Introduction
Bladder cancer (BC) is the most common urinary tract
malignancy, ranking 9th in frequency and 13th in mortality rate
amongst other types of cancer (1).
The global age-standardized incidence rate and age-standardized
mortality rate of this disease per 100,000 population are 5.7 (2.4
in women and 9.6 in men) and 1.9 (0.87 in women and 3.2 in men),
respectively (2). BC tends to
occur more frequently in regions with high Human Development Index,
and its mortality rate is higher in those regions as well (3). The main risk factors contributing to
BC development are tobacco smoking and occupational exposure to
carcinogens (such as various aromatic amines, which are used in dye
and rubber industry), and the incidence rate of BC appears to be
declining in regions with an overall decrease in the smoking
population (4).
BC is known to have high recurrence rate. In a
population-based analysis Chamie et al (5) demonstrated that 39.1% of patients
experienced recurrence without progression, while 33.0% had
recurrence with progression. Therefore, patients require continuous
follow-up over the course of the first 5 years following cancer
resection (every 3-6 months), making BC one of the most costly
human cancers in terms of patient management (6,7).
Cystoscopy and urine cytology are widely accepted as
routine techniques for the detection, diagnosis and surveillance of
BC. Cystoscopy is an effective diagnostic tool, with a sensitivity
of 62-84% and a specificity of 43-98%, depending on the tumor stage
and type (8). However, its
invasive nature, high cost and high intra- and interobserver
variability limit the diagnostic potential of this technique
(9,10). In addition, following cystoscopy,
patients may suffer from various adverse effects, such as pain
during urination (50%), frequent urination (37%), hematuria (17%)
and urinary tract infection (3%) (11,12).
Urine cytology, on the other hand, is a non-invasive approach with
a high sensitivity (up to 100%) for high-grade tumors and a low
sensitivity (4-31%) for low-grade tumors (13). However, interpretation of urine
cytology results may be impeded in the presence of urolithiasis,
infections and low cellular yield (14).
Therefore, there is an urgent need to improve the
methods for BC diagnosis and follow-up. Due to both recent advances
in genomic, epigenomic and proteomic studies of tumor tissue, and
the development of sensitive analytical molecular techniques, a new
approach to cancer detection has emerged, namely liquid biopsy
(15). This technique is based on
the analysis of various tumor-related targets circulating in
biological fluids, such as blood, urine, saliva and cerebrospinal
fluid (16-19).
The aforementioned targets include cell-free tumor DNA (tDNA),
different types of tumor RNAs, peptides, metabolites, exosomes,
endosomes, and even circulating tumor cells (20,21).
Direct liquid biopsy and its modifications have been proven to be
useful in almost every type of cancer, and currently represent an
exponentially growing field of precision medicine (22,23).
As regards BC, urine is the bodily fluid most
suitable for examination, as it bathes the surface of urothelial
cells for an extended period of time, collecting cell-free tDNA and
containing cells exfoliated from the tumor surface. Although the
same, at least to some extent, may be said about blood, it was
proven that the accuracy of BC diagnosis by means of cell-free tDNA
detection is markedly lower in blood compared with urine (24,25).
Despite the fact that the usefulness of urine tDNA-based liquid
biopsy was successfully demonstrated in various regions worldwide,
it is important to mention that the genetic features of a given
population may have an impact on the diagnostic efficiency of a set
biomarker (26-28).
To the best of our knowledge, no clinical studies evaluating the
advantages of the aforementioned approach have been conducted to
date in the Russian Federation, the population of which has been
proven to be genetically distinctive (29,30).
Thus, the aim of the present pilot study was to
assess the diagnostic potential of urine tDNA-based liquid biopsy
in patients with an ongoing oncological process, as well as in
post-resection patients at risk of BC recurrence.
The telomerase reverse transcriptase (TERT) promoter
‘hotspot’ mutations C228T and C250T were selected as tDNA
biomarkers, since they appear to be the most common in BC tumors
(40-75%) and are completely absent in healthy somatic cells
(31-33).
Droplet Digital PCR (ddPCR) was the analytical method of choice due
to its ability to precisely quantify target DNA, even in case of
low allelic frequency (34-37).
This approach to detecting TERT promoter mutations was previously
proven to be comparable to the next-generation sequencing
(NGS)-based validated system ‘UroMuTERT’ in terms of diagnostic
accuracy (38).
Materials and methods
Pilot study design
The study was approved by the Local Ethics Committee
of Medical Research and Educational Center of Lomonosov Moscow
University (protocol no. 4/20, dated 27.04.2020) and conducted
according to the tenets of the Declaration of Helsinki. All
involved patients provided signed informed consent forms. The pilot
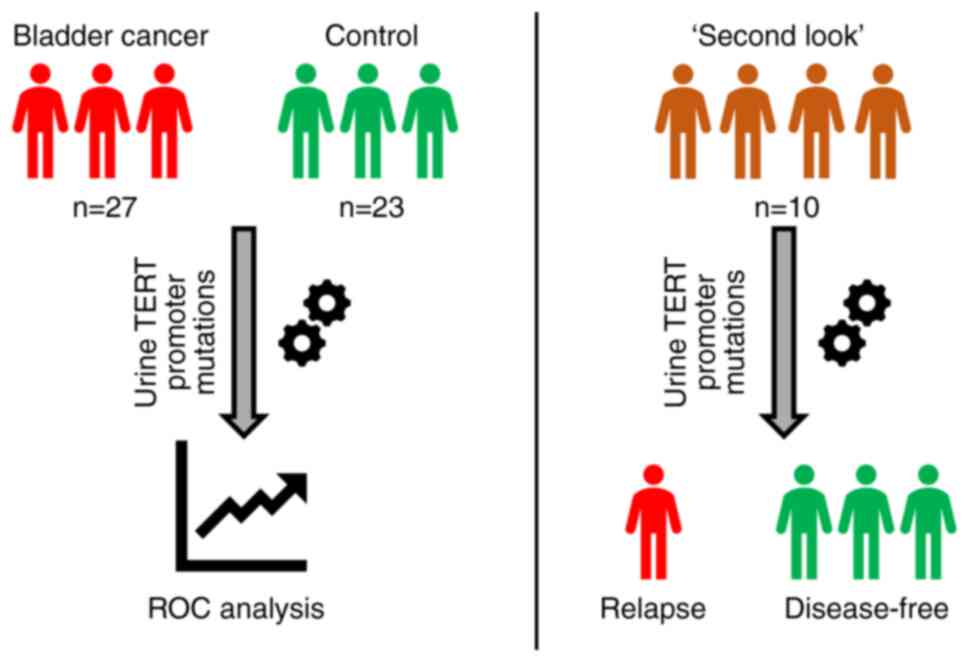
study design is shown in Fig. 1.
In total, 60 patients were enrolled between April 2019 and October
2020; among those, 27 patients had histologically proven
non-muscle-invasive BC (BC group); 23 had either no urological
disease, or cystitis/benign prostatic hyperplasia with no signs of
BC during cystoscopy, which was performed in case of hematuria
(control group); and 10 patients underwent transurethral malignancy
resection 3-6 month prior to study enrollment, with negative
cystoscopy results at the time of biomaterial donation (‘second
look’ group). The clinical and demographic characteristics of the
study participants are summarized in Table I. Tumor size was evaluated via
transabdominal 3D ultrasonography using SonoAce X8 system (Samsung
Medison Co., Ltd.).
 | Table IClinical and demographic
characteristics of study participants. |
Table I
Clinical and demographic
characteristics of study participants.
| Parameters | Bladder cancer
group (n=27) | Control group
(n=23) | ‘Second look’ group
(n=10) |
|---|
| Age,
yearsa | 66 (26-85) | 35 (22-65) | 65 (44-83) |
| Sex, n | | | |
|
Male | 22 | 16 | 9 |
|
Female | 5 | 7 | 1 |
| Smoking, n | 7 | 1 | 3 |
| Tumor stage, n | | | |
|
0a | 0 | N/A | 1b |
|
I | 25 | N/A | 9b |
|
II | 1 | N/A | 0b |
|
III | 1 | N/A | 0b |
| Tumor grade, n | | | |
|
Low | 16 | N/A | N/A |
|
High | 6 | N/A | N/A |
|
Unknown | 5 | N/A | N/A |
Sample collection and processing
Urine samples were collected prior to any
diagnostic/surgical manipulations. Intact urine was aliquoted into
4-ml tubes and immediately stored at -20˚C. After defrosting, each
urine sample was thoroughly mixed by pulse-vortexing. DNA was
isolated from 4 ml of intact urine using QIAamp Circulating Nucleic
Acid Kit (Qiagen GmbH) according to the manufacturer's
instructions. The incubation during the lysis phase was prolonged
for an additional 20 min to ensure that all remaining cell
particles had been eliminated, as the standard manufacturer's
protocol was designed for urine supernatant and not for intact
biomaterial. Such approach allows to enrich the resulting solution
with cell-free tDNA, as well as with genomic tDNA, and to ensure
high DNA concentration in the sample.
Synthetic DNA constructs
The reference plasmids were created using pUC19
vector (cloning sites KpnI and HindIII). The mutant
inserts were obtained with PCR-amplification with the next primers:
Forward primer, 5'-ATAGGTACCAGTGGATTCGCGGGCACAGA-3' and reverse
primer, 5'-AGTCAAAAGCTTCAGCGCTGCCTGAAACT-3' (Evrogen RU, AO).
Genomic DNA isolated from liver cancer cells (originating from the
HepG2 cell line; cat no. 85011430; MilliporeSigma) was used for
amplification of the mutant C228T fragment. To amplify the mutant
insert, Q5® High-Fidelity DNA Polymerase (New England
BioLabs, Inc.) was used. The final stage was blue-white selection
of obtained plasmids. In order to create the plasmid, which
contains both C228T and C250T mutations, site-directed mutagenesis
was applied to the plasmid with the C228T insert, followed by
treatment with DpnI and blue-white selection.
tDNA quantification
Quantification of DNA was performed using the QX200
ddPCR System (Bio-Rad Laboratories, Inc.). Droplet generation,
amplification and analysis were carried out following the
manufacturer's instructions. Detection of TERT promoter C228T and
C250T mutations was achieved using TaqMan Liquid Biopsy dPCR Assays
(TERT_C228T, Assay ID Hs000000092_rm and TERT_C250T, Assay ID
Hs000000093_rm; Thermo Fisher Scientific, Inc.). The thermocycling
conditions were set according to the manufacturer's recommendations
for the aforementioned assays (holding stage at 95˚C for 10 min, 40
cycles of denaturation at 94˚C for 30 sec and annealing at 60˚C for
1 min, holding stage at 98˚C for 10 min). Human Genomic DNA Version
09 (Roche Diagnostics) was used to establish the false-positive
droplet threshold in a series of ddPCR experiments with gradually
increasing control DNA input. This approach is necessary to
distinguish positive and negative clinical samples at lower levels
of DNA. The linearity of the tDNA quantification was assessed in a
series of experiments with gradual dilutions of the aforementioned
synthetic DNA constructs, carrying TERT promoter C228T and C250T
mutations, in the control genomic DNA solution.
Statistical analysis
The results of tDNA quantification are presented as
mutated DNA fraction: Mutated DNA/(wild type + mutated DNA), %.
Data were analyzed using IBM SPSS Statistics 22.0 Software (IBM
Corp). Receiver operating characteristic (ROC) curve analysis was
carried out to establish tDNA fraction cut-off value at optimal
sensitivity and specificity. Area under the ROC curve (AUC),
overall accuracy, positive and negative predictive values were
calculated to evaluate the diagnostic power of TERT promoter
mutations-based urine liquid biopsy. Data distribution was assessed
using Shapiro-Wilk's test. Due to absence of normal distribution,
the correlation between tDNA fraction and tumor size was calculated
using Spearman's rank correlation coefficient. The linear
association of two variables was expressed with the linear
correlation coefficient value (rs). P<0.05 was
considered to indicate a statistically significant difference.
Results
Preclinical assay validation
In order to combat the issue of false-positive
droplets, a series of ddPCR experiments with wild-type control DNA
samples were conducted. While still being in a low input range
(<400 copies/µl per reaction), the concentration of DNA in each
sample was gradually increasing from 50 to 400 copies/µl per
reaction. The maximum number of false-positive droplets per sample
using TERT C228T and C250T primers and probes was registered
(Fig. S1). According to these
results, the false-positive droplet threshold was set to 3
droplets. Thus, all samples with ≤3 positive droplets were
considered as mutation-negative by default.
Determination of the quantitative tDNA analysis
linearity is a crucial step in the preclinical assay validation.
Experimentally calculated tDNA fraction demonstrated a strong
linear association with the expected tDNA fraction, achieved
through gradual dilution of mutation-positive plasmid in control
genomic DNA solution, for both TERT promoter C228T (r=0.94) and
C250T (r=0.99) mutations (Fig.
S2).
Evaluation of liquid biopsy analytical
parameters
All urine-derived DNA samples of patients from the
BC and control groups were successfully analyzed by means of ddPCR.
The results of tDNA quantification in each studied sample are
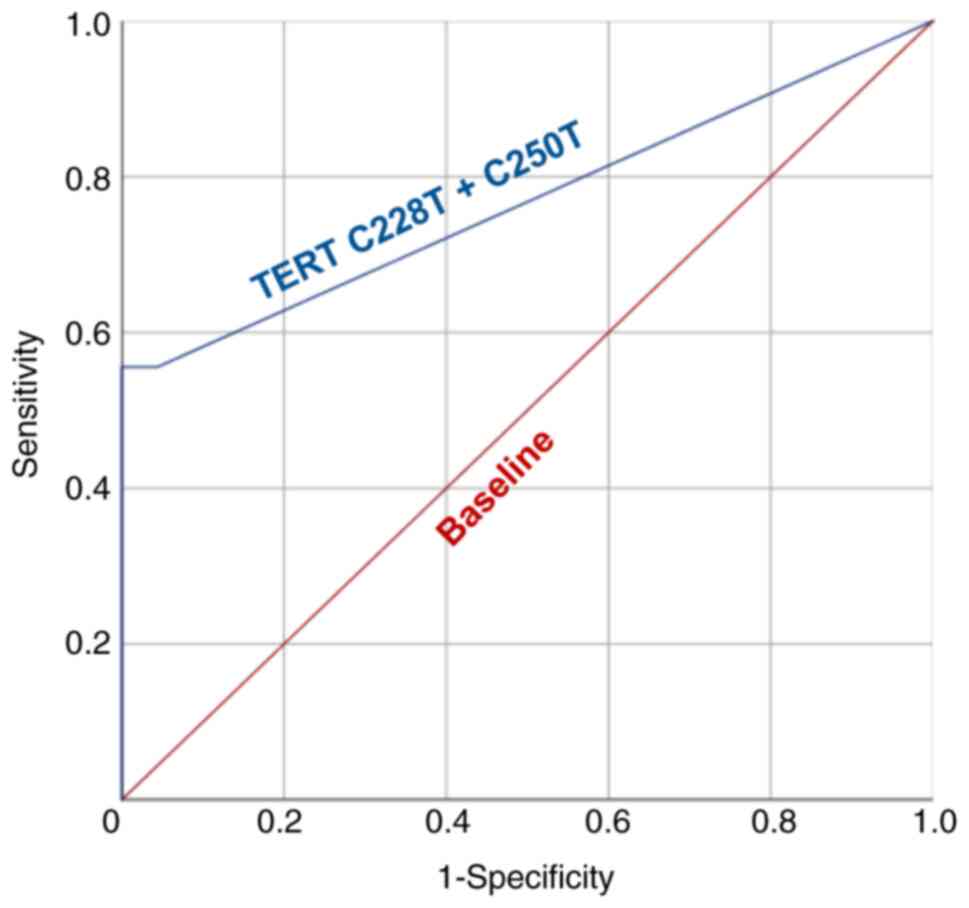
summarized in Table SI. ROC curve
analysis was implemented to establish the tDNA cut-off value and to
determine the analytical parameters of TERT promoter
mutations-based urine liquid biopsy (Fig. 2). The AUC appeared to be 0.768,
indicating a significant diagnostic power of the presented model.
According to the results of ROC curve analysis, the cut-off value
was established at tDNA fraction 0.34%. Samples with >3
mutation-positive droplets, but <0.34% tDNA fraction, were still
considered as mutation-negative.
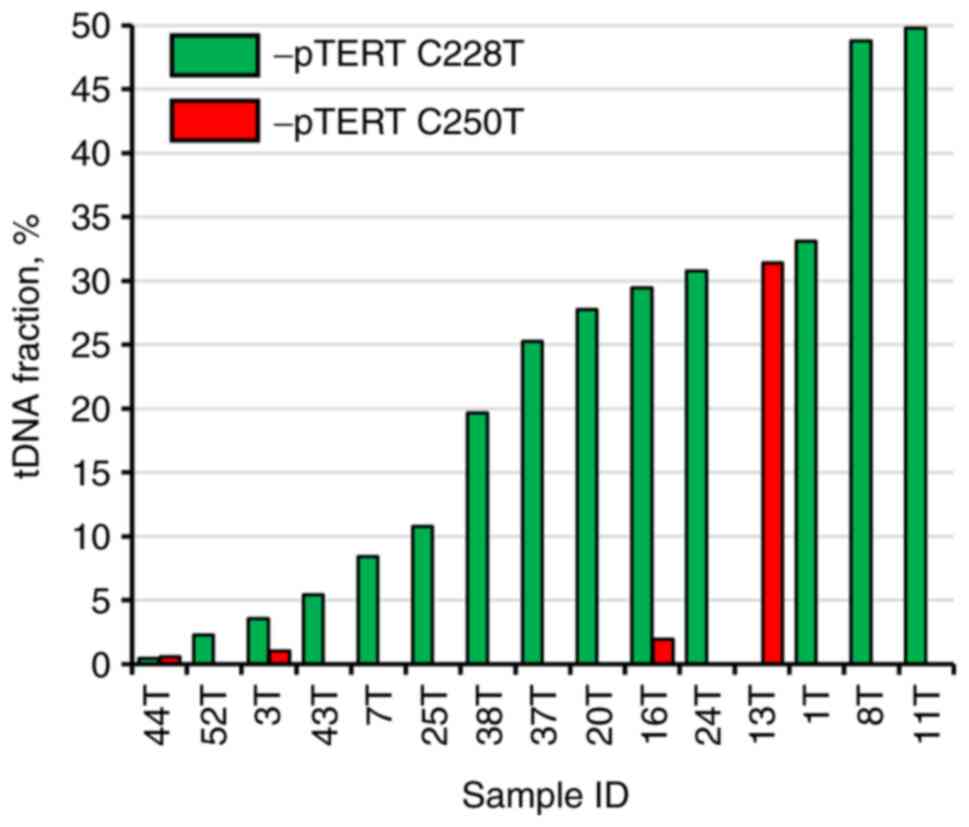
At the set cut-off value, the sensitivity and
specificity were 55.56 and 100%, respectively. tDNA fraction varied
significantly from 0.59 to 48.77% (median, 25.27%; interquartile
range, 5.41-31.39%; Fig. 3).
Examples of ddPCR outcomes of TERT promoter mutations detection in
representative patients are provided in Fig. S3. Nevertheless, no correlation was
observed between tumor size and tDNA fraction (rs=0.174,
P=0.535), or tDNA level (expressed in copies/µl per reaction
mixture; rs=0.129, P=0.646). TERT promoter C228T
mutation appeared to be the most frequent, as it was detected in
14/27 patients, while C250T mutation alone was detected in 4/27
patients, with both mutations simultaneously observed in 3
cases.
Detection of BC recurrence
As previously stated, BC is characterized by a
markedly high recurrence rate; therefore, post-resection patients
require regular follow-up diagnostic procedures every 3-6 months
(5-7).
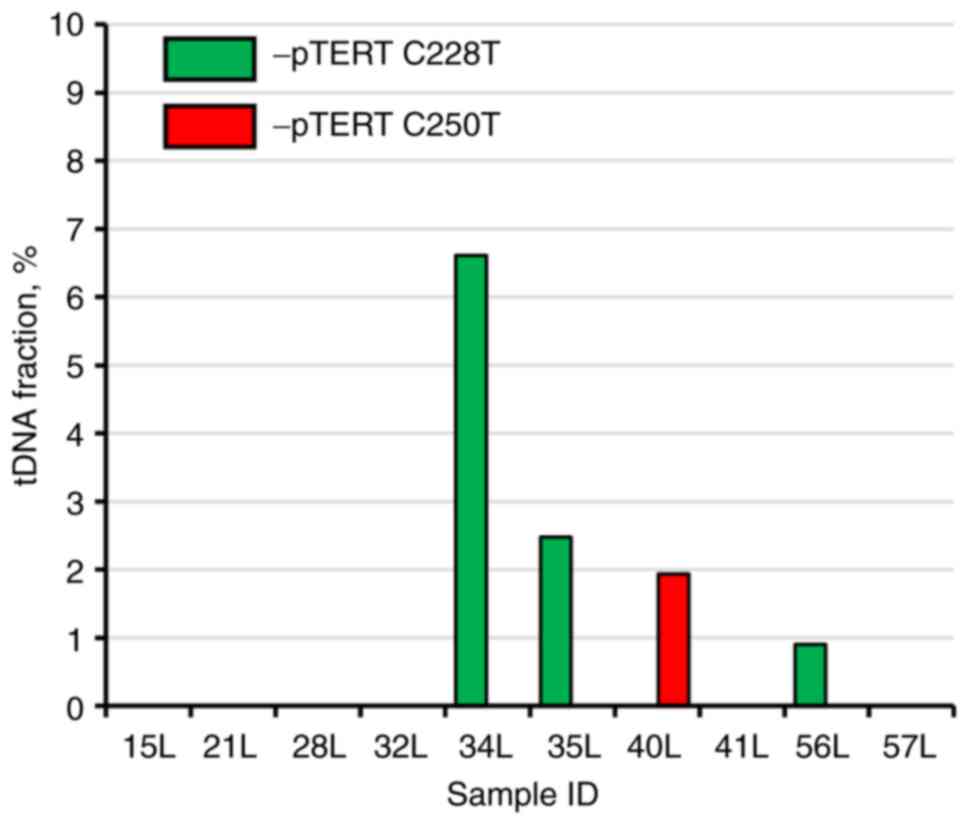
TERT promoter mutations-based liquid biopsy of the ‘second look’
group revealed that 3/10 patients had TERT promoter C228T mutated
DNA molecules in their urine, and 1/10 had C250T mutated genetic
material. The tDNA fraction in mutation-positive patients from the
‘second look’ group was significantly lower compared with that in
the BC group, ranging from 0.90 to 6.61% (Fig. 4). It is important to mention that
cystoscopy was performed immediately after the biomaterial
collection was found to be negative, and from the clinical
perspective it cannot be stated yet that these patients experience
a BC recurrence. However, the fact that false-positive results were
completely absent during the analysis of the control group allows
us to hypothesize that BC recurrence has already occurred at the
cellular level and its clinical diagnosis is expected in the near
future.
Discussion
ddPCR is a robust and precise analytical tool for
absolute quantification of DNA molecules, which is capable of
detection of individual tumor-derived copies of genetic material
(39,40). Theoretically, a single
mutation-positive droplet in a sample indicates the presence of a
malignant cell in the organism; however, ddPCR as a method has
several limitations, such as non-specific amplification and
fluorescent probe degradation, which may cause some droplets to be
detected as false-positive (41).
Generally, the more amplification events occur (high concentration
of DNA in the sample, increased amount of amplification cycles),
the more false-positive droplets are detected. If the DNA input is
sufficiently large, a few false-positive droplets cannot disrupt
the analysis, as the overall tDNA fraction will be close to zero
and the competently established cut-off value (which may be
obtained during comparison of patients with BC with an adequate
group of control patients) will separate false-positive samples
from the true-positive ones. However, this is not the case in
samples with low DNA input, in which even a single false-positive
DNA molecule may easily mislead the operator. In the present study,
a simple approach to diminishing the risk of false-positive
droplets was demonstrated based on a series of experiments with
gradual dilution of wild-type DNA. This approach allows to simplify
the ddPCR plate layout during the analysis of clinical samples,
eliminating the need to use positive control for mutation
calling.
The advent of oncology-related metagenomic projects,
such as ‘Catalogue Of Somatic Mutations In Cancer’ and ‘The Cancer
Genome Atlas Program’, among others, has enabled the development of
targeted genetic approaches to the diagnosis of certain types of
cancer (42-45).
The present pilot study is an example of the implementation of such
an approach. TERT promoter mutations have already been proven to be
one of the most frequent and reliable detectable genetic
alterations in the urine of patients with BC in various populations
(24,31,38,46,47).
The sensitivity of the assay achieved in the pilot
study was 55.56%, which is not considerably high, although, as it
was previously mentioned, it appears that these mutations are
present only in 40-75% of patients BC (31,33).
Furthermore, in other similar urine liquid biopsy studies, TERT
promoter mutations were detected in 52-56% of patients with BC,
which was in complete concordance with our results (46-48).
Both studied mutations activate TERT gene transcription and are
associated with elevated telomerase activity and stable telomere
length (49). However, they are
subject to different regulatory mechanisms, which may find clinical
relevance upon further investigation (50). Thus, it is viable to analyze both
mutations, although in the present study the contribution of the
C250T mutation to the overall sensitivity was significantly lower
compared with that of C228T, as it was detected in only 4/27 vs.
14/27 of the patients, respectively.
TERT promoter mutations, due to their high frequency
in BC, may be used in BC diagnosis on their own with an appropriate
level of sensitivity, yet it is obvious that a more comprehensive
approach (in terms of selected mutations) will have a greater
diagnostic power.
Ou et al (24) developed a NGS-based model, which
included 5 genes for urine supernatant (TERT, FGFR3, TP53, PIK3CA
and KRAS) and 7 genes for urine sediment (TERT, FGFR3, TP53, HRAS,
PIK3CA, KRAS and ERBB2), which yielded AUCs of 0.94 and 0.91,
respectively, in the validation cohort. Moreover, the combination
of mutations (TERT, FGFR3 and HRAS) and methylation biomarkers
(OTX1, ONECUT2 and TWIST1) also led to incredible results, with an
AUC of 0.96, as reported by van Kessel et al (51). ddPCR, which was used in the present
pilot study, is not suitable for a simultaneous analysis of
multiple hotspot mutations in several genes, although extension of
the mutations panel up to 8 targets per urine-derived DNA sample is
easily achievable without any notable time delay or increase in
cost. New perspective genetic BC biomarkers emerge regularly; for
example, Wu et al (52)
recently conducted a study dedicated to the sequencing of
non-coding elements in BC; it was demonstrated that two neighboring
point mutations in the enhancer region of the ADGRG6 gene are quite
frequent (~25% of patients). Thus, the selection of up to 8 most
frequent mutations will not impair the ddPCR-based liquid biopsy
and is expected to make it at least on par with the aforementioned
NGS-based approaches in terms of diagnostic efficiency, as they are
more cost-effective and easier to implement in a common clinical
laboratory.
As previously mentioned, no correlation was observed
in our pilot study between tumor size and level/fraction of
tumor-derived DNA; however, the sample size was not sufficiently
large to reach definitive conclusions. Nonetheless, this appears to
be counterintuitive and it would be reasonable to hypothesize that,
the bigger the tumor, the higher the tDNA fraction, both in the
urine and plasma. In fact, this topic is more complex when several
other factors are considered.
Recent cancer cell culture studies demonstrated that
the cell-free DNA levels did not correlate well with the process of
apoptosis and necrosis, thereby providing evidence for the active
release of cell-free DNA, the dynamics of which are yet to be
uncovered (53,54). Additionally, it is known that tumor
growth may be paired with nutrient deprivation of the surrounding
tissue and the organism as a whole, which may eventually cause an
increase in the overall release of cell-free non-tDNA; thus,
theoretically, with the growth of the tumor its DNA fraction value
will not grow, as actually both elements of its equation (nominator
and denominator) will increase (55). In a large multi-center study it was
demonstrated that the tumor-derived cell-free DNA fraction varies
significantly in patients with the same type and stage of cancer
(56). However, some studies
reported a correlation with advanced-stage disease or with a
sufficiently large tumor size, starting from 27 cm3
(57,58). Absolute tDNA value (expressed in
copies/µl) is an alternative to tDNA fraction, although it is
highly susceptible to DNA isolation inconsistency and, in our case,
to the state of the biomaterial, as intact urine was used, in which
the content of exfoliated tumor cells is expected to vary
significantly among different patients.
Although TERT promoter mutations are generally
considered to be an early event in tumor formation, this does not
indicate that all malignant cells carry these genetic alterations
(59). Amongst the
mutation-positive pilot study participants, 3 patients had TERT
promoter C228T and C250T mutations simultaneously, and the tDNA
fractions were as follows: In the first patient, 0.45 and 0.59%,
respectively; in the second patient, 3.55 and 1.03%, respectively;
and in the third patient, 29.46 and 1.93%, respectively. In the
third patient, the difference was almost 15-fold, indicating that
the C250T mutation possibly occurred notably later in the course of
disease progression. This fact allows us to infer that the same may
be applicable in other cases of BC: The tumor may be large, but
only a certain proportion of its cells carries the studied
mutation. Of note, detection of several hotspot mutations at once
and usage in the following calculations of the one with the highest
fraction or level can substantially decrease the influence of the
discussed phenomenon.
Therefore, a larger sample size, an extended
mutation panel and implementation of another tDNA level
normalization approach are required to assess the ability of tDNA
to reflect the tumor size with more confidence.
Cell-free tDNA-based liquid biopsy not only carries
a great diagnostic potential in patients presenting with at least
some clinical BC manifestations, but it is also capable of
detecting BC up to 10 years prior to clinical diagnosis (60). Moreover, it was recently
demonstrated that post-radical cystectomy patients with metastatic
relapse had significantly higher cell-free plasma tDNA levels
compared with disease-free patients during a 3-year follow-up
(61). These data, as well as the
absence of false-positive results in our pilot study, suggest that
mutation-positive patients from the ‘second look’ group are likely
to experience cancer relapse. All patients from this group will be
participating in our main study, together with additional recruited
participants. We plan to continue their post-malignancy resection
follow-up and to monitor the individual tDNA status in the
urine.
In conclusion, urine liquid biopsy has promising
clinical potential, making it the next possible step in the field
of precision medicine. Future incorporation of this non-invasive
diagnostic approach into the routine clinical workflow may
significantly decrease the number of costly manipulations, such as
cystoscopy. Our plans for the main study include recruitment of
additional patients, extension of the mutation panel, simultaneous
analysis of DNA derived from tumor tissue, plasma and urine,
prolonged follow-up of the post-resection patients, as well as
screening of industrial workers exposed to specific
carcinogens.
There were certain limitations to the present study:
The sample size in the present pilot study was not sufficient to
reliably reflect the TERT promoter mutation frequency in Russian
patients with BC. An increase of the number of study participants
in the main study may alter the calculated sensitivity and
specificity for this biomarker. Absence of genomic DNA derived
directly from the resected tumor limits our knowledge on the actual
tumor genotype in the BC and the ‘second look’ groups. TERT
promoter mutations are also frequently encountered in several other
cancer types, such as glioma, melanoma, pancreatic and thyroid
cancers (62). Thus, there is a
slight possibility that mutation-positive DNA detected in the urine
may have originated from another undiagnosed non-BC primary
tumor.
Supplementary Material
False-positive droplets threshold
determination for: (A) TERT promoter mutation C228T; and (B) TERT
promoter mutation C250T. TERT, telomerase reverse
transcriptase.
Determination of the linearity of the
TERT promoter mutation quantitative analysis. (A) TERT promoter
mutation C228T assay; and (B) TERT promoter mutation C250T assay.
MAF, minor allele frequency; TERT, telomerase reverse
transcriptase. *Linear correlation coefficient
value.
Examples of digital droplet PCR
outcome: (A) Patient with low levels of urine DNA; (B) patient with
high levels of urine DNA.
Results of urine TERT promoter C228T
and C250T detection in each clinical sample.
Acknowledgements
Not applicable.
Funding
The clinical part of the pilot study was carried out within the
state assignment of Lomonosov Moscow State University. The
preclinical experiments were supported by the Russian Foundation
for Basic Research (grant no. 18-29-08040).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
Conceptualization, AK and MZ; methodology, MJ and
EP; software, PK and EP; validation, DK, AT, LS and AB; formal
analysis, DO; investigation, MJ and MZ; resources, DK and AT; raw
data generation, MJ and PK; data curation, AT and DK;
writing-original draft preparation, MJ; writing-review and editing,
MZ and AB; visualization, DO; supervision, LS; project
administration, AK; funding acquisition, AK and MZ. All the authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Local Ethics Committee
of Medical Research and Educational Center of Lomonosov Moscow
University (protocol no. 4/20, dated 27.04.2020) and conducted
according to the tenets of the Declaration of Helsinki. All
involved patients provided signed informed consent forms.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Antoni S, Ferlay J, Soerjomataram I, Znaor
A, Jemal A and Bray F: Bladder cancer incidence and mortality: A
global overview and recent trends. Eur Urol. 71:96–108.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mohammadian M, Safari A, Bakeshei KA, Asti
A, Mohammadian-Hafshejani A, Salehiniya H, Emamiyan M and Khakpour
H: Recent patterns of bladder cancer incidence and mortality: A
global overview. World Cancer Res J. 7(e1464)2020.
|
|
3
|
Dy GW, Gore JL, Forouzanfar MH, Naghavi M
and Fitzmaurice C: Global burden of urologic cancers, 1990-2013.
Eur Urol. 71:437–446. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cumberbatch MGK, Jubber I, Black PC,
Esperto F, Figueroa JD, Kamat AM, Kiemeney L, Lotan Y, Pang K,
Silverman DT, et al: Epidemiology of bladder cancer: A systematic
review and contemporary update of risk factors in 2018. Eur Urol.
74:784–795. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chamie K, Litwin MS, Bassett JC, Daskivich
TJ, Lai J, Hanley JM, Konety BR and Saigal CS: Urologic Diseases in
America Project. Recurrence of high-risk bladder cancer: A
population-based analysis. Cancer. 119:3219–3227. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Picozzi S, Ricci C, Gaeta M, Ratti D,
Macchi A, Casellato S, Bozzini G and Carmignani L: Upper urinary
tract recurrence following radical cystectomy for bladder cancer: A
meta-analysis on 13,185 patients. J Urol. 188:2046–2054.
2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mariotto AB, Robin Yabroff K, Shao Y,
Feuer EJ and Brown ML: Projections of the cost of cancer care in
the United States: 2010-2020. J Natl Cancer Inst. 103:117–128.
2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Jocham D, Stepp H and Waidelich R:
Photodynamic diagnosis in urology: State-of-the-art. Eur Urol.
53:1138–1150. 2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sutton AJ, Lamont JV, Evans R, Williamson
K, O'Rourke D, Duggan B, Sagoo GS, Reid CN and Ruddock MW: An early
analysis of the cost-effectiveness of a diagnostic classifier for
risk stratification of haematuria patients (DCRSHP) compared to
flexible cystoscopy in the diagnosis of bladder cancer. PLoS One.
13(e0202796)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Glatz K, Willi N, Glatz D, Barascud A,
Grilli B, Herzog M, Dalquen P, Feichter G, Gasser TC, Sulser T and
Bubendorf L: An international telecytologic quiz on urinary
cytology reveals educational deficits and absence of a commonly
used classification system. Am J Clin Pathol. 126:294–301.
2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Biardeau X, Lam O, Ba V, Campeau L and
Corcos J: Prospective evaluation of anxiety, pain, and
embarrassment associated with cystoscopy and urodynamic testing in
clinical practice. J Can Urol Assoc. 11:104–110. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Burke DM, Shackley DC and O'Reilly PH: The
community-based morbidity of flexible cystoscopy. BJU Int.
89:347–349. 2002.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lotan Y and Roehrborn CG: Sensitivity and
specificity of commonly available bladder tumor markers versus
cytology: Results of a comprehensive literature review and
meta-analyses. Urology. 61:109–118. 2003.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Raitanen MP, Aine R, Rintala E, Kallio J,
Rajala P, Juusela H and Tammela TLJ: Differences between local and
review urinary cytology in diagnosis of bladder cancer. An
interobserver multicenter analysis. Eur Urol. 41:284–289.
2002.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jung A and Kirchner T: Liquid biopsy in
tumor genetic diagnosis. Dtsch Arztebl Int. 115:169–174.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rothé F, Laes JF, Lambrechts D, Smeets D,
Vincent D, Maetens M, Fumagalli D, Michiels S, Drisis S, Moerman C,
et al: Plasma circulating tumor DNA as an alternative to metastatic
biopsies for mutational analysis in breast cancer. Ann Oncol.
25:1959–1965. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Millholland JM, Li S, Fernandez CA and
Shuber AP: Detection of low frequency FGFR3 mutations in the urine
of bladder cancer patients using next-generation deep sequencing.
Res reports Urol. 4:33–40. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Pan W, Gu W, Nagpal S, Gephart MH and
Quake SR: Brain tumor mutations detected in cerebral spinal fluid.
Clin Chem. 61:514–522. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liang Y, Han J, Jia C, Ma Y, Lan Y, Li Y
and Wang S: Effect of endometrial injury on secretion of
endometrial cytokines and IVF outcomes in women with unexplained
subfertility. Mediators Inflamm. 2015(757184)2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Arneth B: Update on the types and usage of
liquid biopsies in the clinical setting: A systematic review. BMC
Cancer. 18(527)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mathai R, Vidya R, Reddy B, Thomas L,
Udupa K, Kolesar J and Rao M: Potential utility of liquid biopsy as
a diagnostic and prognostic tool for the assessment of solid
tumors: Implications in the precision oncology. J Clin Med.
8(373)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Remon J, García-Campelo R, de Álava E,
Vera R, Rodríguez-Peralto JL, Rodríguez-Lescure Á, Bellosillo B,
Garrido P, Rojo F and Álvarez-Alegret R: Liquid biopsy in oncology:
A consensus statement of the spanish society of pathology and the
spanish society of medical oncology. Clin Transl Oncol. 22:823–834.
2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chen M and Zhao H: Next-generation
sequencing in liquid biopsy: Cancer screening and early detection.
Hum Genomics. 13(34)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ou Z, Li K, Yang T, Dai Y, Chandra M, Ning
J, Wang Y, Xu R, Gao T, Xie Y, et al: Detection of bladder cancer
using urinary cell-free DNA and cellular DNA. Clin Transl Med.
9(4)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Avogbe PH, Manel A, Vian E, Durand G,
Forey N, Voegele C, Zvereva M, Hosen MI, Meziani S, De Tilly B, et
al: Urinary TERT promoter mutations as non-invasive biomarkers for
the comprehensive detection of urothelial cancer. EBioMedicine.
44:431–438. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lodewijk I, Dueñas M, Rubio C,
Munera-Maravilla E, Segovia C, Bernardini A, Teijeira A, Paramio JM
and Suárez-Cabrera C: Liquid biopsy biomarkers in bladder cancer: A
current need for patient diagnosis and monitoring. Int J Mol Sci.
19(2514)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang K, Liu T, Liu C, Meng Y, Yuan X, Liu
L, Ge N, Liu J, Wang C, Ren H, et al: TERT promoter mutations and
TERT mRNA but Not FGFR3 mutations are urinary biomarkers in han
chinese patients with urothelial bladder cancer. Oncologist.
20:263–269. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yuan X, Liu C, Wang K, Liu L, Liu T, Ge N,
Kong F, Yang L, Björkholm M, Fan Y, et al: The genetic difference
between Western and Chinese urothelial cell carcinomas: Infrequent
FGFR3 mutation in Han Chinese patients. Oncotarget. 7:25826–25835.
2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Stepanov VA, Balanovsky OP, Melnikov AV,
Lash-Zavada AY, Khar'kov VN, Tyazhelova TV, Akhmetova VL, Zhukova
OV, Shneider YV, Shil'nikova IN, et al: Characteristics of
populations of the russian federation over the panel of fifteen
loci used for DNA identification and in forensic medical
examination. Acta Naturae. 3:56–67. 2011.PubMed/NCBI
|
|
30
|
Zhernakova DV, Brukhin V, Malov S, Oleksyk
TK, Koepfli KP, Zhuk A, Dobrynin P, Kliver S, Cherkasov N, Tamazian
G, et al: Genome-wide sequence analyses of ethnic populations
across Russia. Genomics. 112:442–458. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hayashi Y, Fujita K, Matsuzaki K,
Matsushita M, Kawamura N, Koh Y, Nakano K, Wang C, Ishizuya Y,
Yamamoto Y, et al: Diagnostic potential of TERT promoter and FGFR3
mutations in urinary cell-free DNA in upper tract urothelial
carcinoma. Cancer Sci. 110:1771–1779. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Russo IJ, Ju Y, Gordon NS, Zeegers MP,
Cheng KK, James ND, Bryan R and Ward DG: Toward personalised liquid
biopsies for urothelial carcinoma: Characterisation of ddPCR and
urinary cfDNA for the detection of the TERT 228 G>A/T mutation.
Bladder Cancer. 4:41–48. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Siraj AK, Bu R, Iqbal K, Parvathareddy SK,
Siraj N, Siraj S, Diaz MRF, Rala DR, Benito AD, Sabido MA, et al:
Telomerase reverse transcriptase promoter mutations in cancers
derived from multiple organ sites among middle eastern population.
Genomics. 112:1746–1753. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Demaree B, Weisgerber D, Dolatmoradi A,
Hatori M and Abate AR: Direct quantification of EGFR variant allele
frequency in cell-free DNA using a microfluidic-free digital
droplet PCR assay. Methods Cell Biol. 148:119–131. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Crimi S, Falzone L, Gattuso G, Grillo CM,
Candido S, Bianchi A and Libra M: Droplet digital PCR analysis of
liquid biopsy samples unveils the diagnostic role of
hsa-miR-133a-3p and hsa-miR-375-3p in oral cancer. Biology (Basel).
9(379)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Falzone L, Musso N, Gattuso G, Bongiorno
D, Palermo CI, Scalia G, Libra M and Stefani S: Sensitivity
assessment of droplet digital PCR for SARS-CoV-2 detection. Int J
Mol Med. 46:957–964. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
La Rocca F, Grieco V, Ruggieri V, Zifarone
E, Villani O, Zoppoli P, Russi S, Laurino S, Falco G, Calice G, et
al: Superiority of droplet digital PCR over real-time quantitative
PCR for JAK2 V617F allele mutational burden assessment in
myeloproliferative neoplasms: A retrospective study. Diagnostics
(Basel). 10(143)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hosen MI, Forey N, Durand G, Voegele C,
Bilici S, Avogbe PH, Delhomme TM, Foll M, Manel A, Vian E, et al:
Development of sensitive droplet digital PCR assays for detecting
urinary TERT promoter mutations as non-invasive biomarkers for
detection of urothelial cancer. Cancers (Basel).
12(3541)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Mao X, Liu C, Tong H, Chen Y and Liu K:
Principles of digital PCR and its applications in current
obstetrical and gynecological diseases. Am J Transl Res.
11:7209–7222. 2019.PubMed/NCBI
|
|
40
|
Zhang H, Liu R, Yan C, Liu L, Tong Z,
Jiang W, Yao M, Fang W and Chen Z: Advantage of next-generation
sequencing in dynamic monitoring of circulating tumor DNA over
droplet digital PCR in cetuximab treated colorectal cancer
patients. Transl Oncol. 12:426–431. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ruiz-Villalba A, van Pelt-Verkuil E, Gunst
QD, Ruijter JM and van den Hoff MJ: Amplification of nonspecific
products in quantitative polymerase chain reactions (qPCR). Biomol
Detect Quantif. 14:7–18. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Tate JG, Bamford S, Jubb HC, Sondka Z,
Beare DM, Bindal N, Boutselakis H, Cole CG, Creatore C, Dawson E,
et al: COSMIC: The catalogue of somatic mutations in cancer.
Nucleic Acids Res. 47:D941–D947. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Tomczak K, Czerwińska P and Wiznerowicz M:
The Cancer Genome Atlas (TCGA): An immeasurable source of
knowledge. Contemp Oncol (Pozn). 19:A68–A77. 2015.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Horn S, Figl A, Rachakonda PS, Fischer C,
Sucker A, Gast A, Kadel S, Moll I, Nagore E, Hemminki K, et al:
TERT promoter mutations in familial and sporadic melanoma. Science.
339:959–961. 2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin
L and Garraway LA: Highly recurrent TERT promoter mutations in
human melanoma. Science. 339:957–959. 2013.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Ward DG, Baxter L, Gordon NS, Ott S,
Savage RS, Beggs AD, James JD, Lickiss J, Green S, Wallis Y, et al:
Multiplex PCR and next generation sequencing for the non-invasive
detection of bladder cancer. PLoS One. 11(e0149756)2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Critelli R, Fasanelli F, Oderda M,
Polidoro S, Assumma MB, Viberti C, Preto M, Gontero P, Cucchiarale
G, Lurkin I, et al: Detection of multiple mutations in urinary
exfoliated cells from male bladder cancer patients at diagnosis and
during follow-up. Oncotarget. 7:67435–67448. 2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Batista R, Lima L, Vinagre J, Pinto V,
Lyra J, Máximo V, Santos L and Soares P: TERT promoter mutation as
a potential predictive biomarker in BCG-treated bladder cancer
patients. Int J Mol Sci. 21(947)2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Borah S, Xi L, Zaug AJ, Powell NM, Dancik
GM, Cohen SB, Costello JC, Theodorescu D and Cech TR: Cancer. TERT
promoter mutations and telomerase reactivation in urothelial
cancer. Science. 347:1006–1010. 2015.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Li Y, Zhou QL, Sun W, Chandrasekharan P,
Cheng HS, Ying Z, Lakshmanan M, Raju A, Tenen DG, Cheng SY, et al:
Non-canonical NF-κB signalling and ETS1/2 cooperatively drive C250T
mutant TERT promoter activation. Nat Cell Biol. 17:1327–1338.
2015.PubMed/NCBI View Article : Google Scholar
|
|
51
|
van Kessel KEM, Beukers W, Lurkin I,
Ziel-van der Made A, van der Keur KA, Boormans JL, Dyrskjøt L,
Márquez M, Ørntoft TF, Real FX, et al: Validation of a DNA
methylation-mutation urine assay to select patients with hematuria
for cystoscopy. J Urol. 197:590–595. 2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Wu S, Ou T, Xing N, Lu J, Wan S, Wang C,
Zhang X, Yang F, Huang Y and Cai Z: Whole-genome sequencing
identifies ADGRG6 enhancer mutations and FRS2 duplications as
angiogenesis-related drivers in bladder cancer. Nat Commun.
10(720)2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Wang W, Kong P, Ma G, Li L, Zhu J, Xia T,
Xie H, Zhou W and Wang S: Characterization of the release and
biological significance of cell-free DNA from breast cancer cell
lines. Oncotarget. 8:43180–43191. 2017.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Aucamp J, Bronkhorst AJ, Peters DL, Van
Dyk HC, Van der Westhuizen FH and Pretorius PJ: Kinetic analysis,
size profiling, and bioenergetic association of DNA released by
selected cell lines in vitro. Cell Mol Life Sci. 74:2689–2707.
2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Bronkhorst AJ, Ungerer V and Holdenrieder
S: The emerging role of cell-free DNA as a molecular marker for
cancer management. Biomol Detect Quantif. 17(100087)2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Bettegowda C, Sausen M, Leary RJ, Kinde I,
Wang Y, Agrawal N, Bartlett BR, Wang H, Luber B, Alani RM, et al:
Detection of circulating tumor DNA in early- and late-stage human
malignancies. Sci Transl Med. 6(224ra24)2014.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Abbosh C, Birkbak NJ, Wilson GA,
Jamal-Hanjani M, Constantin T, Salari R, Le Quesne J, Moore DA,
Veeriah S, Rosenthal R, et al: Phylogenetic ctDNA analysis depicts
early-stage lung cancer evolution. Nature. 545:446–451.
2017.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Parkinson CA, Gale D, Piskorz AM, Biggs H,
Hodgkin C, Addley H, Freeman S, Moyle P, Sala E, Sayal K, et al:
Exploratory analysis of TP53 mutations in circulating tumour DNA as
biomarkers of treatment response for patients with relapsed
high-grade serous ovarian carcinoma: A retrospective study. PLoS
Med. 13(e1002198)2016.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Leão R, Lee D, Figueiredo A, Hermanns T,
Wild P, Komosa M, Lau I, Mistry M, Nunes NM, Price AJ, et al:
Combined genetic and epigenetic alterations of the TERT promoter
affect clinical and biological behavior of bladder cancer. Int J
Cancer. 144:1676–1684. 2019.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Hosen MI, Sheikh M, Zvereva M, Scelo G,
Forey N, Durand G, Voegele C, Poustchi H, Khoshnia M, Roshandel G,
et al: Urinary TERT promoter mutations are detectable up to 10
years prior to clinical diagnosis of bladder cancer: Evidence from
the Golestan Cohort Study. EBioMedicine. 53(102643)2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Birkenkamp-Demtröder K, Christensen E,
Nordentoft I, Knudsen M, Taber A, Høyer S, Lamy P, Agerbæk M,
Jensen JB and Dyrskjøt L: Monitoring treatment response and
metastatic relapse in advanced bladder cancer by liquid biopsy
analysis. Eur Urol. 73:535–540. 2018.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Vinagre J, Almeida A, Pópulo H, Batista R,
Lyra J, Pinto V, Coelho R, Celestino R, Prazeres H, Lima L, et al:
Frequency of TERT promoter mutations in human cancers. Nat Commun.
4(2185)2013.PubMed/NCBI View Article : Google Scholar
|