Introduction
In 2018, cancers of the head and neck accounted for
approximately 3.7% of new cancer diagnoses in the United States,
with about 64,700 new cases and 13,700 deaths (1). These cancers may occur at any site in
the head and neck, with some of the more common sites including the
oral cavity, pharynx, and larynx. Tobacco and alcohol abuse have
historically been the most common risk factors associated with head
and neck cancers, however cases associated with human
papillomavirus (HPV) infection have been increasing (2). Up to 90% of head and neck cancers are
of squamous cell carcinoma (SCC) histology, and over 90% of those
have overexpression of epidermal growth factor receptor (EGFR)
(1). While the majority of
patients present with early stage or locally advanced disease, up
to 15% of patients present with distant metastases (3). Prognosis in the recurrent or
metastatic (R/M) setting is poor, with a median overall survival
(OS) of less than one year (4).
Until recently, chemotherapy was used in the
first-line setting for R/M disease. The preferred regimen is based
on results of the EXTREME trial (1,5). In
this study, 442 patients with R/M SCCHN were randomized to a
standard chemotherapy regimen containing a platinum (cisplatin or
carboplatin) plus 5-fluorouracil (5-FU), with or without cetuximab,
an anti-EGFR monoclonal antibody. Patients in the
cetuximab-containing arm had significantly improved OS (10.1 months
vs. 7.4 months, P=0.04) and progression free survival (PFS, 5.6
months vs. 3.3 months, P<0.001) compared to the chemotherapy
alone arm. While this was the first study to demonstrate
significantly improved OS in this patient population, the EXTREME
regimen is not without adverse effects. Patients in the cetuximab
arm experienced significantly higher rates of sepsis (including
septic shock) and skin reactions compared to patients receiving
chemotherapy alone. Despite FDA approval and a Category 1
recommendation in the NCCN guidelines, use of the platinum, 5-FU,
cetuximab regimen is limited in clinical practice due to toxicity.
The KEYNOTE-048 trial introduced immunotherapy into the first-line
setting for R/M SCCHN. This trial assessed 882 patients who
received either pembrolizumab monotherapy, standard chemotherapy
with the EXTREME regimen, or pembrolizumab plus a platinum and
5-FU. Pembrolizumab monotherapy and pembrolizumab plus chemotherapy
led to a significant improvement in OS with a median of 11.5 and 13
months, respectively, compared to the EXTREME regimen at 10.7
months. Grade 3-5 adverse events were seen in 54.7% with
pembrolizumab, 85.1% with pembrolizumab plus chemotherapy, and
83.3% with the EXTREME regimen.
An alternative to the EXTREME regimen for first-line
treatment of R/M SCCHN is a regimen containing a platinum, taxane,
plus cetuximab. In the TPEx trial, 54 patients received four cycles
of cisplatin, docetaxel, and cetuximab every 3 weeks followed by
cetuximab maintenance therapy every two weeks thereafter (6). Overall response rates were 44.4% with
a median PFS of 6.2 months and a median OS of 14 months. Although
these results were promising, in clinical practice carboplatin and
paclitaxel are often substituted for cisplatin and docetaxel,
respectively, due to enhanced tolerability (1). Narveson et al is the only
study that evaluated the weekly administration schedule of
carboplatin and paclitaxel (3 weeks on, 1 week off for a maximum of
six cycles, with weekly cetuximab continued during and after
completion of chemotherapy) in 41 patients with very advanced or
metastatic head and neck cancer (7). Partial responses were achieved in 37%
of patients, with a median PFS of 4.6 months and median OS of 5.25
months. Although PFS and OS were lower in this study compared to
the TPEx trial, the authors commented that 96% of patients had an
Eastern Cooperative Oncology Group (ECOG) performance status of 1
or 2, indicating an inferior baseline performance status compared
to patients typically eligible for clinical trials.
Standard practice at our institution is to
administer paclitaxel, carboplatin, and cetuximab (PCC) as the
first/second-line regimen of chemotherapy choice in the R/M setting
for patients with SCCHN, with chemotherapy administered weekly or
every 3 weeks depending on patient's age, performance status,
comorbidities, and physician preference. The weekly regimen studied
by Narveson et al (7) uses
higher doses given 3 weeks on, 1 week off. The break was included
to help limit toxicity. Our institution's protocol uses lower doses
of carboplatin and paclitaxel given weekly with no break to limit
toxicity while providing a convenient schedule as cetuximab is
administered weekly. Therefore, the primary objective of this study
was to evaluate safety and toxicity outcomes in patients receiving
weekly vs. every 3 weeks PCC. Secondary objectives were to compare
median weekly relative dose intensity, PFS, and OS between
groups.
Materials and methods
Treatment protocols
This was a retrospective, single-center, cohort
study evaluating patients who had received treatment with weekly or
every 3 weeks paclitaxel, carboplatin, and cetuximab. This study
included patients with a diagnosis of R/M SCCHN who were 18 years
of age and older. Patients received at least 3 weeks (one cycle) of
the weekly regimen of PCC (paclitaxel 45 mg/m2 IV weekly
+ carboplatin AUC 1.5 IV weekly + cetuximab 400 mg/m2 IV
week 1, then 250 mg/m2 IV weekly thereafter) or one
cycle of the every 3 weeks regimen of PCC (paclitaxel 175
mg/m2 IV every 3 weeks + carboplatin AUC 5 IV every 3
weeks + cetuximab 400 mg/m2 IV week 1, then 250
mg/m2 IV weekly thereafter).
Study population
Patients who were prisoners, pregnant, diagnosed
with nasopharyngeal carcinoma or salivary gland carcinoma, changed
from paclitaxel to docetaxel, or received PCC as an induction
regimen prior to radiation or surgery were excluded. All eligible
patients treated at The James Cancer Hospital at The Ohio State
University from January 1, 2013 to July 31, 2018 were included.
Patients were stratified by age (≥65 or <65 years); performance
status (ECOG 0-1 vs. ECOG 2-3); line of therapy (1st vs. 2nd and
beyond in the R/M SCCHN setting); and those who recurred/progressed
within 6 months of primary chemoradiation.
This study was approved by The James Cancer Hospital
at The Ohio State University's Investigational Review Board and
Clinical Scientific Review Committee. Data were collected for
patients who met the pre-specified inclusion criteria. Data
extracted from electronic medical records included patient
demographics, disease characteristics, chemotherapy dosing, and
toxicity considerations.
Toxicity evaluation
Toxicity was graded utilizing the Common Terminology
Criteria for Adverse Events (CTCAE) version 4.03 and was determined
as documented by the treating oncologist and review of the
electronic medical record (8). PFS
was defined as time from first dose of PCC to progression (as
determined by oncologist's documentation in the electronic medical
record, radiologist interpretation of imaging, and/or change in
therapy) or death from any cause. OS was defined as time from first
dose of PCC to death from any cause. Analysis time was censored at
the last follow-up date for patients without PFS/OS events.
Relative dose intensity (RDI) was defined as the cumulative dose of
the agent delivered during treatment in milligrams per square meter
(mg/m2) divided by the intended dose in mg/m2
multiplied by 100. This was reported as a percentage and determined
for each agent: paclitaxel, carboplatin, cetuximab.
Grade 3/4 toxicity rates, PFS, and OS were compared
between the weekly and every 3 weeks PCC treatment groups using
inverse probability of treatment weighting (IPTW) to account for
potential differences in patient characteristics between the
groups. IPTW is a propensity score-based methodology that yields an
unbiased treatment effect estimate. In this analysis, propensity
scores for IPTW were calculated using probabilities from a logistic
regression model with treatment regimen as the outcome and age,
sex, race, baseline ECOG performance status, tobacco use, previous
radiation, previous surgery, PCC initiation within 6 months of
chemoradiation, and line of therapy in R/M setting as the
independent variables. Standardized differences between groups for
patient characteristics were evaluated with and without application
of IPTW to assess the balance achieved by IPTW. Although not
absolute, one rule of thumb is that a standardized difference of
>0.2 suggests imbalance between groups.
An IPTW-adjusted logistic regression model with IPTW
was used to test group differences in incidence of grade 3/4
toxicities. The treatment group comparison of Grade 3/4 toxicity
rates in the logistic regression model was assessed at the α=0.05
significance level. Kaplan-Meier curves with IPTW were generated
for each group for PFS and OS outcomes. Since the PFS and OS
outcomes were hypothesized not to differ between the treatment
regimens, statistical hypothesis tests were not performed for these
outcomes. A non-significant result for these outcomes would not
lead to the conclusion of equivalence or non-inferiority, and the
sample size was inadequate for sufficiently powered statistical
tests of equivalence or non-inferiority. Therefore, the PFS and OS
outcomes were presented descriptively by the Kaplan-Meier curves
and median time to event and/or event estimates at select follow-up
times for each treatment group. Chemotherapy dose intensity,
frequency of discontinuation, dose reductions, and dose delays were
reported for each group.
In order to characterize PFS and OS for high-risk
subgroups receiving the weekly PCC regimen, median survival and
estimated survival at 1 year were calculated for patients receiving
the weekly PCC treatment group with age >65, baseline ECOG of 2
or 3, line of therapy >1, and recurrence/progression within 6
months of primary chemoradiation. Since there was no direct
comparison group for these subgroups, the survival estimates were
unweighted.
Results
Patient characteristics
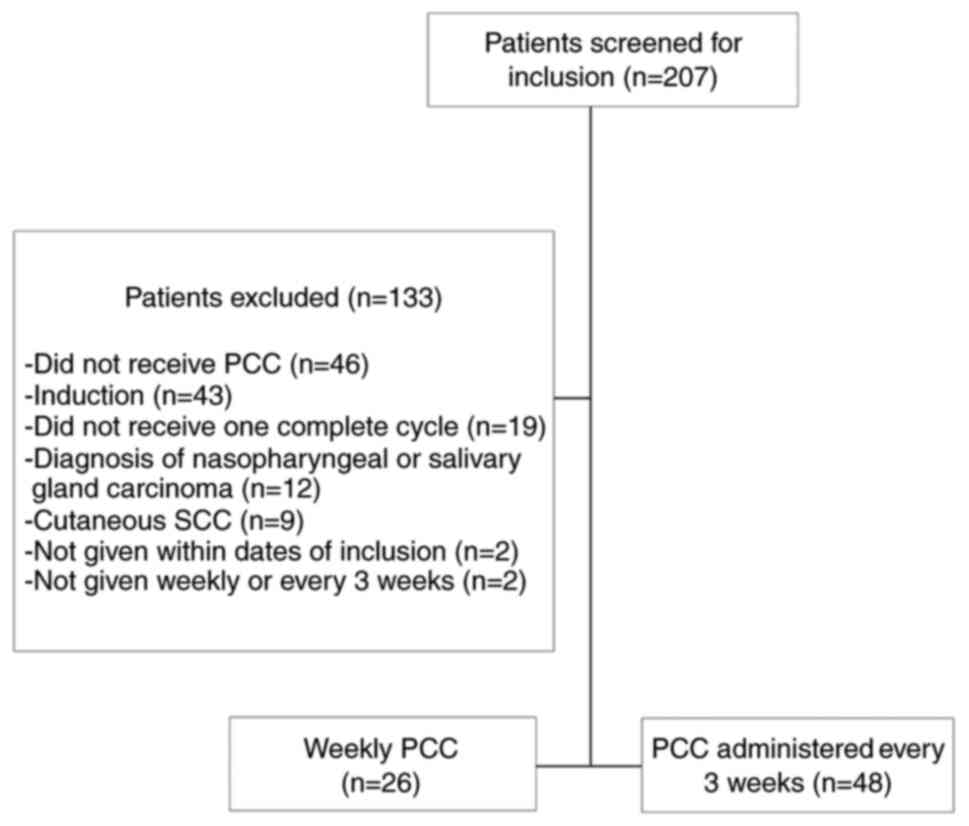
A total of 207 patients with R/M SCCHN were screened
for inclusion (Fig. 1). 133
patients were excluded with the most common reasons being that the
patients did not receive all 3 PCC agents with the regimen, PCC was
used as an induction regimen prior to surgery or radiation, the
patients did not receive one complete cycle of PCC, or patients had
nasopharyngeal or salivary sites of disease. As a result, 74
patients were included with 26 patients in the weekly PCC cohort
and 48 patients in the every 3 weeks PCC cohort.
Table I illustrates
baseline characteristics. The majority of the patients were males,
white, and had received prior chemotherapy. The oropharynx was the
most common site of disease for patients in both the weekly PCC
group at 54% and the every 3 weeks PCC group at 56%. Of the 14
patients with disease of the oropharynx in the weekly PCC group, 6
(43%) were HPV positive and 9 (64%) were p16 positive. Of the 27
patients with disease of the oropharynx in the every 3 weeks PCC
group, 12 (44%) were HPV positive and 14 (52%) were p16 positive.
Prior to IPTW adjustment, differences between groups (Std.Diff
>0.2) at baseline were notable as follows: the weekly PCC group
compared to the every 3 weeks PCC group included more patients who
were older (median 65 years of age vs. 60), white (92 vs. 85%), had
a worse ECOG performance status of 2 or 3 (27 vs. 17%), were more
heavily pretreated with radiation (92 vs. 85%) and surgery (58 vs.
40%), had PCC initiated within 6 months of chemoradiation (46 vs.
27%), and received PCC as third line or greater for R/M disease (23
vs. 2%). However, after IPTW adjustment, study groups were similar
with the exception of the weekly PCC group having an older
population (median 63 years of age vs. 60) and being more heavily
pretreated with radiation (95 vs. 89%) and surgery (58 vs.
46%).
 | Table IBaseline characteristics. |
Table I
Baseline characteristics.
| | Unweighted | IPTW Adjustment |
|---|
| Characteristic | Weekly (n=26) | Every 3 weeks
(n=48) | Std.
diffa | Weekly (n=23.7) | Every 3 weeks
(n=45.6) | Std.
diffa |
|---|
| Age, median
(IQR) | 64.5 (60-71) | 59.5 (54-64) | 0.89 | 63 (59-65) | 60 (53-64) | 0.56 |
| Sex, male | 22 (84.6) | 40 (83.3) | 0.04 | 22 (91.1) | 39 (86.4) | 0.15 |
| Race | | | 0.22 | | | 0.03 |
|
Black | 2 (7.7) | 6 (12.5) | | 3 (14.2) | 5(10) | |
|
White | 24 (92.3) | 41 (85.4) | | 20 (85.8) | 40 (86.8) | |
| Baseline ECOG PS | | | 0.25 | | | 0.09 |
|
0-1 | 19 (73.1) | 40 (83.3) | | 18 (77.2) | 37 (80.7) | |
|
2-3 | 7 (26.9) | 8 (16.7) | | 5 (22.8) | 9 (19.3) | |
| Primary site of
disease | | | 0.33 | | | 0.26 |
|
Oral
cavity | 6 (23.1) | 7 (14.6) | | 5 (20.9) | 7 (14.5) | |
|
Oropharynx | 14 (53.8) | 27 (56.3) | | 13 (56.6) | 26 (56.8) | |
|
Pharynx | 1 (3.8) | 3 (6.3) | | 0 (1.5) | 2 (5.4) | |
|
Larynx | 2 (7.7) | 7 (14.6) | | 3 (14.5) | 7 (16.4) | |
|
Paranasal | 3 (11.5) | 4 (8.3) | | 2 (6.6) | 3(7) | |
| Tobacco use | 19 (73.1) | 31 (64.6) | 0.18 | 18 (77.1) | 31 (68.5) | 0.19 |
| Alcohol use | 9 (34.6) | 19 (39.6) | 0.10 | 10 (41.6) | 19 (41.8) | 0.01 |
| Previous
treatment | | | | | | |
|
Chemotherapy | 20 (76.9) | 37 (77.1) | 0.01 | 19 (79.4) | 37 (82.3) | 0.07 |
|
Radiation | 24 (92.3) | 41 (85.4) | 0.22 | 23 (95.3) | 40 (88.9) | 0.24 |
|
Surgery | 15 (57.7) | 19 (39.6) | 0.37 | 14 (57.6) | 21 (45.6) | 0.24 |
| PCC initiated within
6 months of chemoradiation | 12 (46.2) | 13 (27.1) | 0.4 | 9 (36.1) | 14 (31.7) | 0.09 |
| Line of therapy in a
recurrent/metastatic setting | | | 0.71 | | | 0.15 |
|
First | 18 (69.2) | 45 (93.8) | | 20 (84.9) | 41 (88.9) | |
|
Second | 2 (7.7) | 2 (4.2) | | 1 (4.6) | 2 (4.7) | |
|
Third or
greater | 6 (23.1) | 1 (2.1) | | 3 (10.6) | 3 (6.3) | |
Toxicities
The incidence of grade 3/4 toxicity was greater in
the every 3 weeks PCC group at 66% compared to the weekly PCC group
at 25% (P=0.01) (Table II). The
odds of having a grade 3/4 toxicity was 82% lower with weekly PCC
compared to every 3 weeks PCC [HR 0.18 (0.05-0.64)]. The most
common grade 3/4 adverse events seen in the every 3 weeks group vs.
the weekly group were neutropenia (53 vs. 8%), anemia (32 vs. 15%),
and fatigue (10 vs. 3%). The incidence of grade 3/4 toxicity or any
grade toxicity requiring dose modification or discontinuation was
77% in the every 3 weeks PCC group vs. 74% in the weekly PCC group
(P=0.78).
 | Table IIToxicity with inverse probability of
treatment weighting adjustment. |
Table II
Toxicity with inverse probability of
treatment weighting adjustment.
| Outcome | Weekly (n=23.7) | Every 3 weeks
(n=45.6) |
|---|
| Grade 3/4 toxicity, n
(%)a | 6 (25.4) | 30 (65.7) |
| Specific Grade 3/4
toxicity, n (%) | | |
|
Neutropenia | 2 (7.6) | 24 (52.5) |
|
Thrombocytopenia | 0 (0.0) | 3 (6.6) |
|
Anemia | 4 (15.1) | 14 (31.7) |
|
Fatigue | 1 (2.7) | 5(10) |
|
Skin
rash | <1 (1.7) | 0 (0) |
|
Neuropathy | 0 (0) | 1 (1.9) |
|
Other | <1 (1.7) | 1 (1.5) |
| Grade 3/4 toxicity
or toxicity requiring dose modification or discontinuation, n
(%)b | 18 (73.8) | 35 (77.0) |
Survival
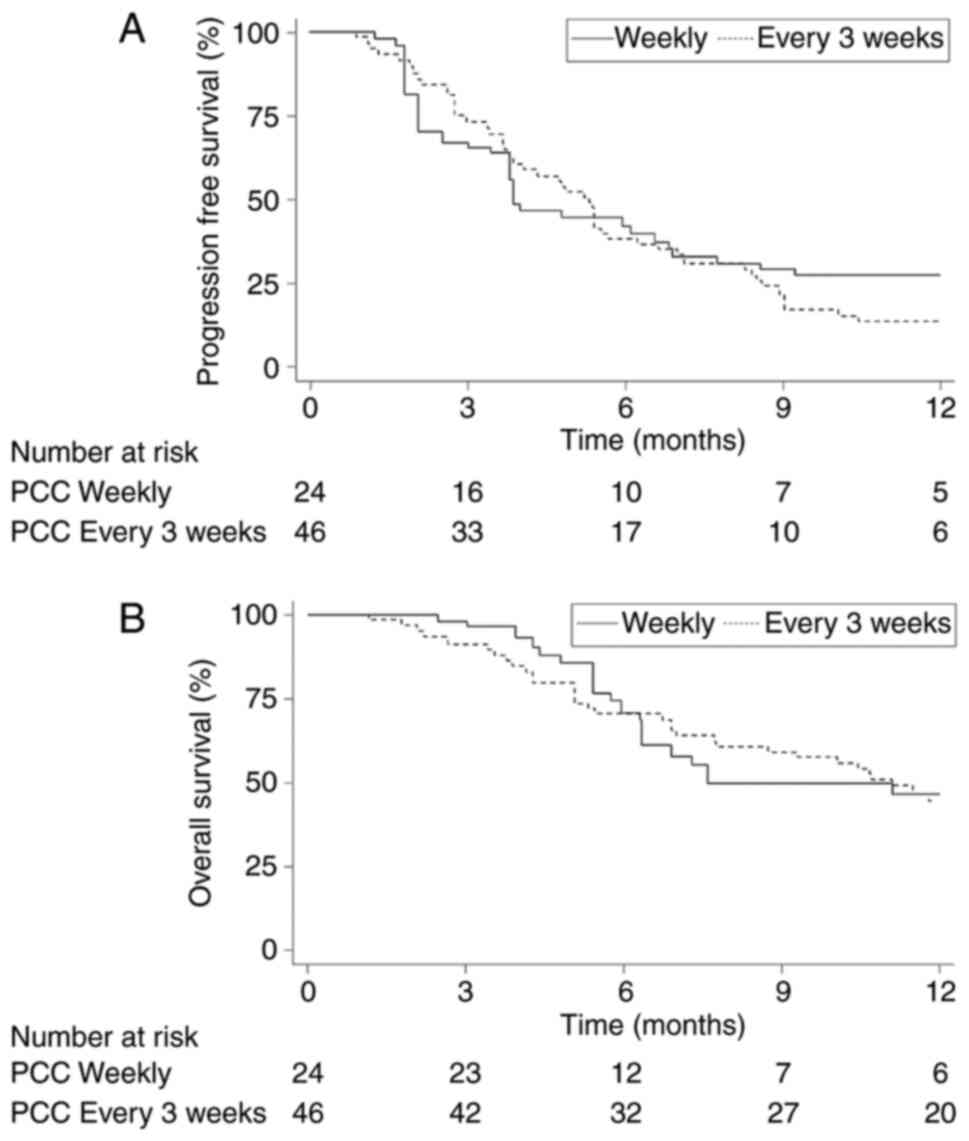
The median PFS in the weekly PCC group was 3.9
months compared to 5.3 months in the every 3 weeks PCC group. The
12-month PFS rate was 27 vs. 13%, respectively (Fig. 2A). The median OS in the weekly PCC
group was 7.6 months compared to 11.1 months in the every 3 weeks
PCC group. The 12-month OS rate was 46% compared to 44% (Fig. 2B). The overall response rate was
39% in the weekly PCC group vs. 27% in the every 3 weeks PCC group.
No formal statistical comparisons were performed on secondary
outcomes due to small sample size for a non-inferiority
hypothesis.
Subgroup analyses assessing survival outcomes of
patients in the weekly PCC group included patients who were >65
years old, had a poor performance status of 2 or 3, or received PCC
as a second line therapy or greater in the R/M setting (Table III). The median PFS for these
subgroups was 4.8 months for patients >65 years old, 6.1 months
for patients with a poor performance status of 2 or 3, 3.9 months
for patients who received PCC as a second line therapy or greater
in the R/M setting, and 6.1 months for patients who
recurred/progressed within 6 months of primary chemoradiation
compared to the median PFS for the weekly PCC cohort overall at 4.8
months. The PFS rates at 1 year were 0, 14, 0, and 17%
respectively, which were similar to the weekly PCC cohort overall
at 18%. The median OS was similar for each subgroup at 6.3, 7.6,
6.9, and 7.6 months compared to the weekly PCC cohort overall at
7.3 months. However, the OS rates at 1 year were 21, 14, 38, and
31% respectively, similar to the 32% seen in the weekly PCC cohort
overall.
 | Table IIISubgroup analysis of Paclitaxel,
Carboplatin and Cetuximab treatment. |
Table III
Subgroup analysis of Paclitaxel,
Carboplatin and Cetuximab treatment.
| Unweighted | Median PFS
(months) | PFS rate at 1 year
(%) | Median OS
(months) | OS rate at 1 year
(%) |
|---|
| Age >65
(n=13) | 4.8 | 0 | 6.3 | 20.5 |
| Baseline ECOG PS
value of 2 or 3 (n=7) | 6.1 | 14.3 | 7.6 | 14.3 |
| Line of therapy
>1 (n=8) | 3.9 | 0 | 6.9 | 37.5 |
| Recurred/progressed
within 6 months of primary chemoradiation (n=12) | 6.1 | 16.7 | 7.6 | 31.3 |
| Full weekly cohort
(n=26) | 4.8 | 18.5 | 7.3 | 32.1 |
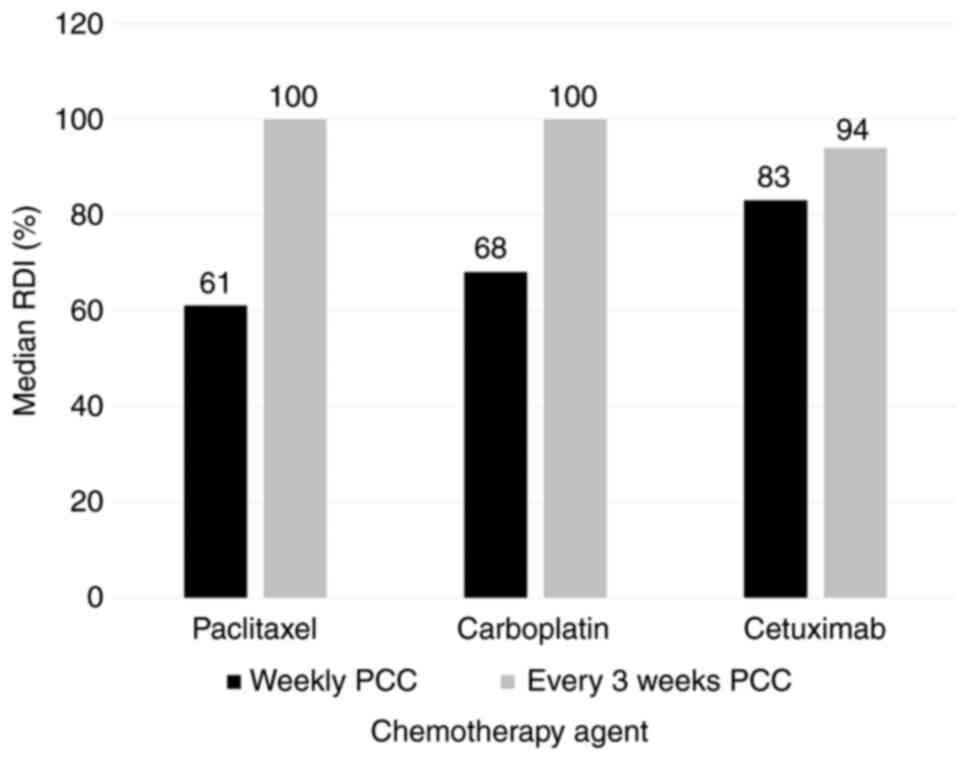
The median RDI of paclitaxel for the weekly PCC
group was 61 vs. 100% in the every 3 weeks PCC group. The RDI of
carboplatin was 68 vs. 100% and the RDI of cetuximab was 83 vs.
94%, respectively (Fig. 3). A
total of 31 patients in the every 3 weeks PCC group were able to
receive 100% of the doses intended leading to the high median in
this group. Of the 17 patients with documented progression in the
weekly PCC group, 12 received immunotherapy as a next line of
treatment at progression. Zero patients were candidates for further
chemotherapy at progression and 5 patients chose not to continue
with a next line of therapy. Of the 35 patients with documented
progression in the every 3 weeks PCC group, 8 received
immunotherapy as a next line of treatment at progression. A total
of 14 patients were candidates for further chemotherapy at
progression and 13 patients chose not to continue with a next line
of therapy.
Discussion
This retrospective study of the treatment of R/M
SCCHN showed that a weekly PCC regimen was associated with lower
toxicity compared to an every 3 weeks regimen of PCC. The odds of
having a grade 3/4 toxicity was 82% higher with every 3 weeks PCC,
and the most common adverse events seen in this group were
neutropenia, anemia, and fatigue. This is consistent with previous
trials for R/M SCCHN. In the TPEx trial, patients received
paclitaxel 175 mg/m2 every 3 weeks in the PCC regimen
with a 6% incidence of grade 4 neutropenia (6). At The James Cancer Hospital at The
Ohio State University, the every 3 weeks PCC regimen had a higher
incidence of grade 3/4 neutropenia at 53%. Narveson et al
utilized a weekly PCC regimen with a dose of paclitaxel of 80
mg/m2 given 3 weeks on, 1 week off. The total cumulative
dose for a cycle was 240 mg/m2, resulting in 39% of
patients experiencing grade 3/4 neutropenia (7). Comparing the Narveson et al
weekly regimen to the weekly regimen used in this study, PCC uses
lower doses of paclitaxel 45 mg/m2 weekly. Since the
total cumulative dose of paclitaxel is 135 mg/m2 each
cycle, this could explain why the incidence of grade 3/4
neutropenia was only 8% and lower than seen with any other group
studied.
Secondary efficacy outcomes were assessed in each of
these trials in the R/M setting. At The James Cancer Hospital,
patients with a good performance status are considered to be able
to tolerate the toxicities associated with the every 3 weeks PCC
regimen, while patients with worse performance status and poor
performance status are thought to derive more benefit from the
weekly PCC regimen. As a result, the every 3 weeks PCC group had a
median OS of 11.1 months, OS rate at 1 year of 44%, median PFS of
5.3 months, PFS rate at 1 year of 13%, and an ORR 27%. This is
comparable to the TPEx trial which resulted in a median OS of 15.3
months, median PFS of 7.1 months, and an ORR of 44% (6). In contrast, the weekly PCC regimen
was studied by Narveson et al in patients with poorer ECOG
performance status of 1-2. This regimen resulted in a median OS of
5.25 months, median PFS of 4.6 months, and an ORR of 41% (7). The weekly PCC group for the purposes
of this current study had a median OS of 7.6 months, OS rate at 1
year of 46%, median PFS of 3.9 months, PFS rate at 1 year of 27%,
and an ORR 39%. While the every 3 weeks PCC group had a numerically
higher median OS than the weekly PCC group (11.1 mos vs. 7.6 mos),
the OS rates at 1 year were similar (46 vs. 44%), indicating that
weekly PCC is a safe and effective option for patients with poor
performance status and older age.
It was important to stratify the weekly cohort into
high-risk subgroups as the practice at The James Cancer Hospital is
to give the weekly PCC regimen to patients who were older, had a
poor performance status, and/or were more heavily pretreated. The
results of the subgroup analysis indicates that these patients
achieved similar survival outcomes as the overall cohort receiving
the weekly regimen. The weekly PCC regimen provides a treatment
option for older patients with worse performance status who are
heavily pretreated and would not be able to receive every 3 weeks
PCC due to toxicity. There is very limited data on the use of
weekly carboplatin and paclitaxel in combination with cetuximab,
especially in the second line setting.
The patients in the every 3 weeks PCC group were
able to maintain higher median dose intensity compared to the
weekly PCC regimen throughout treatment and received more
chemotherapy as a result. A reason for the difference in RDI
relates to how the regimens are scheduled. The every 3 weeks PCC
group was seen weekly due to cetuximab being given every week. Labs
were drawn and systems assessments were completed that often showed
grade 3/4 toxicity during weeks 2 and 3 of a cycle. There were no
dose modifications made as a result of toxicity as long as the
toxicity resolved to grade ≤1 by the start of the next cycle.
However, the weekly regimen was seen each week to receive all 3 PCC
agents. If there was toxicity seen on laboratory or systems
assessment, dose modifications for grade 1/2 toxicity were more
likely. As a result, this led to more toxicity in the every 3 weeks
group but fewer dose reductions, leading to greater dose intensity.
This rationale supports the reason why there was no difference
between groups in toxicity when dose modifications were included
because the weekly group was more likely to be dose reduced for
grade 1/2 toxicities.
There were several limitations to this study. It was
a retrospective chart review, yielding a small sample size of 74
patients. Limited sample size is the major limitation of this
study. Treatment selection was based not only on patients'
performance status and age but also on physician preference which
may have introduced bias. Incidences of adverse events as well as
rationale for dose modifications were reliant on documentation in
the medical record. However, one strength of this study was that
the weekly regimen of PCC included a dosing strategy that has not
previously been reported. Also, survival analysis on RDI was not
performed as patients receiving weekly PCC were older, had worse
performance status, were more heavily pretreated, and were more
likely to experience a chemotherapy dose reduction if they
experienced grade 2 toxicity.
In conclusion, the weekly PCC regimen represents a
viable treatment option for R/M SCCHN due to an improved
tolerability profile and similar efficacy compared to every 3 weeks
PCC. In older, heavily pretreated patients with a decreased
performance status, weekly PCC can help achieve goals of care by
prolonging survival, improving quality of life, and limiting
toxicity.
Acknowledgements
The abstract was presented at the American Society
for Radiation Oncology (ASTRO) conference in Chicago, IL in
September 2019 and published as abstract no. 351 in International
Journal of Radiation Oncology on April 1, 2020.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Author's contributions
VK and MB conceived and designed the current study.
LG prepared the original draft of the manuscript. LG, VK and MB
wrote, reviewed and edited the manuscript. LG, MI, TES and SEH
collected the data. LG and TES confirmed the authenticity of all
the raw data. KP defined the statistical methodology, utilized
statistical software tools and conducted formal statistical
analysis. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The current research was conducted in accordance
with the World Medical Association Declaration of Helsinki. As this
is a retrospective study, the protocol was reviewed and the
requirement for written and informed consent was waived by the
Buck-Institutional Review Board of Ohio State University. The study
protocol was approved by the aforementioned Institutional Review
Board (Study ID, 2018C0169).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Adelstein D, Gillison ML, Pfister DG,
Spencer S, Adkins D, Brizel DM, Burtness B, Busse PM, Caudell JJ,
Cmelak AJ, et al: NCCN guidelines insights: Head and neck cancers,
version 2.2017. J Natl Compr Canc Netw. 15:761–770. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chaturvedi AK, Engels EA, Anderson WF and
Gillison ML: Incidence trends for human papillomavirus-related and
-unrelated oral squamous cell carcinomas in the United States. J
Clin Oncol. 26:612–619. 2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Duprez F, Berwouts D, De Neve W, Bonte K,
Boterberg T, Deron P, Huvenne W, Rottey S and Mareel M: Distant
metastases in head and neck cancer. Head Neck. 39:1733–1743.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Price KA and Cohen EE: Current treatment
options for metastatic head and neck cancer. Curr Treat Options
Oncol. 13:35–46. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Vermorken JB, Mesia R, Rivera F, Remenar
E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol
D, et al: Platinum-based chemotherapy plus cetuximab in head and
neck cancer. N Engl J Med. 359:1116–1127. 2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Guigay J, Fayette J, Dillies AF, Sire C,
Kerger JN, Tennevet I, Machiels JP, Zanetta S, Pointreau Y, Moal
LB, et al: Cetuximab, docetaxel, and cisplatin as first-line
treatment in patients with recurrent or metastatic head and neck
squamous cell carcinoma: A multicenter, phase II GORTEC study. Ann
Oncol. 26:1941–1947. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Narveson L, Kathol E, Rockey M, Henry D,
Grauer D and Neupane P: Evaluation of weekly paclitaxel,
carboplatin, and cetuximab in head and neck cancer patients with
incurable disease. Med Oncol. 33(107)2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Common Terminology Criteria for Adverse
Events (CTCAE). Version 4.03. US Department of Health and Human
Services, National Institutes of Health and National Cancer
Institute, 2010. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf.
Accessed December 10, 2018.
|