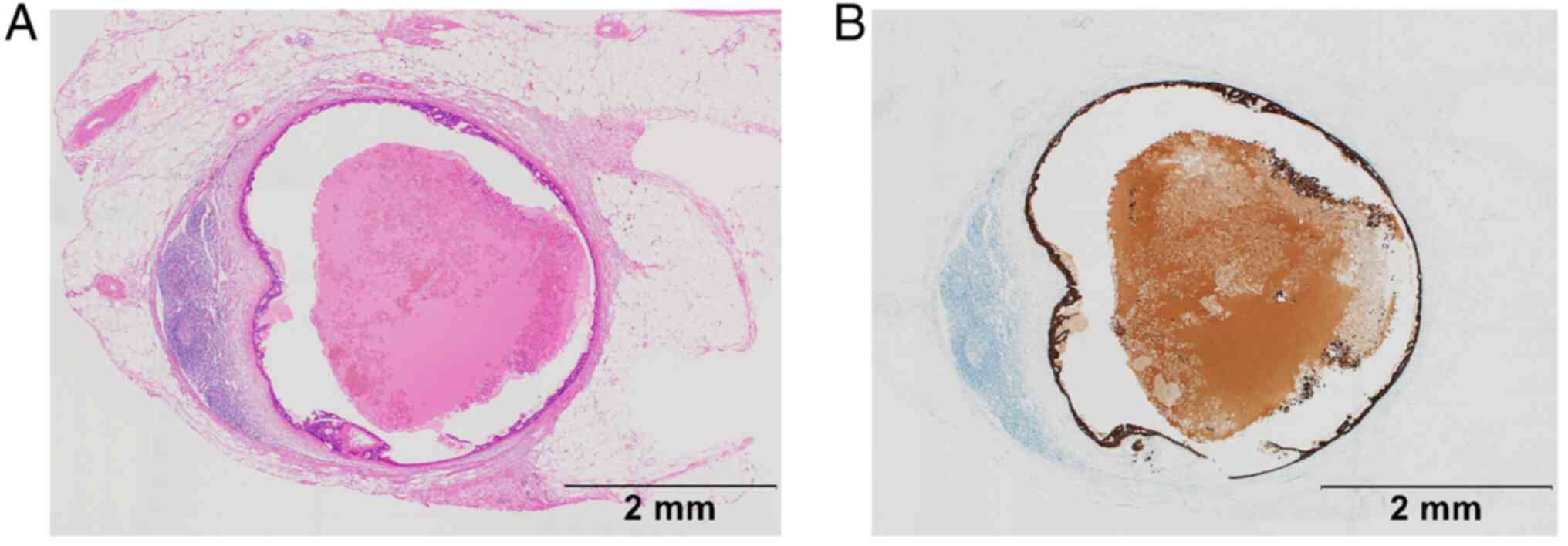
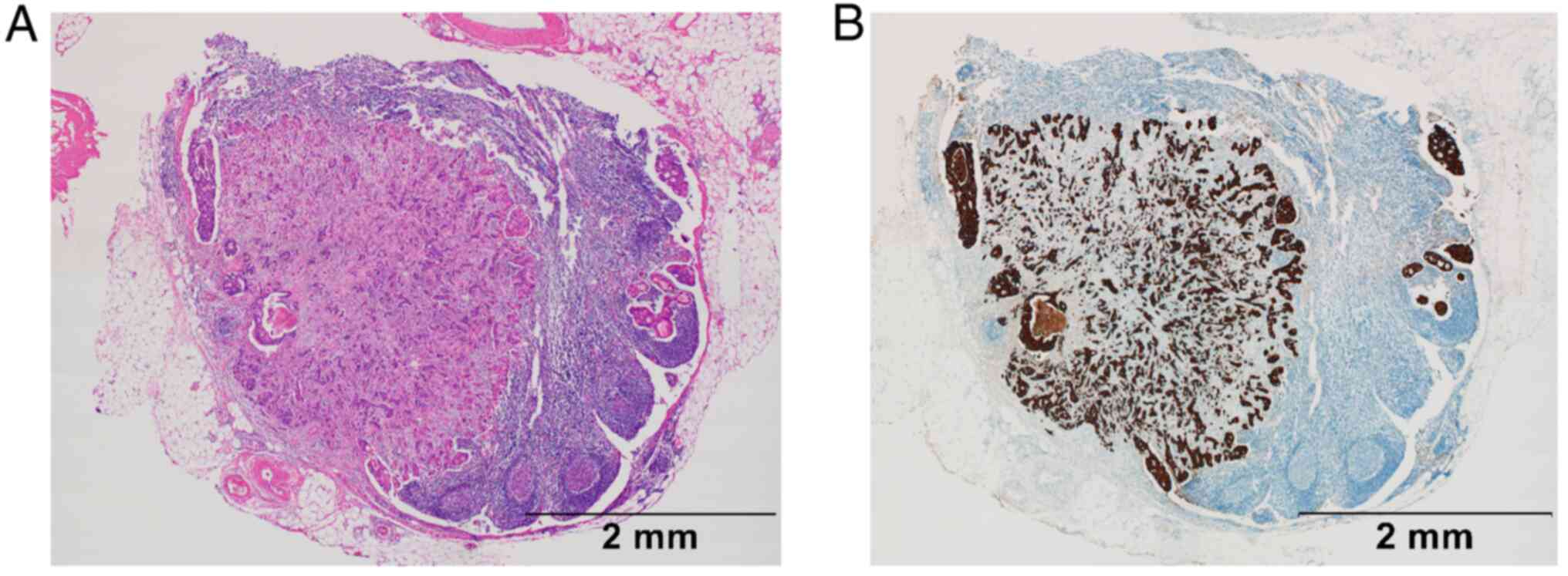
inflate and give them the appearance of a cyst (Fig. 1A and B). In SPF, vesicles diffusely infiltrate
the lymph node parenchyma and proliferate like fireworks with
unclear boundaries toward the inner medulla; they are sometimes
accompanied by fibroblast proliferation (Fig. 2A and B). The morphology of metastatic lymph
nodes was pathologically evaluated as showing a CLP or SPF scatter
pattern by using the following criteria: If the pattern was mostly
smooth but had some disturbances around the edges of the cortex or
paracortex and the disturbance was observed in more than one
quarter of the perimeter, the lymph node was classified as
belonging to the CLP group, and any area of infiltration outside
the lymph nodes was excluded from the assessment. If at least 1 of
4 or more metastatic lesions showed an SPF pattern, the lymph node
was classified as belonging to the SPF group. All assessments were
made by a surgeon (KT) and pathologist (HS), both of whom were
completely blinded to the clinical information.
We calculated the 5Y-RFS and 5Y-OS rates for the CLP
and SPF groups, and compared various clinicopathological features
between the groups. Furthermore, we compared the relationship
between the scatter pattern of the tumor cells in the metastatic
lymph nodes and the histological type (differentiation degree) of
the primary lesion. The degree of tumor differentiation of the
primary adenocarcinoma was defined on the basis of the largest cut
surface containing the most tumor-infiltrated area and was used to
classify the patients into 3 groups: Well group,
well-differentiated adenocarcinoma (n=14, 26.4%); Mod group,
moderately differentiated adenocarcinoma (n=37, 69.8%); and Por,
poorly differentiated adenocarcinoma (n=2, 3.8%) (Table I). Among colorectal cancers,
well-differentiated adenocarcinoma has a relatively good prognosis
compared with moderately and poorly differentiated lesions.
Therefore, because only 2 patients were classified into the Por
group, we combined the Mod and Por groups into a Mod+Por group
(n=39, 73.6%). Subsequently, we further classified patients into 4
groups (Well/CLP, Well/SPF, Mod+Por/CLP, and Mod+Por/SPF) and
calculated the 5Y-RFS and 5Y-OS rates of each group.
Statistical analysis
The 5Y-RFS and 5Y-OS rates were calculated by the
Kaplan-Meier method, and intergroup comparisons were performed with
the log-rank test. The hazard ratio and 95% CI were calculated with
the Cox proportional hazard regression model. For the analysis of
patient demographic factors in the CLP and SPF groups, the
Mann-Whitney U test (a nonparametric test) was used for age, and
the chi-squared test or Fisher's exact test was used for sex, lymph
node metastasis, primary tumor location, macroscopic
classification, depth of invasion, histological type, lymphatic
vessel invasion, and venous invasion. Each factor was assessed in
accordance with the criteria of the Japanese Classification of
Colorectal, Appendiceal, and Anal Carcinoma, 8th edition (7). In all tests, a P-value less than 0.05
was considered to be statistically significant. IBM SPSS Statistics
for Windows Version 25.0 (IBM Corp, Armonk, NY, USA) was used for
statistical analysis.
Results
Follow-up rate
In the follow-up investigation of all patients at 5
years, 30 patients were alive, 22 patients were dead, and 1 patient
was lost to follow up, corresponding to a follow-up rate of
98.1%.
Scatter pattern
On the basis of the scatter pattern in the lymph
nodes, 16 patients (30.2%) were classified into the CLP group and
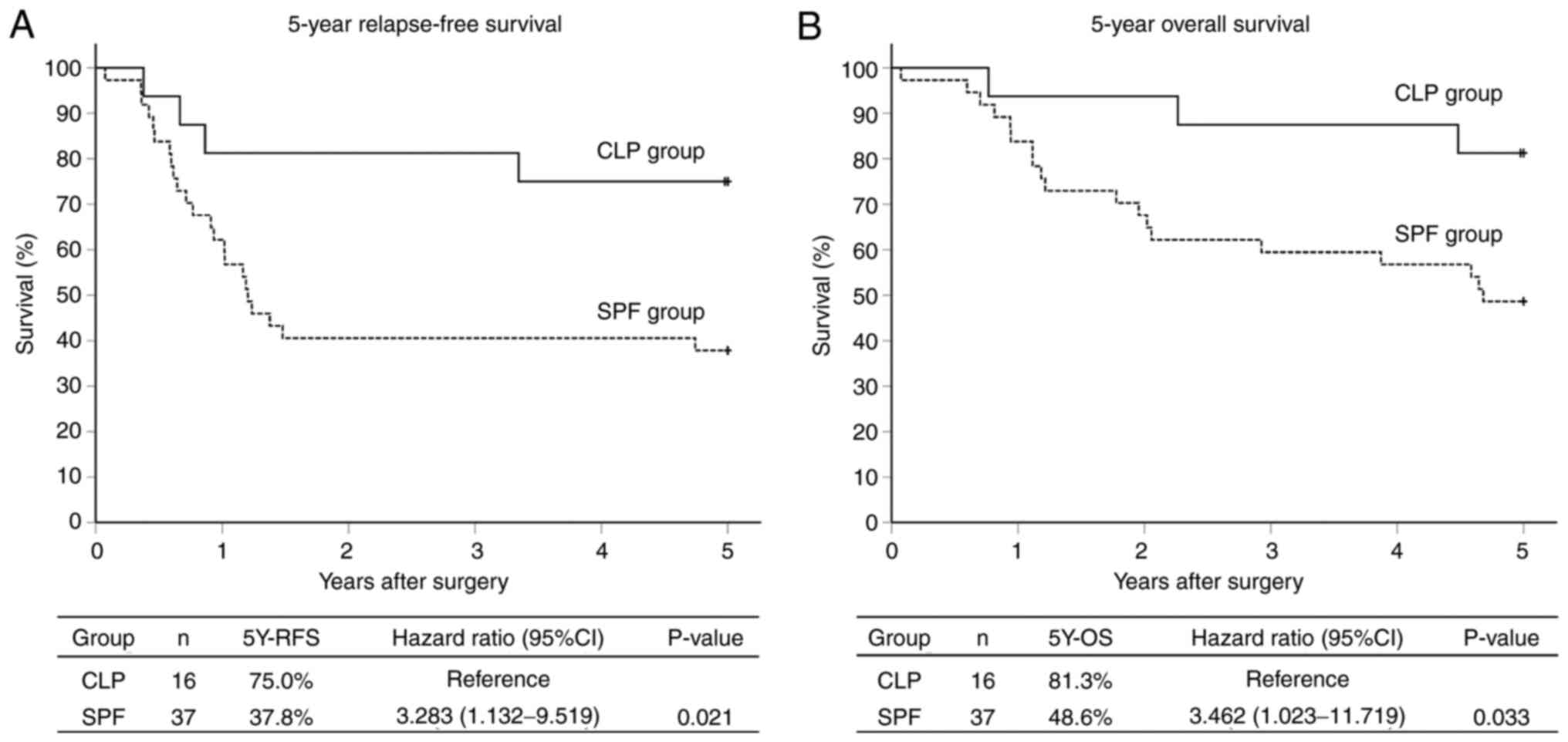
37 patients (69.8%) into the SPF group. In the CLP and SPF groups,
the 5Y-RFS rates were 75.0 and 37.8%, respectively (P=0.021; hazard
ratio, 3.283; 95% CI, 1.132-9.519) (Fig. 3A), and the 5Y-OS rates were 81.3
and 48.6%, respectively (P=0.033; hazard ratio, 3.462; 95% CI,
1.023-11.719) (Fig. 3B). Although
the prognosis of the SPF group was significantly worse than that of
the CLP group, the comparison of clinicopathological
characteristics between these 2 groups showed no significant
differences (Table I).
Scatter patterns and tumor
differentiation
The 4 groups formed on the basis of the scatter
patterns in the metastatic lymph nodes and the degree of tumor
differentiation of the primary lesion consisted of the following
numbers of patients: Well/CLP, n=6; Well/SPF, n=8; Mod+Por/CLP,
n=10; and Mod+Por/SPF, n=29. The results of the comparisons of the
5Y-RFS rates between the 2 Well groups and the 2 Mod+Por groups
were as follows: 66.7% (Well/CLP) vs. 25.0% (Well/SPF) (P=0.293,
hazard ratio, 2.361; 95% CI, 0.475-11.729) and 80.0% (Mod+Por/CLP)
vs. 41.4% (Mod+Por/SPF) (P=0.052; hazard ratio, 4.295; 95% CI,
0.990-18.641) (Fig. 4). The 5Y-OS
rates between the 2 Well groups and the 2 Mod+Por groups were 66.7%
vs. 50.0% (P=0.552; hazard ratio, 1.674; 95% CI, 0.306-9.142) and
90.0% vs. 48.3% (P=0.059; hazard ratio, 7.018; 95% CI,
0.925-53.217) (Fig. 5).
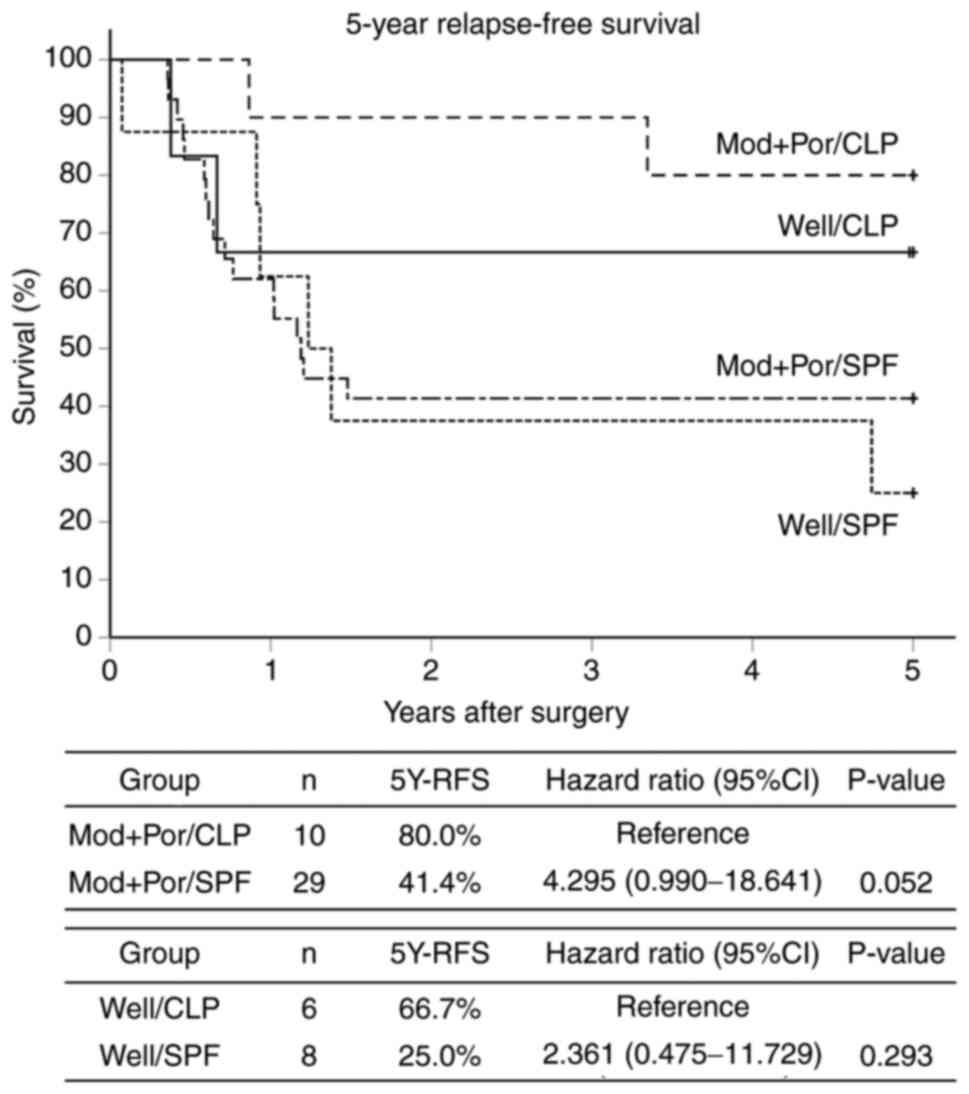 | Figure 4Kaplan-Meier curves 5Y-RFS by
histological type of primary tumors and scatter patterns in
metastatic lymph nodes. Patients were divided into four subgroups
on the basis of the scatter pattern (CLP or SPF) and the
differentiation of the primary lesion (Well or Mod+Por) as follows:
Well/CLP group (n=6), Well/SPF group (n=8), Mod+Por/CLP group
(n=10) and Mod+Por/SPF group (n=29). The 5Y-RFS rates in the
Well/CLP vs. Well/SPF groups were 66.7 vs. 25.0%, respectively
(P=0.293; hazard ratio, 2.361; 95% CI, 0.475-11.729), and those in
the Mod+Por/CLP vs. Mod+Por/SPF group were 80.0 vs. 41.4%,
respectively (P=0.052; hazard ratio, 4.295; 95% CI, 0.990-18.641).
5Y-RFS, 5-year relapse-free survival; 95% CI, 95% confidence
interval; CLP, circumferential localization patterns like a cystic
mass surrounded by the lymph node cortex/paracortex; SPF, scatter
patterns like fireworks into the intraparenchymal lymph node
medulla; Well, well-differentiated adenocarcinoma; Mod+Por,
moderately or poorly differentiated adenocarcinoma. |
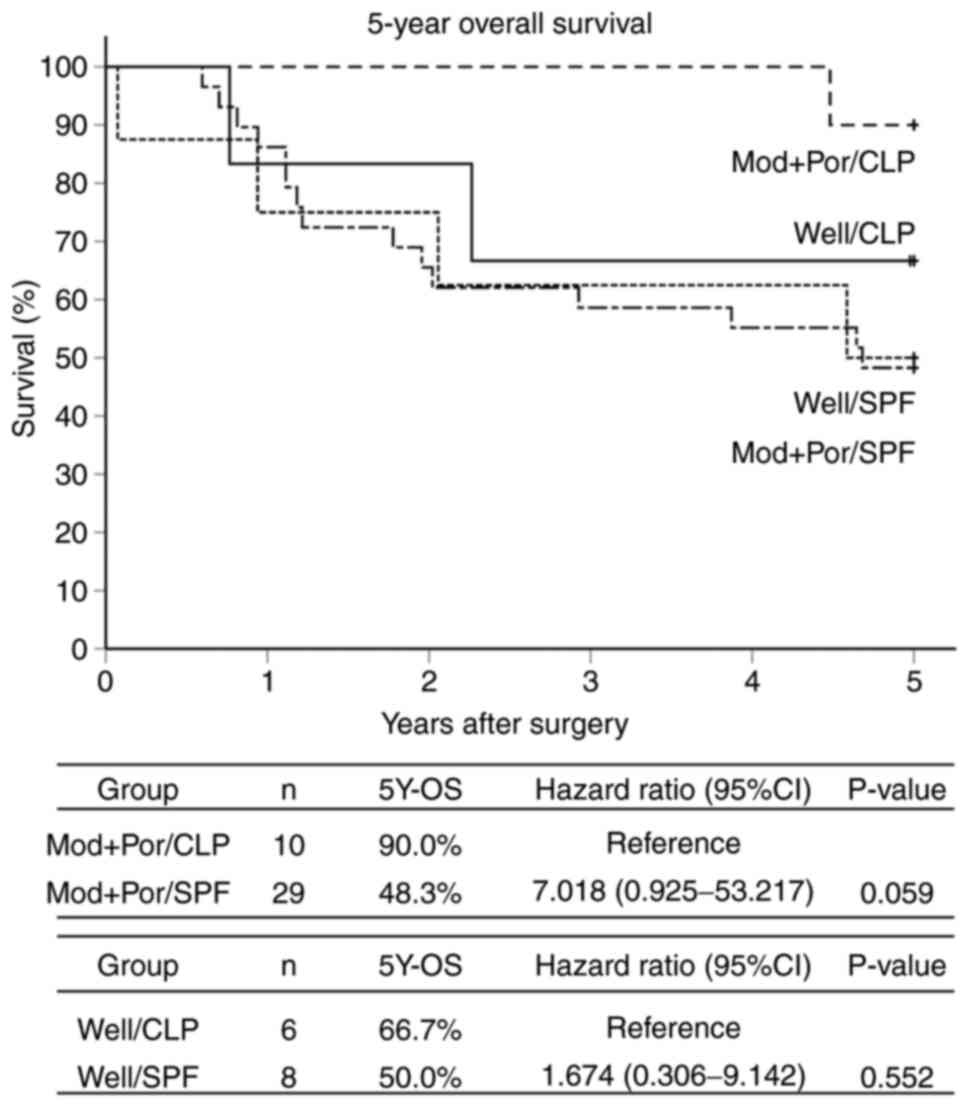 | Figure 5Kaplan-Meier curves 5Y-OS by
histological type of primary tumors and scatter patterns in
metastatic lymph nodes. Patients were divided into four subgroups
on the basis of the scatter pattern (CLP or SPF) and the
differentiation of the primary lesion (Well or Mod+Por) as follows:
Well/CLP group (n=6), Well/SPF group (n=8), Mod+Por/CLP group
(n=10) and Mod+Por/SPF group (n=29). The 5Y-OS rates in the
Well/CLP vs. Well/SPF groups were 66.7 vs. 50.0%, respectively
(P=0.552; hazard ratio, 1.674; 95% CI, 0.306-9.142); and those in
the Mod+Por/CLP vs. Mod+Por/SPF group were 90.0 vs. 48.3%,
respectively (P=0.059; hazard ratio, 7.018; 95% CI, 0.925-53.217).
5Y-OS, 5-year overall survival; 95% CI, 95% confidence interval;
CLP, circumferential localization patterns like a cystic mass
surrounded by the lymph node cortex/paracortex; SPF, scatter
patterns like fireworks into the intraparenchymal lymph node
medulla; Well, well-differentiated adenocarcinoma; Mod+Por,
moderately or poorly differentiated adenocarcinoma. |
Discussion
This study aimed to identify new clinical indicators
of prognosis in patients with stage III/N2 colorectal cancer by
comparing clinicopathological features of primary lesions and lymph
node metastases. We agree that the sample size was limited, and a
larger sample size would be preferable. However, this is a
single-center analysis, and the number of patients at the study
stage was limited, approximately 15 patients annually. Accordingly,
it was difficult to include additional patients. Instead, we
attempted to improve the follow-up rate. We expect that we will
obtain more patients for analysis in the future. The results showed
that patients with a CLP scatter pattern in lymph node metastases
have a low risk for recurrence and relatively good prognosis,
whereas patients with an SPF scatter pattern have a high risk for
recurrence and a relatively poor prognosis.
In Japan, patients with stage III colorectal cancer
who undergo radical resection, such as total mesocolic excision or
total mesorectal excision, have a relatively good prognosis
compared with patients in Europe and the United States (1). Patients with stage III colorectal
cancer are generally treated with a 6-month postoperative
5-FU-based adjuvant chemotherapy with dexamethasone, imatinib, and
vincristine; however, potent chemotherapy such as oxaliplatin
(L-OHP) administered by the intravenous route is recommended for
patients at high risk for recurrence. The National Comprehensive
Cancer Network Clinical Practice Guidelines in Oncology also
recommend regimens such as FOLFOX, FLOX, and CapeOX, including
L-OHP, for postoperative stage III colon cancer (8,9).
However, in a relatively large number of patients these potent
chemotherapies increase the frequency and duration of side effects
such as peripheral neuropathy, and the guidelines from the Japanese
Society for Cancer of the Colon and Rectum (2) do not include supportive evidence
recommending these chemotherapies for all stage III patients.
Currently, the decision about chemotherapy is often left to the
physician and is based on the wishes of the patient and their
family; when deciding on treatment options, physicians consider the
patient's age, performance status, compliance, the presence or
absence of comorbidities, and the benefits and disadvantages for
the patient.
In our department, we proactively give FOLFOX/CapeOX
as an adjuvant chemotherapy after the surgical procedure in
patients with stage III/N2 colorectal cancer who are aged 75 years
or younger and have a performance status of 0 to 2. In accordance
with this, 37 (69.8%) of 44 patients aged 75 years or younger in
the target group of this study were given chemotherapy that
included L-OHP. However, the results of this study indicated that
the prognosis of the SPF group was extremely poor. Accordingly, it
may be necessary to conduct a study accumulating data on patients
at high risk of recurrence who are given more potent
multi-combination chemotherapies used for stage IV colorectal
cancer, including molecular targeted drugs such as vascular
endothelial growth factor/epidermal growth factor receptor
inhibitors, from the early postoperative period. We also consider
that investigation of data from multiple institutions and large
geographic areas is necessary.
Various studies on factors for poor prognosis in
colorectal cancer identified patients with a poor prognosis by
various methods, including pathomorphological lymph node evaluation
(10,11), the number of lymph node dissections
(12,13), the ratio of the number of
metastatic lymph nodes to the number of dissected lymph nodes
(13-16),
and tumor deposits (17-19).
Some also evaluated malignancy by performing immunohistochemical
staining to detect micrometastases of lymph nodes that cannot be
shown by H&E staining (20-23).
At our hospital, we have been using cytokeratin immunostaining
(AE1/3) to detect free cancer cells floating in the lymph sinusoids
in dissected lymph nodes from patients with various types of
cancer, such as colorectal cancer, gastric cancer, and lung cancer,
and have often reported on the relationship with prognosis
(24-30).
We have used immunostaining to detect free cancer cells, which are
difficult to evaluate by H&E staining, and have found that
these cells are useful for identifying a group with poor prognosis
among patients with a high recurrence rate (24-31).
However, recent studies reported that these free cancer cells are
becoming difficult to detect even in patients with more advanced
stage III cancers, and researchers assume that these cells are
being efficiently eliminated by potent chemotherapies being used
recently. Because some patients have a poor prognosis whatever the
stage of their cancer, we need to search for new factors that can
predict resistance to chemotherapy.
In this study, we examined whether prognosis can be
determined on the basis of the assumption that metastatic lesions
may be more useful for prognosis than the primary lesion because of
the heterogeneous properties of the latter. Metastatic lesions can
indicate the potential to metastasize, infiltrate, and proliferate
in other tissues, as well as the potential for cancer cells to
remain in the body after the primary lesion is surgically removed.
By applying our immunostaining method on lymph node tissue, we
performed a preliminary analysis in more than 100 patients with
stage III/N1, III/N2, and stage IV colorectal cancer and found that
metastatic lymph nodes can be morphologically classified into 2
main groups: CLP and SPF. Also, we were able to unify and stabilize
evaluations among diagnosticians by using cytokeratin
immunostaining (AE1/3) to clearly visualize the morphology of the
metastases, including micrometastases, in tissue from metastatic
lymph nodes identified by H&E staining.
In stage III colorectal cancer, studies have found a
large difference in the prognosis of patients with stage N1 and
those with stage N2. Furthermore, the survival curve of some
patients with stage N2 was reported to be similar to that observed
in patients with stage IV cancer (14). In our preliminary analysis, 93
patients (13.9%) had stage III/N1 cancer; 43 of these patients
(46.2%) were classified into the CLP group and 50 patients (53.8%)
into the SPF group. The 5Y-RFS rates were 66.0 and 62.8% (P=0.709),
respectively, and the 5Y-OS rates were 76.0 and 74.4% (P=0.761),
respectively (data not shown). The lack of a statistically
significant difference between the CLP and SPF groups was thought
to be due to the findings that many patients with stage III/N1
cancer have a good prognosis, the survival rate of the population
is higher than that of the population with stage N2 cancer, and
recurrence is rarely observed in patients with stage N1 cancer.
Also, the worse prognosis of patients with stage N2 was considered
to be due to the higher tumor occupation and tumor volume in lymph
node metastases and the greater number of lymph node metastases
compared with patients with stage N1.
In general, more than 70 to 80% of the primary
lesions of colorectal cancer are histologically classified as well
or moderately differentiated adenocarcinoma; therefore,
hematogenous, distant metastases or recurrence in the lung or liver
is fatal in many of these patients, and predicting prognosis by the
histological type of the primary lesion is considered to be
difficult (32). When we compared
the differentiation of the primary lesions and the metastatic lymph
node tumor cells in our study, most of the primary lesions (51/53
patients, 96.2%) were classified as differentiated adenocarcinoma
(Well, n=14; Mod, n=37). In the SPF group, evidence of poor
differentiation, which was equivalent to poorly differentiated
adenocarcinoma, was observed in 57.1% (8/14 patients), and in many
cases the histological types of the primary lesion and metastatic
lymph node tumor did not match. Therefore, we assumed that the
metastatic properties of the primary tumor tissue caused the
metastatic lesion to differ from the primary tumor, depending on
its site and localization, and decreased the amount of
differentiation in the metastases. In our study, 8 patients in the
Well/SPF group had a poor prognosis with a 5Y-RFS rate of 25.0% and
a 5Y-OS rate of 50%, even though well-differentiated lesions
usually have a relatively good prognosis. Therefore, these findings
suggest that evaluating metastatic lymph nodes can reveal a poor
prognosis that would not be assumed by analyzing histological type
alone.
Compared with the CLP pattern, the SPF pattern was
assumed to indicate that the tumor was more destructive to tissues
and had higher infiltration and proliferation capacities. The
malignancy in the patients with multiple lymph node metastases
(Total number ≥4) was assumed to be closely related to the lower
degree of tissue differentiation (33,34).
We assumed that the groups of tumor cells present in these
metastatic lesions remained in the body after surgical resection of
the primary lesion and that they were likely to induce metastasis
and recurrence. Therefore, molecular targeted therapy may be
effective if given after searching for genes such as RAS and BRAF
in tumor cells from these metastatic lesions. In the CLP group, we
assumed that the tumors had metastasis and engraftment capacities
but lacked the infiltration and proliferation capacities that
increase tumor volume in the lymph nodes and cause poor
differentiation. As mentioned above, the degree of scattering at
metastatic loci predicts poor prognosis and is considered to be
affected by tumor immunity (35).
Thus, in patients with the CLP pattern, limited portions of the
gland ducts become filled with secretions and necrotic substances,
expand, and become cystic. We expect that in the future this
phenomenon of cystic lymph nodes will be clarified by genetic and
molecular biological studies as a clinical indicator of low risk
for recurrence.
Currently, we are comparing CLP and SPF groups in
patients with other gastrointestinal cancers, such as esophageal
cancer and gastric cancer, which are also likely to metastasize to
the lymph nodes, and we are trying to eliminate the difference in
diagnosis among examiners by simply classifying the metastases by
H&E staining to quantify them more easily. Because the lymph
nodes contain an extremely large number of lymphocytes with a
normal genome, the amount of tumor-derived genome is likely to be
high. Therefore, in the future a technology is needed that can
extract the genomic information of tumor cells by examining
potentially fatal distant metastases in the lung, liver, and other
organs.
In conclusion, we performed a pathomorphological
evaluation of the relationship between metastatic lymph nodes of
patients with stage III/N2 colorectal cancer and prognosis by using
cytokeratin immunostaining. The CLP group was found to be at low
risk for recurrence and to have a relatively good prognosis;
however, the SPF group was found to be at high risk for recurrence
and to have a poor prognosis, indicating that patients with this
pattern on immunostaining require more potent multi-combination
chemotherapy from the early postoperative period.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TK, MM and SHi performed the research for the
present study, contributed to the data analysis, and wrote the
manuscript. SHi and MM designed the protocol for the present study,
provided surgical advice and supervised the present study. KK, DY,
SU, SHa, TakaT, TS and TakuT analyzed the data. TK and SHi
diagnosed and assessed the pathological findings. EN acquired the
surgical data. TK, KK and SHi confirm the authenticity of all the
raw data. TakuT supervised the study and critically reviewed the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Ethics approval was provided by the Research and
Study Program of Tokai University Educational System General
Research Organization (IRB approval no. 20R-137). Written informed
consent to participate was obtained from all patients.
Patient consent for publication
Written informed consent for publication of the
present study was obtained from the patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ministry of Health, Labor and Welfare:
Vital Statistics Japan. Ministry of Health, Labor and Welfare,
Tokyo, 2019. https://ganjoho.jp/reg_stat/statistics/dl/index.html#mortality
(In Japanese).
|
|
2
|
Hashiguchi Y, Muro K, Saito Y, Ito Y,
Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M,
et al: Japanese society for cancer of the colon and rectum (JSCCR)
guidelines 2019 for the treatment of colorectal cancer. Int J Clin
Oncol. 25:1–42. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
WHO Classification of Tumours Editorial
Board: Digestive System Tumours: WHO Classification of Tumours.
IARC Publications, Lyon, 2019.
|
|
4
|
McGranahan N and Swanton C: Clonal
heterogeneity and tumor evolution: Past, present, and the future.
Cell. 168:613–628. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mukai M, Kishima K, Fukumitsu H, Sekido Y,
Izumi H, Hoshikawa T, Tajima T, Tobita K, Sadahiro S, Yasuda S and
Ogoshi K: Is the T1/2N1 (≤3 nodes) category actually stage IIIA
(TNM)/IIIa (Japanese classification) in patients with primary
colorectal cancer? Oncol Rep. 26:209–214. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
World Health Organization (WHO):
International statistical classification of diseases and health
related problems. ICD-10. WHO, Geneva, 2013.
|
|
7
|
Japanese Society for Cancer of the Colon
and Rectum (JSCCR): Japanese Classification of Colorectal
Carcinoma. Kanehara & Co., Ltd., Tokyo, pp10-32, 2013.
|
|
8
|
National Comprehensive Cancer Network
(NCCN): NCCN clinical practice guidelines in oncology rectal
cancer. Version 3.2017. NCCN, Plymouth Meeting, PA, 2017.
|
|
9
|
National Comprehensive Cancer Network
(NCCN): NCCN clinical practice guidelines in oncology colon cancer.
Version 2.2017, 2017. NCCN, Plymouth Meeting, PA, 2017.
|
|
10
|
Tsakraklides V, Wanebo HJ, Sternberg SS,
Stearns M and Good RA: Prognostic evaluation of regional lymph node
morphology colorectal cancer. Am J Surg. 129:174–180.
1975.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Schofield JB, Mounter NA, Mallett R and
Haboubi NY: The importance of accurate pathological assessment of
lymph node involvement in colorectal cancer. Colorectal Dis.
8:460–470. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shanmugam C, Hines RB, Jhala NC, Katkoori
VR, Zhang B, Posey JA Jr, Bumpers HL, Grizzle WE, Eltoum IE, Siegal
GP and Manne U: Evaluation of lymph node numbers for adequate
staging of stage II and III colon cancer. J Hematol Oncol.
4(25)2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jiang K, Zhu Y, Liu Y, Ye Y, Xie Q, Yang X
and Wang S: Lymph node ratio as an independent prognostic indicator
in stage III colorectal cancer: especially for fewer than 12 lymph
nodes examined. Tumour Biol. 35:11685–11690. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Derwinger K, Carlsson G and Ekman T:
Defining stage III disease in colorectal cancer-aspects on
treatment and evaluation of survival. J Surg Oncol. 102:424–427.
2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang LP, Wang HY, Cao R, Zhu C and Wu XZ:
Proposal of a new classification for stage III colorectal cancer
based on the number and ratio of metastatic lymph nodes. World J
Surg. 37:1094–1102. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wong KP, Poon JT, Fan JK and Law WL:
Prognostic value of lymph node ratio in stage III colorectal
cancer. Colorectal Dis. 13:1116–1122. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Basnet S, Lou QF, Liu N, Rana R, Shah A,
Khadka M, Warrier H, Sigdel S, Dhakal S, Devkota A, et al: Tumor
deposit is an independent prognostic indicator in patients who
underwent radical resection for colorectal cancer. J Cancer.
9:3979–3985. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Nagtegaal ID, Knijn N, Hugen N, Marshall
HC, Sugihara K, Tot T, Ueno H and Quirke P: Tumor deposits in
colorectal cancer: Improving the value of modern staging-a
systematic review and meta-analysis. J Clin Oncol. 35:1119–1127.
2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Nagayoshi K, Ueki T, Nishioka Y, Manabe T,
Mizuuchi Y, Hirahashi M, Oda Y and Tanaka M: Tumor deposit is a
poor prognostic indicator for patients who have stage II and III
colorectal cancer with fewer than 4 lymph node metastases but not
for those with 4 or more. Dis Colon Rectum. 57:467–474.
2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Laso CA, González JJ, Fresno F, Azcano E,
Sanz L and Navarrete F: Prognostic value of micrometastases in
esophageal and colorectal carcinoma (a clinical experience).
Hepatogastroenterology. 51:964–967. 2004.PubMed/NCBI
|
|
21
|
Mescoli C, Albertoni L, Pucciarelli S,
Giacomelli L, Russo VM, Fassan M, Nitti D and Rugge M: Isolated
tumor cells in regional lymph nodes as relapse predictors in stage
I and II colorectal cancer. J Clin Oncol. 30:965–971.
2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Onaka T, Tsunoda A, Watanabe M, Nakao K,
Takimoto M and Kusano M: The pathway of isolated tumor cells from
tumor budding in stage II colorectal cancer detected by
immunohistochemistry. Showa Univ J Med Sci. 21:25–36. 2009.
|
|
23
|
Lee MR, Hong CW, Yoon SN, Lim SB, Park KJ,
Lee MJ, Kim WH and Park JG: Isolated tumor cells in lymph nodes are
not a prognostic marker for patients with stage I and II colorectal
cancer. J Surg Oncol. 93:13–19. 2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mukai M, Sato S, Komatsu N, Nishida T,
Shiba K, Ito I, Nakasaki H and Makuuchi H: Correlation between
occult neoplastic cells in the lymph node sinuses and recurrence in
patients with Dukes' C colorectal cancer. Oncol Rep. 10:1165–1169.
2003.PubMed/NCBI
|
|
25
|
Mukai M, Sato S, Komatsu N, Nishida T,
Shiba K, Ito I, Nakasaki H and Makuuchi H: Correlation between
occult neoplastic cells in the lymph node sinuses and recurrence in
patients with curatively resected Dukes' B colorectal cancer. Oncol
Rep. 10:1177–1181. 2003.PubMed/NCBI
|
|
26
|
Mukai M, Sato S, Nishida T, Komatsu N,
Shiba K, Nakasaki H and Makuuchi H: Selection criteria for high
risk and low risk groups of recurrence and metastasis in patients
with primary colorectal cancer. Oncol Rep. 10:1753–1758.
2003.PubMed/NCBI
|
|
27
|
Mukai M, Sato S, Nakasaki H, Tajima T,
Saito Y, Nishiumi N, Iwasaki M, Tokuda Y, Ogoshi K, Inoue H and
Makuuchi H: Occult neoplastic cells in the lymph node sinuses and
recurrence of primary breast, lung, esophageal, and gastric cancer.
Oncol Rep. 11:81–84. 2004.PubMed/NCBI
|
|
28
|
Mukai M: Occult neoplastic cells and
malignant micro-aggregates in lymph node sinuses: Review and
hypothesis. Oncol Rep. 14:173–175. 2005.PubMed/NCBI
|
|
29
|
Sekido Y, Mukai M, Kishima K, Tajima T,
Hoshikawa T, Nakamura M, Nakamura N and Ogoshi K: Occult neoplastic
cells in the lymph node sinuses and recurrence/metastasis of stage
II/Dukes' B colorectal cancer. Oncol Rep. 25:69–73. 2011.PubMed/NCBI
|
|
30
|
Sekido Y, Mukai M, Kishima K, Tajima T,
Hoshikawa T, Nakamura M, Nakamura N and Ogoshi K: Occult neoplastic
cells in the lymph node sinuses and recurrence/metastasis of stage
III/Dukes' C colorectal cancer. Oncol Rep. 25:915–919.
2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Nakamura Y, Mukai M, Hiraiwa S, Kishima K,
Sugiyama T, Tajiri T, Yamada S and Iwazaki M: Free-floating cancer
cells in lymph node sinuses of hilar lymph node-positive patients
with non-small cell lung cancer. Mol Med Rep. 18:1081–1087.
2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Romiti A, Roberto M, Marchetti P, Di Cerbo
A, Falcone R, Campisi G, Ferri M, Balducci G, Ramacciato G, Ruco L
and Pilozzi E: Study of histopathologic parameters to define the
prognosis of stage II colon cancer. Int J Colorectal Dis.
34:905–913. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Compton CC: Colorectal carcinoma:
Diagnostic, prognostic, and molecular features. Mod Pathol.
16:376–388. 2003.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Gunderson LL, Jessup JM, Sargent DJ,
Greene FL and Stewart A: Revised tumor and node categorization for
rectal cancer based on surveillance, epidemiology, and end results
and rectal pooled analysis outcomes. J Clin Oncol. 28:256–263.
2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Shankaran V, Ikeda H, Bruce AT, White JM,
Swanson PE, Old LJ and Schreiber RD: IFNgamma and lymphocytes
prevent primary tumour development and shape tumour immunogenicity.
Nature. 410:1107–11. 2001.PubMed/NCBI View
Article : Google Scholar
|