Introduction
Regorafenib is a novel oral multikinase inhibitor
that has shown antitumor activity in various gastrointestinal
cancers, including colorectal cancer and gastrointestinal stromal
tumor (1). The CORRECT and CONCUR
trials have been significant benchmarks in the management of
metastatic colorectal cancer (mCRC), demonstrating that regorafenib
can slow disease progression and increase the median overall
survival (OS) in patients with mCRC (2,3). Those
double-blind phase 3 studies showed that the addition of
regorafenib to best supportive care prolongs the median OS by up to
2.5 months and progression-free survival (PFS) by up to 1.5 months
compared with the addition of placebo in patients with mCRC who had
progressed after standard therapy failure. Although disease control
[partial response (PR) plus stable disease] was achieved in 51% of
the patients, only 4% showed a PR, and none showed a complete
response (CR). Yoshino et al (4) recently reported a case of mCRC that
responded well to regorafenib, resulting in a 2-year-long therapy
with a PR. Here, we report the very first case of mCRC that was
refractory to conventional chemotherapy such as fluorouracil,
leucovorin and irinotecan (FOLFIRI) with cetuximab and
fluorouracil, leucovorin and oxaliplatin (FOLFOX) with bevacizumab,
but showed a CR to regorafenib. Our patient was a MSI-H, right
sided advanced colon cancer patient who had extensive sunlight
exposure to her torso. We believe these factors may have
contributed to the patient's excellent response to regorafenib,
resulting in a prolonged state of CR even after treatment
cessation. The mechanism behind CR should be further elucidated in
future studies; for now, we have suggested some possible
theories.
Case report
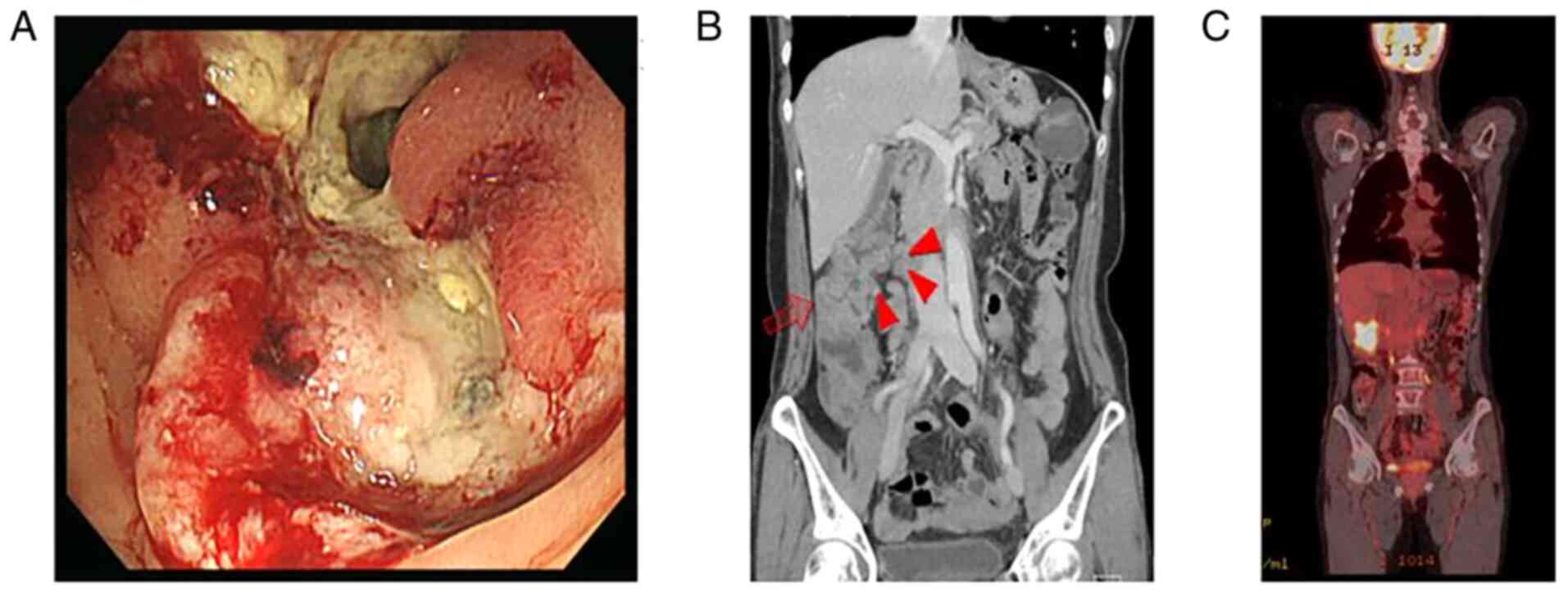
A 54-year-old woman with abdominal pain and nausea
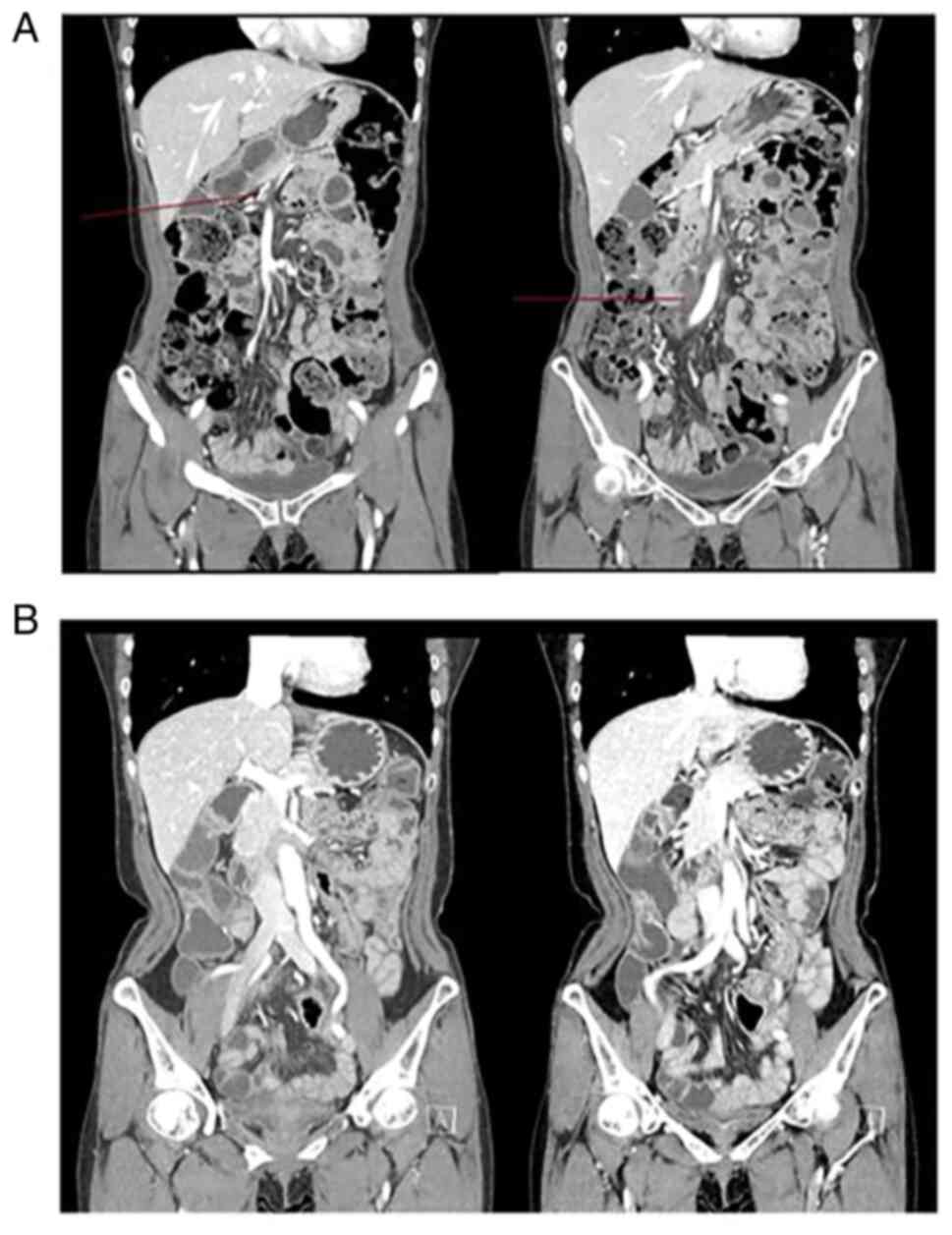
was diagnosed with hepatic flexure colon cancer obstruction with
peritoneal seeding in March 2016 (Fig.
1). The patient underwent palliative laparoscopic right
hemicolectomy due to obstruction signs, with pathologic report of
T4aN2bM1 moderately differentiated adenocarcinoma with
lymphovascular invasion and perineural invasion. The tumor also had
epidermal growth factor (EGFR) positivity, high microsatellite
instability (MSI-H), wild-type KRAS/NRAS, and safety proximal and
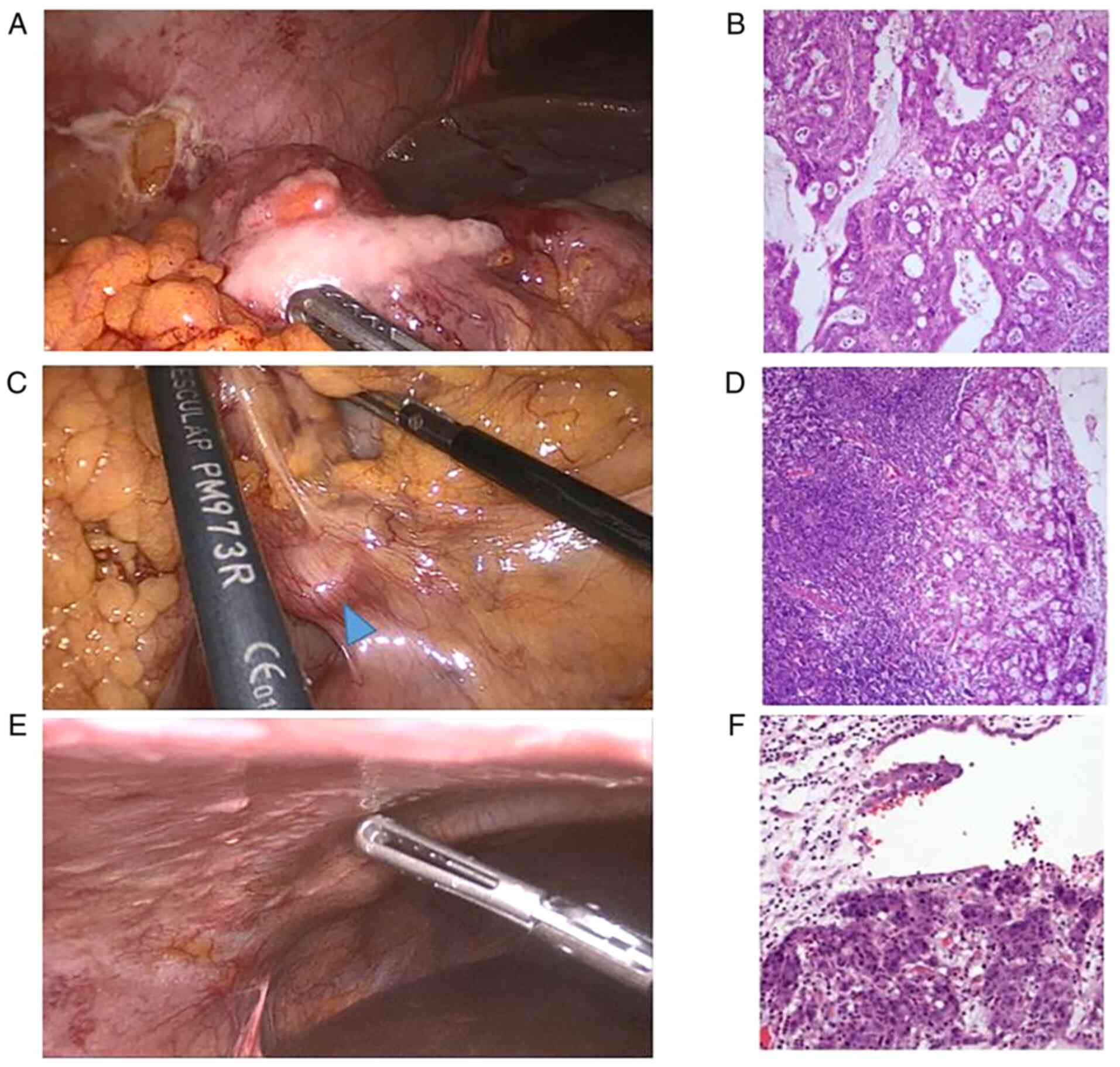
distal margins of 7 and 11 cm, respectively. The peritoneal seeding
mass was partially removed for biopsy confirmation, which revealed
pathologically metastatic adenocarcinoma (Fig. 2). Shortly after palliative
laparoscopic right hemicolectomy, the patient received 12 cycles of
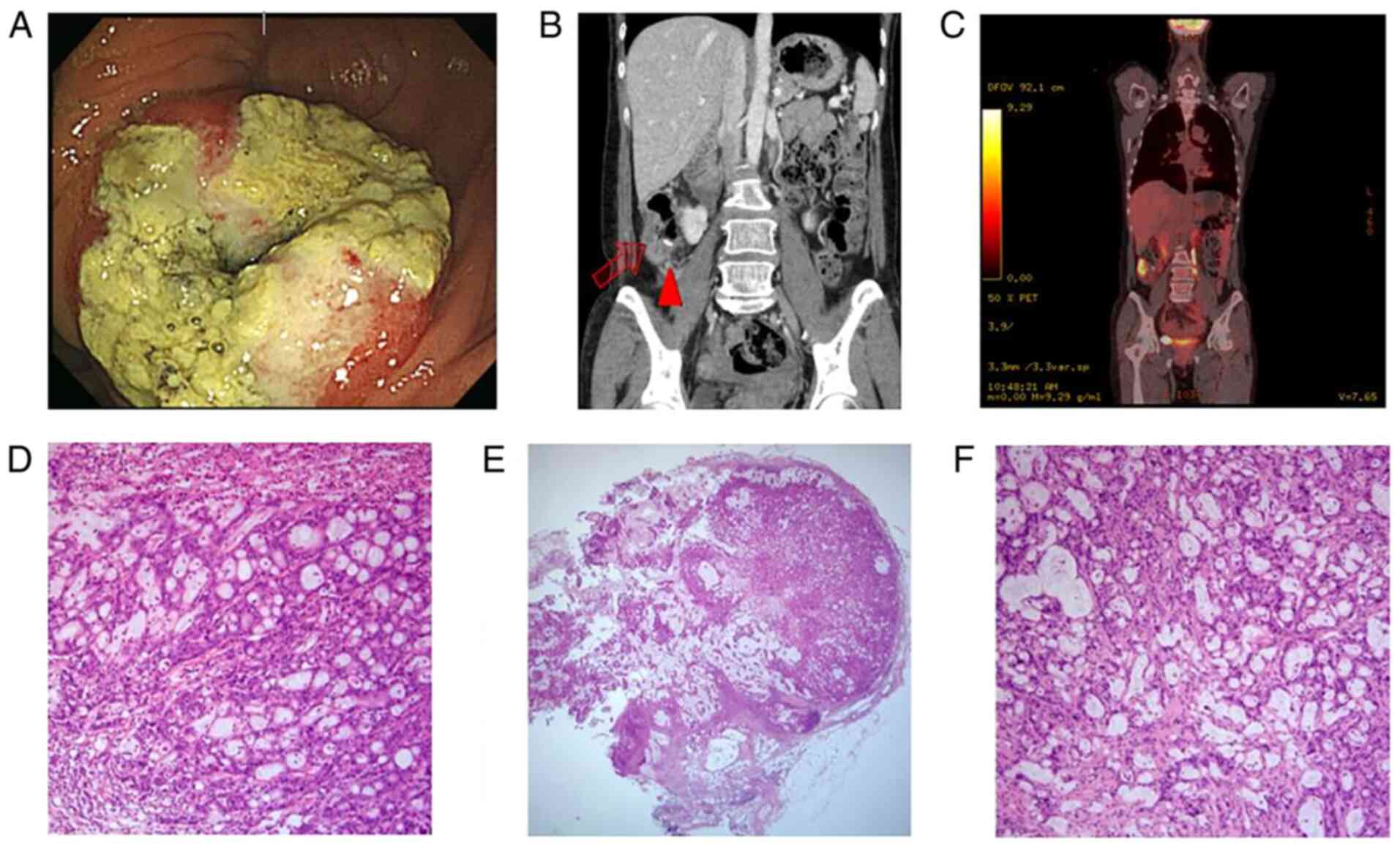
FOLFIRI with cetuximab. After the 12th cycle, local anastomosis
site recurrence and pericolic lymph node metastasis were observed
(Fig. 3). In October 2016, the
patient underwent palliative colectomy with lymph node dissection,
which revealed 10/18 positive lymph nodes. During surgery, SMA
lymph node metastasis was evident, which was confirmed by selective
lymph node retrieval and permanent biopsy. Metal clips were placed
in the area of lymph node metastasis for follow-up. Following
recovery, the patient received second-line chemotherapy consisting
of FOLFOX with bevacizumab. Progression of metastasis to the right
iliac lymph nodes was detected in January 2017 after only four
cycles of FOLFOX with bevacizumab (Fig.
4). Thereafter, the patient received regorafenib, starting with
160 mg for two cycles, which was quickly reduced to 120 mg for five
cycles according to the Common Terminology Criteria for Adverse
Events (CTCAE version 5) indicating grade 3 palmar-plantar
erythrodysesthesia syndrome (5).
Symptoms were partially alleviated after dose reduction, but
because grade 2 palmar-plantar erythrodysesthesia syndrome
persisted, the patient requested a further dose reduction;
therefore, she received 80 mg of regorafenib during cycles 8-12.
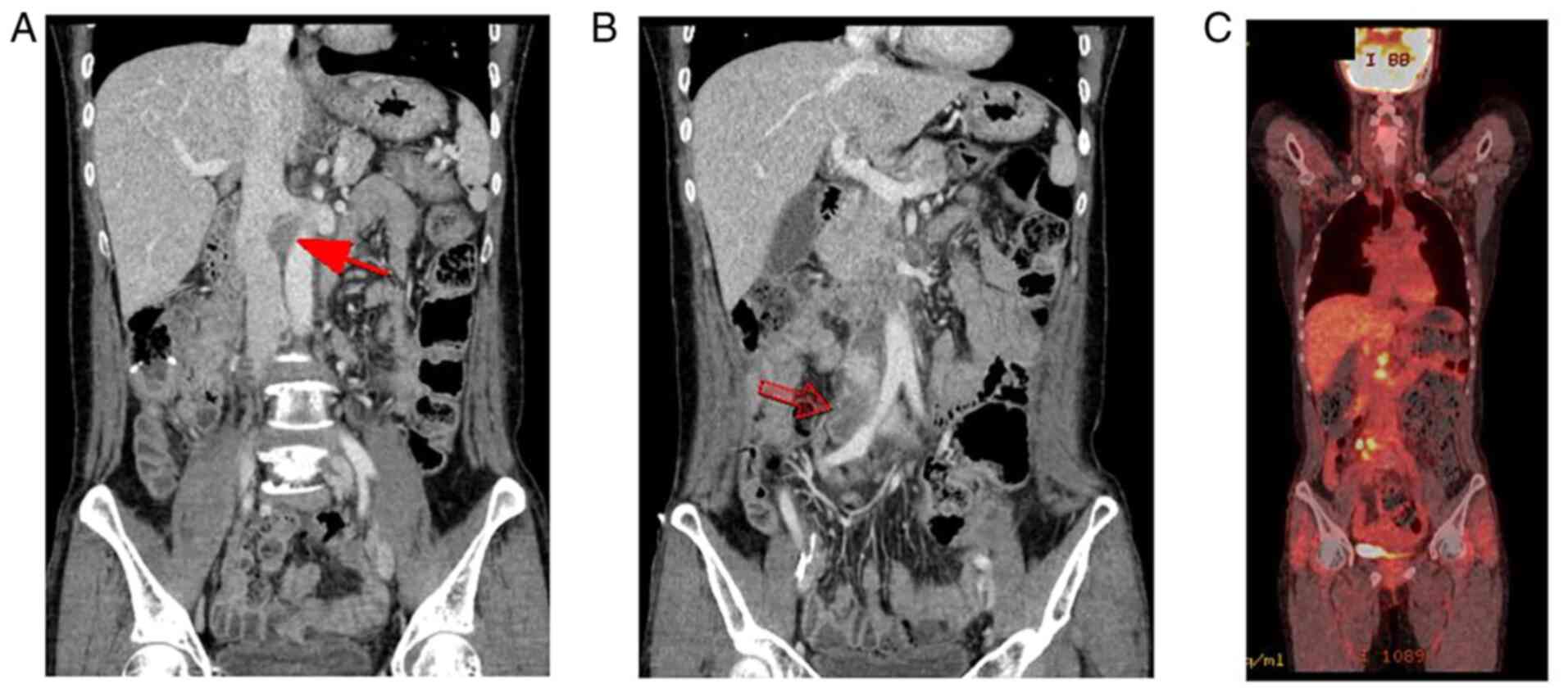
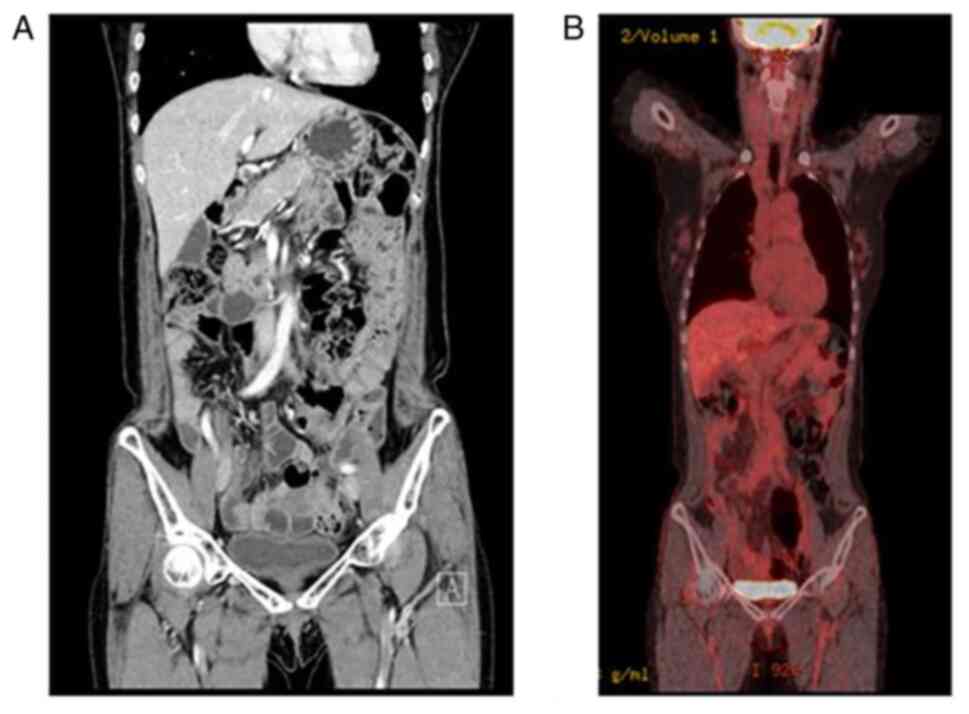
After the seventh cycle, the previously confirmed SMA lymph node
metastasis had disappeared on computed tomography (CT) (Fig. 5A). From cycle 13 to 17, the patient
requested a further dose reduction to 40 mg due to general
weakness. The right common iliac lymph node metastasis was no
longer visible on CT after the cycle 16 (Fig. 5B). The patient decided to terminate
chemotherapy and has not experienced recurrence at 2 years since
treatment cessation. The clinical effect of regorafenib monotherapy
was classified as a CR according to the Response Evaluation
Criteria in Solid Tumors (RECIST) v1.1(6). CT was used for periodic screening, and
18F-fluorodeoxyglucose positron emission tomography
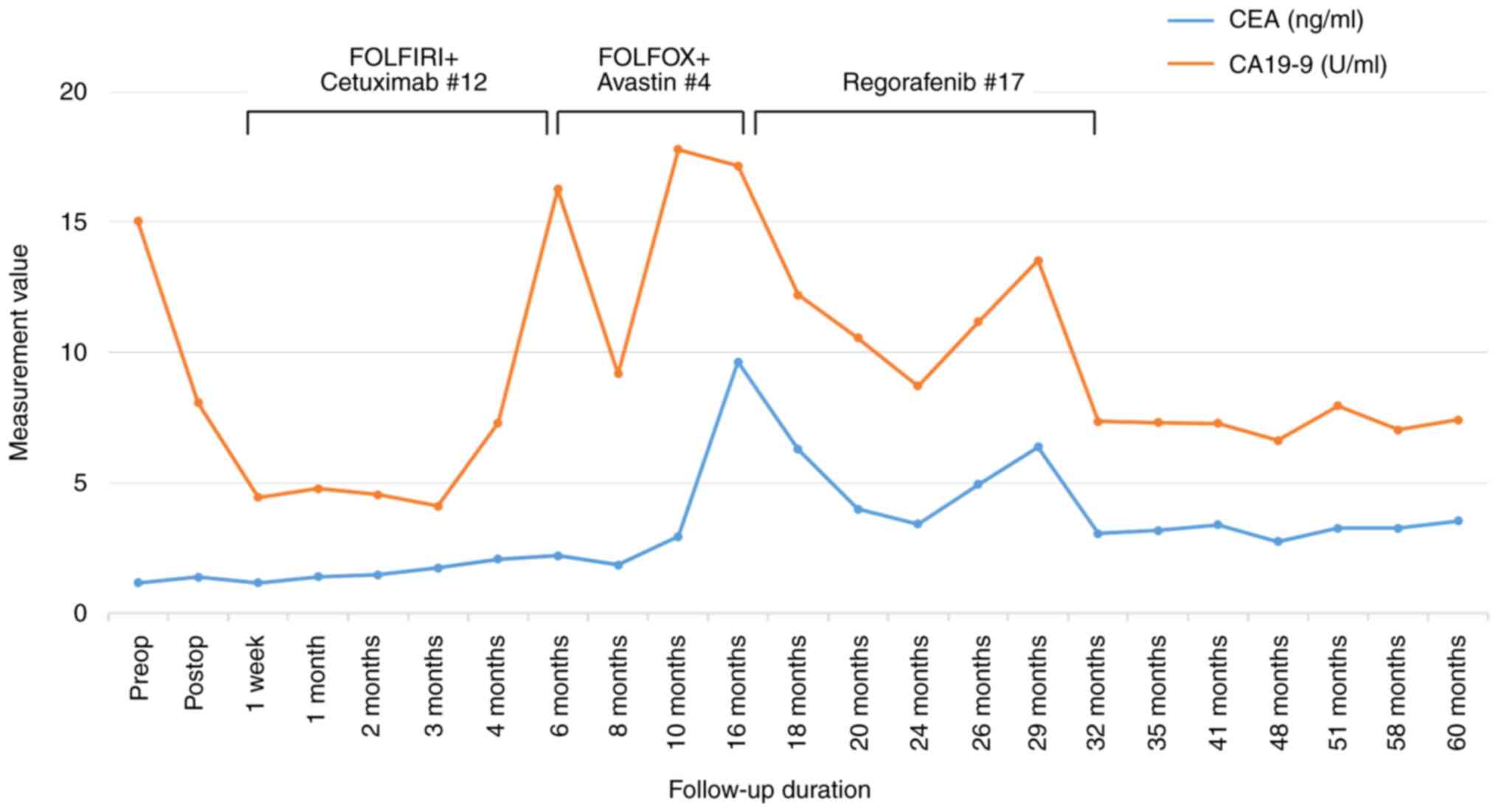
(PET)-CT was used to confirm the CR (Fig. 6). The levels of two tumor markers,
carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19-9,
were higher during chemotherapy, but decreased during regorafenib
treatment and became within normal range at the time of termination
(3.05 ng/ml and 7.35 U/ml, respectively) (Fig. 7). The patient did not suffer from
any adverse events aside from palmar-plantar erythrodysesthesia
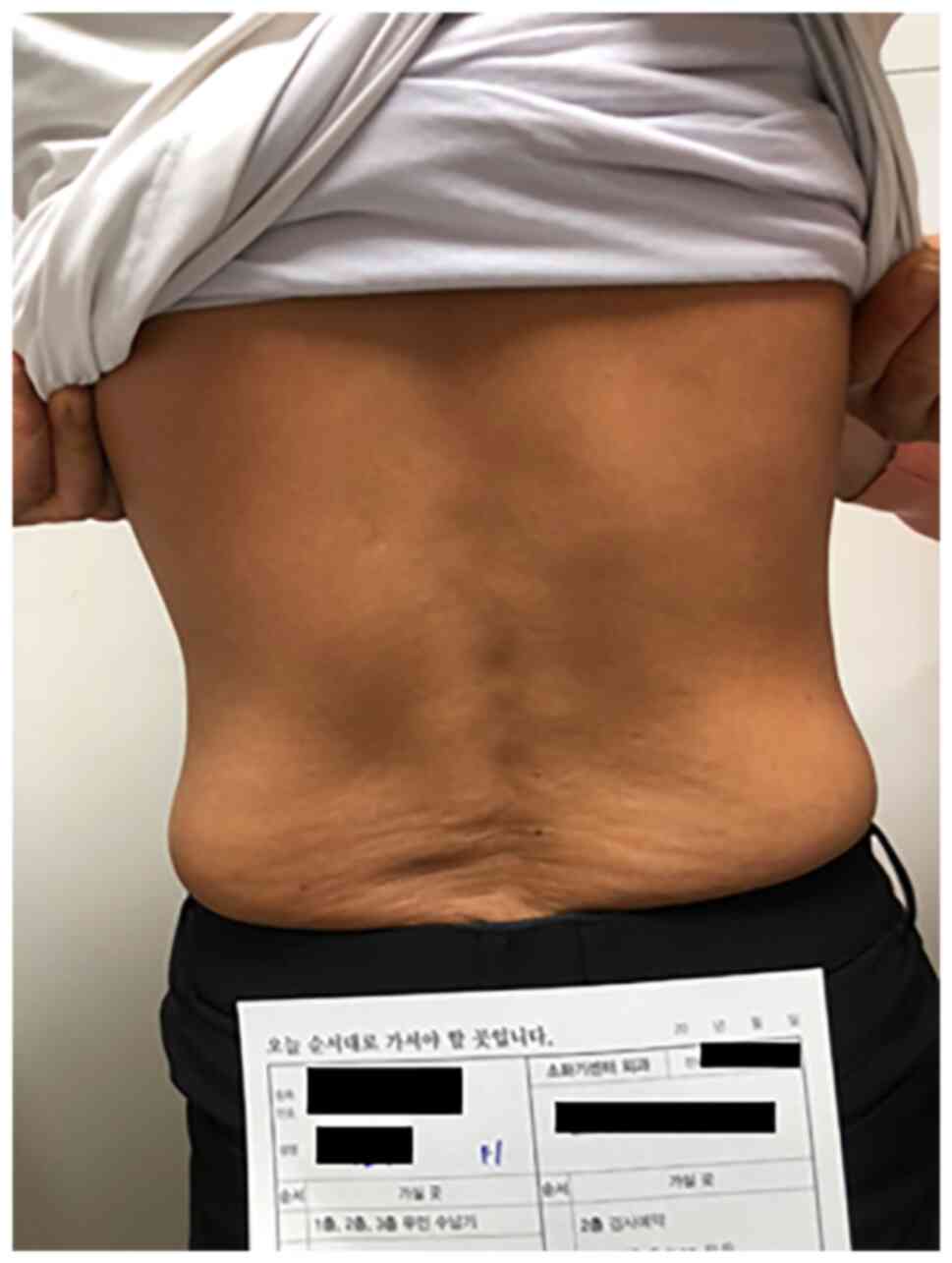
syndrome and mild fatigue. While the patient underwent regorafenib
monotherapy, she exposed herself to extensive sunlight for at least
1 h per day (Fig. 8). The patient's
vitamin D ((1α25-(OH)2Vitamin D3) level was
not recorded during therapy but was much higher (68.49 pg/ml) than
the normal range (19.6-54.3 pg/ml) after CR achievement, without
any supplements.
Discussion
Prior to the introduction of regorafenib for mCRC
treatment, patients who were not responsive to conventional
palliative chemotherapy basically had no other treatment option.
However, the CORRECT and CONCUR trials showed that both the PFS and
OS of mCRC patients were prolonged following regorafenib treatment,
compared with the placebo groups (P<0.0001 and P=0.0016), with a
high disease control rate of up to 51% (2,3).
Unfortunately, no patients in either study showed a CR, and only a
few previously reported cases indicated radiological responses
(4,7). More recent studies of regorafenib have
reported similar results, with no patients showing a CR and only a
few showing a PR (0-3%) (8,9). Our patient, who initially had
MSI-high, right-sided colon cancer with peritoneal carcinomatosis,
progressed to SMA and paraaortic lymph node metastases despite
conventional chemotherapy. Progression led to cessation of
chemotherapy and administration of regorafenib, which resulted in a
CR according to both radiological studies and serum tumor marker
levels, consistent with the RECIST criteria. Furthermore, the
patient has not experienced recurrence at 2 years since cessation
of regorafenib. The CEA level was initially normal but increased
dramatically due to diffuse paraaortic lymph node metastasis and
then approached the normal range soon after the patient started
regorafenib, remaining normal even after treatment cessation.
However, the CA 19-9 level appeared to be the more sensitive marker
in this patient, showing significant elevation at the time of
cancer progression. Despite first- and second-line chemotherapy and
targeted therapy, the CA 19-9 level continued to increase with
cancer progression and only gradually decreased during regorafenib
treatment (Fig. 7).
To our knowledge, this is the first case of a CR in
a patient with mCRC treated with regorafenib; only a handful of
other gastrointestinal cancer cases have shown a CR following
regorafenib treatment. Notably, the patient's cancer not only
stopped progressing but completely regressed. We hypothesize the
following reasons for the sudden disappearance of the previously
chemotherapy-resistant tumor following regorafenib treatment.
MSI-high tumors have a high mutational burden, which increases the
likelihood of tumor exposure to the innate immune system (10,11).
High MSI is also an effective biomarker of the response to
immune-check inhibitor (ICI) in colon cancer, compared with the
previously established marker PD-1. Thus, the mutational burden
appears to be a more significant factor in immune system escape
compared with PD-1; consequently, high MSI is an important
surrogate marker (12,13). Increased antitumor immunity is among
the pharmacologic traits of regorafenib (14,15),
which inhibits CSF1R, a tyrosine kinase receptor involved in
macrophage proliferation. CSF1R inhibition by regorafenib may
reduce the recruitment of tumor-associated macrophages to the tumor
bed, limiting their function, as demonstrated in a model of highly
aggressive murine CT26 metastatic colon cancer (16,17).
Regorafenib also increases cytotoxic T cells and inhibits the MAPK
and JAK1/2-STAT axis, thereby attenuating IFNγ-induced PD-L1 and
IDO1 expression and inducing immune cell attacks (18). The REGONIVO study reported steady
tumor regression after treatment with a combination of regorafenib
and ICI (19). Based on these
studies, increased tumor recognition after regorafenib treatment
and decreased tumor immune system escape after ICI treatment appear
very promising. Therefore, we suggest that the increased tumor
burden due to MSI-H status led to elevated tumor antigenicity,
along with enhanced tumor recognition of the immune cells, by
regorafenib in our patient, thereby inducing remission. However,
this hypothesis should be further evaluated in larger sample
sizes.
Another factor that may have influenced our
patient's outcome is that she exposed her torso to sunlight for at
least 1 h per day (Fig. 8). Her
vitamin D level was higher than the of normal range at the time of
the CR, although we did not record her initial vitamin D level. The
patient took no vitamin D supplements or injections; therefore we
infer that her high vitamin D level was due solely to the direct
absorption of sunlight. Recent studies investigating the
relationship between vitamin D and cytotoxic T cells that attack
cancer suggested that an increased vitamin D level may promote
CD8+ T cell tumor infiltration, which would result in a
lower tumor burden. Karkeni et al (20) have reported that vitamin D
supplements contributed to significant increases in CD8+
T cell recruitment to the tumor site and CD8+ T cell
activation compared with the control. We cautiously infer that
vitamin D synthesized by sunshine may have played a role in
activating cytotoxic T cells, which contributed to the patient's
CR. This theory should be explored in both laboratory and clinical
trials.
Our patient had a right-sided tumor harboring
wild-type RAS. In their CORRELATE study, Ducreux et al
(21) showed similar OS and PFS
after regorafenib treatment between left- and right-sided mCRC
patients; however, Yoon et al (9) found that left-sided primary tumors
were associated with improved outcomes, including PFS, after
treatment in their multivariate analysis (2.6 vs. 1.9 months,
P=0.04). The role of the RAS mutation status remains controversial;
in the CORRECT study, no relationship was detected between the KRAS
mutation status and outcomes following regorafenib treatment,
whereas in the REBECCA study, mutations were found to be a
prognostic factor for poor survival (2,22).
Further studies are essential to confirm whether these factors
influence the outcomes of regorafenib treatment.
As suggested by Yoshino et al (4), we stopped regorafenib treatment after
the 17th cycle due to the lack of remnant tumor evident on both CT
and PET-CT. Remarkably, the patient has not experienced recurrence
2 years since treatment cessation; no similar findings have been
reported previously. Although the regorafenib dose had to be
reduced due to palmar-plantar erythrodysesthesia syndrome, there
were no other severe side effects such as liver function
deterioration or hypertension. Notably, the dose reduction did not
appear to diminish the antitumor effect of regorafenib; however,
further studies are needed for confirmation.
In summary, this case study suggests that the high
mutational burden carried by MSI-H tumor acts as a neoantigen in
the innate immune system, and that regorafenib plays an additional
role in enhancing tumor antigen recognition by immune cells. An
increased vitamin D level via extensive sunlight exposure may also
have encouraged cytotoxic T cell recruitment and activation to the
tumor microenvironment. All of these factors appear to have
contributed to the patient's CR. However, well-designed preclinical
and clinical studies are required to demonstrate the mechanism
behind this response.
In conclusion, this case report presents the first
patient with MSI-H mCRC to achieve a CR to regorafenib treatment
after failure of conventional palliative chemotherapy. The
mechanism behind this response requires further evaluation in
larger studies, to identify which mCRC patients are most likely to
benefit from regorafenib.
Acknowledgements
Not applicable.
Funding
The present study was supported by the 2015 Inje University
Busan Paik Hospital Research Grant (Busan Paik-2015).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HB was involved in manuscript writing and
acquisition of data. HJL wrote the manuscript. JuP designed the
study and gathered patient information. HYP was involved in
pathology review and case review. JiP was involved in image (CT)
review. SL was involved in image (PET) review. KBB was involved in
conception of the case report and analysis of the case. HB and KBB
confirm the authenticity of all the raw data. HB, HJL and KBB have
reviewed all the raw data separately, and have confirmed that the
results are correctly described. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
This type of study does not require ethics approval
due to its retrospective nature. The patient consented to the
treatment.
Patient consent for publication
Informed oral consent was obtained from the
patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wilhelm SM, Dumas J, Adnane L, Lynch M,
Carter CA, Schütz G, Thierauch KH and Zopf D: Regorafenib (BAY
73-4506): A new oral multikinase inhibitor of angiogenic, stromal
and oncogenic receptor tyrosine kinases with potent preclinical
antitumor activity. Int J Cancer. 129:245–255. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Grothey A, Van Cutsem E, Sobrero A, Siena
S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, et
al: Regorafenib monotherapy for previously treated metastatic
colorectal cancer (CORRECT): An international, multicentre,
randomised, placebo-controlled, phase 3 trial. Lancet. 381:303–312.
2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, Xu
J, Bai Y, Chi Y, Wang L, et al: Regorafenib plus best supportive
care versus placebo plus best supportive care in Asian patients
with previously treated metastatic colorectal cancer (CONCUR): A
randomised, double-blind, placebo-controlled, phase 3 trial. Lancet
Oncol. 16:619–629. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yoshino K, Manaka D, Kudo R, Kanai S,
Mitsuoka E, Kanto S, Hamasu S, Konishi S and Nishitai R: Metastatic
colorectal cancer responsive to regorafenib for 2 years: A case
report. J Med Case Rep. 11(227)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
U.S. Department of Health and Human
Services: Common Terminology Criteria for Adverse Events (CTCAE).
Version 5.0. U.S. Department of Health and Human Services,
Washington, DC, 2017. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf.
|
|
6
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kawasaki K, Hamamoto Y, Adachi M, Kanai T
and Takaishi H: Early tumor cavitation with regorafenib in
metastatic colorectal cancer: A case report. Oncol Lett.
11:231–233. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Moriwaki T, Fukuoka S, Taniguchi H,
Takashima A, Kumekawa Y, Kajiwara T, Yamazaki K, Esaki T, Makiyama
C, Denda T, et al: Propensity score analysis of regorafenib versus
trifluridine/tipiracil in patients with metastatic colorectal
cancer refractory to standard chemotherapy (REGOTAS): A Japanese
society for cancer of the colon and rectum multicenter
observational study. Oncologist. 23:7–15. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yoon SE, Lee SJ, Lee J, Park SH, Park JO,
Lim HY, Kang WK, Park YS and Kim ST: The impact of primary tumor
sidedness on the effect of regorafenib in refractory metastatic
colorectal cancer. J Cancer. 10:1611–1615. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Schrock AB, Ouyang C, Sandhu J, Sokol E,
Jin D, Ross JS, Miller VA, Lim D, Amanam I, Chao J, et al: Tumor
mutational burden is predictive of response to immune checkpoint
inhibitors in MSI-high metastatic colorectal cancer. Ann Oncol.
30:1096–1103. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mandal R, Samstein RM, Lee KW, Havel JJ,
Wang H, Krishna C, Sabio EY, Makarov V, Kuo F, Blecua P, et al:
Genetic diversity of tumors with mismatch repair deficiency
influences anti-PD-1 immunotherapy response. Science. 364:485–491.
2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Innocenti F, Ou FS, Qu X, Zemla TJ,
Niedzwiecki D, Tam R, Mahajan S, Goldberg RM, Bertagnolli MM,
Blanke CD, et al: Mutational analysis of patients with colorectal
cancer in CALGB/SWOG 80405 identifies new roles of microsatellite
instability and tumor mutational burden for patient outcome. J Clin
Oncol. 37:1217–1227. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
André T, Shiu KK, Kim TW, Jensen BV,
Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs
P, et al: Pembrolizumab in microsatellite-instability-high advanced
colorectal cancer. N Engl J Med. 383:2207–2218. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Grothey A, Prager G and Yoshino T: The
mechanism of action of regorafenib in colorectal cancer: A guide
for the community physician. Clin Adv Hematol Oncol. 12 (Suppl
17):S1–S19. 2019.PubMed/NCBI
|
|
15
|
Arai H, Battaglin F, Wang J, Lo JH, Soni
S, Zhang W and Lenz HJ: Molecular insight of regorafenib treatment
for colorectal cancer. Cancer Treat Rev. 81(101912)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Abou-Elkacem L, Arns S, Brix G, Gremse F,
Zopf D, Kiessling F and Lederle W: Regorafenib inhibits growth,
angiogenesis, and metastasis in a highly aggressive, orthotopic
colon cancer model. Mol Cancer Ther. 12:1322–1331. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cannarile MA, Weisser M, Jacob W, Jegg AM,
Ries CH and Rüttinger D: Colony-stimulating factor 1 receptor
(CSF1R) inhibitors in cancer therapy. J Immunother Cancer.
5(53)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wu RY, Kong PF, Xia LP, Huang Y, Li ZL,
Tang YY, Chen YH, Li X, Senthilkumar R, Zhang HL, et al:
Regorafenib promotes antitumor immunity via inhibiting PD-L1 and
IDO1 expression in melanoma. Clin Cancer Res. 25:4530–4541.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fukuoka S, Hara H, Takahashi N, Kojima T,
Kawazoe A, Asayama M, Yoshii T, Kotani D, Tamura H, Mikamoto Y, et
al: Regorafenib plus nivolumab in patients with advanced gastric or
colorectal cancer: An open-label, dose-escalation, and
dose-expansion phase Ib trial (REGONIVO, EPOC1603). J Clin Oncol.
38:2053–2061. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Karkeni E, Morin SO, Tayeh BB, Goubard A,
Josselin E, Castellano R, Fauriat C, Guittard G, Olive D and Nunès
JA: Vitamin D controls tumor growth and CD8+ T cell
infiltration in breast cancer. Front Immunol.
10(1307)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ducreux M, Petersen LN, Öhler L, Bergamo
F, Metges JP, de Groot JW, Wang JY, Paredes BC, Dochy E,
Fiala-Buskies S, et al: Safety and effectiveness of regorafenib in
patients with metastatic colorectal cancer in routine clinical
practice in the prospective, observational CORRELATE study. Eur J
Cancer. 123:146–154. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Adenis A, de la Fouchardiere C, Paule B,
Burtin P, Tougeron D, Wallet J, Dourthe LM, Etienne PL, Mineur L,
Clisant S, et al: Survival, safety, and prognostic factors for
outcome with regorafenib in patients with metastatic colorectal
cancer refractory to standard therapies: Results from a multicenter
study (REBECCA) nested within a compassionate use program. BMC
Cancer. 16(412)2016.PubMed/NCBI View Article : Google Scholar
|