Introduction
Cervical cancer is a major health burden worldwide;
there were an estimated 13,170 new cases of cervical cancer and
4,250 deaths in 2019(1). The
standard treatment for localized inoperable cervical cancer is
external beam radiotherapy (EBRT) with concurrent cisplatin
followed by brachytherapy (2).
Dose optimization using intracavitary applicators has been shown to
significantly decrease doses to organs at risk (OARs) and morbidity
(3). OARs, such as bladder, rectum
and sigmoid colon, are dose-limiting as radiation above certain
levels can cause further complications. Brachytherapy induces
enteritis and cystitis due to irradiation to pelvic OARs.
Therefore, it is crucial to optimize the dose to OARs to minimize
the risk of complications (4-6).
The volume and position of OARs in the pelvis affect the position
of the tumor and the dose to both OARs and tumor. Bladder volume is
one of the most important variables during brachytherapy insertions
because of the notable changes in bladder contents. The effect of
bladder volume on doses to OARs and tumor is unclear. There is no
consensus in clinical practice on the optimal bladder volume during
brachytherapy (7-9).
Certain studies have suggested that smaller bladder volume is
better to achieve a lower dose to OARs (5) while others have shown that full
bladder is non-inferior with regard to bladder dose (7,9).
Another study suggested that bladder volume exhibits different
dosimetric effects on different OARs (10). Various bladder filling protocols
have been recommended with limited evidence, including empty
bladder with indwelling Foley's catheter (11), a known limited filling status (50
cc) (12) and full bladder
(7). The International Commission
on Radiation Units and Measurements (ICRU) 89 report had no
recommendations on bladder volume status (full, empty or other)
during pelvic intracavitary brachytherapy (13). Ongoing clinical study Embrace II
(14) by European Brachytherapy
Group-European Society for Radiotherapy and Oncology Gynecology
(GEC-ESTRO GYN) working group has not specified bladder volume
control during brachytherapy. To the best of our knowledge, few
studies have assessed the effect of bladder volume on OAR dose and
tumor during image-guided adaptive brachytherapy for cervical
cancer or the treatment results.
The present study aimed to determine the optimal
bladder volume to guide clinical practice by investigating the
effect of bladder volume on cervical cancer OARs and primary tumor
during image-guided adaptive brachytherapy and its impact on
treatment outcome.
Materials and methods
Patients
The present study was approved by the Medical Ethics
Committee of the University of Hong Kong-Shenzhen Hospital
(Shenzhen, China). All 109 patients with cervical cancer who
received radical radiotherapy at Oncology Medical Center of the
University of Hong Kong-Shenzhen Hospital between January 2015 and
July 2019 were included in the retrospective study and relevant
information was obtained from medical records. The inclusion
criteria were as follows: i) age ≥18 years, ii) ECOG performance
status ≤2, iii) pathology-confirmed, International Federation of
Gynecology and Obstetrics (FIGO; 2009) (15) stage IB1-IVB (retroperitoneal lymph
nodes metastasis only) cervical cancer, and iv) patients were
treated with EBRT with concurrent cisplatin followed by
image-guided adaptive brachytherapy. Patients were excluded if they
had a history of pelvic surgery or irradiation or recurrent
cervical cancer.
Brachytherapy applicator insertion
procedure
Intracavitary brachytherapy was performed 2-3 weeks
after initiation of EBRT. Brachytherapy applicators were inserted
under general anesthesia. A Foley's catheter was inserted and 150
cc normal saline was injected into bladder after general
anesthesia. The intrauterine tandem was inserted under ultrasound
guidance. The tandem length with stopper was selected based on the
length of the uterine cavity. Ring or ovoid shaped applicators were
selected based on disease extent and vaginal distensibility.
Following the completion of applicator insertion, the Foley's
catheter was kept open with connection to a urine bag until the end
of each brachytherapy session. The Foley's catheter was removed
after each brachytherapy session. Simulation, delineation of
bladder volume/wall and treatment were performed in the empty
bladder state.
Imaging and contouring
All patients underwent CT simulation with 1 mm axial
image slices in treatment position from the 10th thoracic vertebra
to the upper third of the femur. The CT images were transferred to
Varian eclipse treatment planning system, (version 15.0; Varian
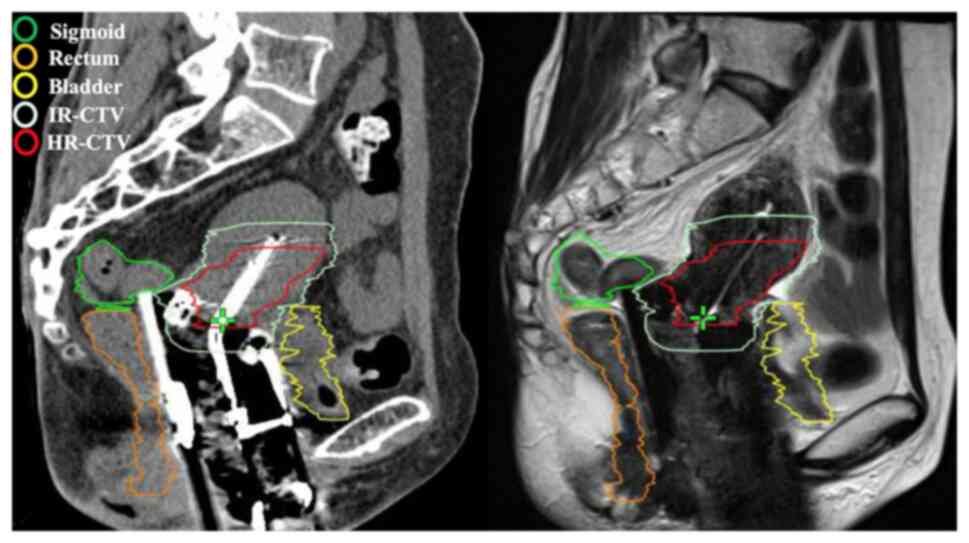
Medical Systems). HR-CTV and OARs (bladder, rectum and sigmoid
colon) were delineated on the CT image (Fig. 1) with reference to available
information (including gynecological examination, magnetic
resonance imaging at the time of diagnosis and before
brachytherapy) by a radiation oncologist according to the GEC-ESTRO
GYN working group recommendations (2) and were confirmed by a radiation
oncologist consultant. OARs were defined by Radiation Therapy
Oncology Group Consensus Panel Atlas (16). Rectum contouring started inferiorly
from the lowest level of the ischial tuberosities (right or left)
and ended superiorly before the rectum lost its round shape in the
axial plane and connected anteriorly with the sigmoid. The volume
of OARs and HR-CTV were obtained from CT simulation scan by the
Varian eclipse treatment planning system (version 15.0). Bladder,
rectum and sigmoid colon volume comprised the entire volume,
including the wall and contents of bladder, rectum and sigmoid
colon.
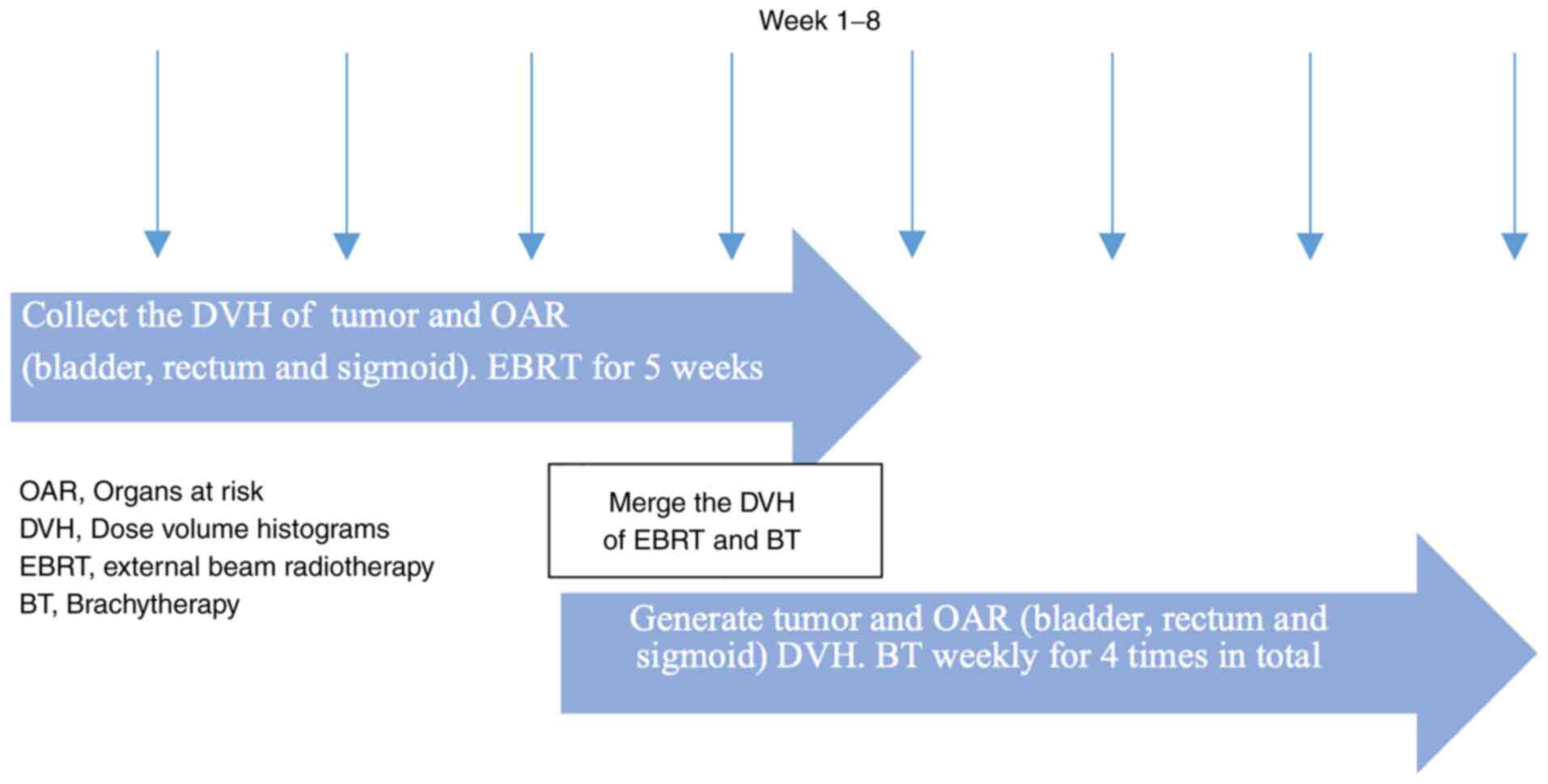
Treatment planning
EBRT was delivered on a 6 MV linear accelerator;
prescription dose was 45 Gy in 25 fractions to whole pelvis with a
simultaneous integrated boost of 55.0-57.5 Gy to pelvic or
retroperitoneal metastatic lymph nodes (Fig. 2). Concurrent cisplatin at 40
mg/m2 was given weekly during EBRT. All 109 patients
received the same dose of external beam therapy but different doses
of brachytherapy in the treatment planning section. The
image-guided brachytherapy plan was constructed using the Varian
eclipse treatment planning system (version 15.0). The prescription
dose for each high-dose-rate (HDR) brachytherapy was 6-7 Gy to
HR-CTV, four times in total. Equivalent dose (EQD2) (17) was calculated for HDR treatments
using the linear quadratic model normalized to 2 Gy/fraction as
follows: EQD2=n x d x , where n represents radiotherapy
session number, d represents the dose and α/β ratio (18) represents radiotherapy sensitivity
of tumor and normal tissue. The full course treatment plan,
including external radiotherapy and brachytherapy evaluation
criteria of OARs for all patients, were as follows: Rectum,
D2cc<75 Gy (EQD2); sigmoid colon, D2cc<75 Gy (EQD2) and
bladder D2cc<90 Gy (EQD2). Dose prescription of HR-CTV D90 was
as follows: Stage IB-IIIA, >84 Gy (EQD2) and ≥stage IIIB, >90
Gy (EQD2). Dose constraint of OARs had a higher priority than
HR-CTV. Dose of external irradiation and brachytherapy were
converted to EQD2, then HR-CTV D90 and D2cc of rectum, sigmoid and
bladder during external radiotherapy and brachytherapy were
calculated using deformable registration by medical imaging
software version 6.9.5, MIM Software Inc. Doses to HR-CTV and the
D2 and D1cc of bladder, rectal and sigmoid wall were obtained from
dose-volume histograms (DVHs) automatically generated by the Varian
eclipse treatment planning system (version 15.0). Toxicity during
concurrent chemoradiotherapy (CCRT) was graded according to Common
Terminology Criteria for Adverse Events 4.03 criteria (19).
Statistical analysis
Data analysis was performed using SPSS version 19.0
for Windows (IBM, Corp.), GraphPad prism 8 (GraphPad Software,
Inc.) and R Programming Language version 3.6.1 (R Studio, Inc.).
Clinicopathological and treatment-associated characteristics were
assessed using descriptive statistics. Paired t-test (mean ± SD)
was used to compare means between two groups. Association between
bladder volume and dose to OARs and HR-CTV were analyzed using
linear regression model. Cumulative HR-CTV D90 represented the
total dose received by HR-CTV during the whole course of RT and was
defined as the integrated dose to 90% of HR-CTV including both EBRT
and brachytherapy. Local failure was defined as local tumor
residual at the end of treatment or local recurrence during follow
up. Local control was defined as no local residual at the end of
treatment and no local recurrence during follow up. Overall
survival (OS) was the time from the start of EBRT to the date of
death from any cause or the last confirmed date of survival. Local
control and OS of all patients were based on both brachytherapy and
external beam therapy. Receiver operating characteristic (ROC)
curve was used to select cutoff values of cumulative HR-CTV D90
predicting local failure. Survival was evaluated using Kaplan-Meier
curves with the log-rank test and Cox proportional hazard model.
Bilateral P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
A total of consecutive 109 patients with 421
intracavitary brachytherapy insertions were included. The median
age was 53 years (range, 33-77 years). The FIGO (2009) stage
distribution was 7 (6.4), 51 (46.8), 43 (39.4%) and 8 (7.3%) for
stage I-III and IV, respectively. A total of 101 patients (92.7%)
had squamous cell carcinoma. A total of 99 patients (90.8%)
completed the scheduled four brachytherapy insertions. Patient and
clinicopathological characteristics are presented in Table I. A total of two patients refused
to continue brachytherapy due to uterine perforation and vaginal
injury during the last brachytherapy; eight patients changed to
stereotactic body radiation therapy or external radiation boost due
to large residual volume following external radiotherapy with
expected poor dose coverage by intracavity brachytherapy.
 | Table IPatient and clinicopathological
characteristics. |
Table I
Patient and clinicopathological
characteristics.
| Characteristic | Number (%),
n=109 |
|---|
| Median age (range),
years | 53.00
(33.00-77.00) |
| Median BMI
(range) | 22.67
(15.87-29.80) |
| ECOG score | |
|
0-1 | 97.00 (89.00) |
|
2 | 12.00 (11.00) |
| Histology | |
|
Squamous
cell carcinoma | 101.00 (92.70) |
|
Adenocarcinoma | 4.00 (3.70) |
|
Other | 4.00 (3.70) |
| FIGO stage | |
|
IB1 | 5.00 (4.60) |
|
IB2 | 2.00 (1.80) |
|
IIA1 | 4.00 (3.70) |
|
IIA2 | 9.00 (8.30) |
|
IIB | 38.00 (34.90) |
|
IIIA | 4.00 (3.70) |
|
IIIB | 39.00 (35.80) |
|
IVA | 3.00 (2.80) |
|
IVB | 5.00 (4.60) |
| Brachytherapy
insertion | |
|
1 | 1.00 (0.90) |
|
2 | 3.00 (2.80) |
|
3 | 6.00 (5.50) |
|
4 | 99.00 (90.80) |
| Applicator type | |
|
Ring | 95.00 (22.60) |
|
Ovoid | 326.00 (77.40) |
Bladder volume during
brachytherapy
The Foley's catheter was kept open with connection
to a urine bag during brachytherapy. The median volume of bladder,
rectum, sigmoid colon and HR-CTV of all 421 brachytherapy
insertions on simulation CT was 60.1 (range, 13.5-318.1), 28.3
(8.6-104.0), 49.6 (6.0-274.6) and 22.6 (5.6-108.1 cc),
respectively. Linear regression model, including volumes of rectum,
sigmoid colon and HR-CTV, corpus angle, age and body mass index
(BMI), showed that sigmoid [odds ratio (OR), 0.190; 95% CI,
0.102-0.278; P<0.001] and rectum volume (OR, 0.222; 95% CI,
-0.018-0.462; P=0.070) were associated with bladder volume during
brachytherapy. HR-CTV volume, corpus angle, age and BMI did not
affect bladder volume during brachytherapy (Table II). With the increase of
brachytherapy insertion time, paired t-test (Table III) showed that bladder volume at
the fourth insertion increased significantly compared with the
first insertion (mean ± SD; 73.7±37.7 vs. 65.1±24.8 cc;
P=0.015).
 | Table IILinear regression model of factors
associated with bladder volume during brachytherapy. |
Table II
Linear regression model of factors
associated with bladder volume during brachytherapy.
| | 95% CI limit | |
|---|
| Factor | OR | Lower | Upper | P-value |
|---|
| Rectum volume | 0.222 | -0.018 | 0.462 | 0.070 |
| Sigmoid volume | 0.190 | 0.102 | 0.278 | <0.001 |
| HR-CTV volume | 0.168 | -0.053 | 0.389 | 0.136 |
| Corpus angle | 0.135 | -0.058 | 0.327 | 0.170 |
| Age | 0.105 | -0.185 | 0.395 | 0.477 |
| BMI | 0.087 | -0.850 | 1.025 | 0.855 |
 | Table IIIChange in bladder volume between
brachytherapy insertions (paired t-test). |
Table III
Change in bladder volume between
brachytherapy insertions (paired t-test).
| Pair | | N | Bladder volume
(mean ± SD), cc | Change in bladder
volume (mean ± SD), cc | t | P-value |
|---|
| 1 | 2nd insertion | 108 | 67.5±36.9 | 1.9±34.6 | 0.582 | 0.562 |
| | 1st insertion | 108 | 65.5±24.8 | | | |
| 2 | 3rd insertion | 105 | 65.8±29.8 | 0.9±30.3 | 0.302 | 0.763 |
| | 1st insertion | 105 | 64.9±24.9 | | | |
| 3 | 4th insertion | 99 | 73.7±37.7 | 8.6±34.7 | 2.472 | 0.015 |
| | 1st insertion | 99 | 65.1±24.8 | | | |
Effect of bladder volume on dose to
OARs and tumor (HR-CTV)
Linear regression analysis showed that bladder
volume was associated significantly and linearly with D1 (R, 0.315;
P<0.001) and D2 cc (R, 0.346; P<0.001) of the bladder wall
(data not shown). There was a 1 Gy increase in D1 and D2cc with
every 83 or 90 cc increase in bladder volume, respectively. Linear
regression models were repeatedly constructed to evaluate the
effect of bladder volume on dose to OARs and tumor (HR-CTV) by
adjusting confounding factors, including rectum, sigmoid and HR-CTV
volume, corpus angle, applicator length and BMI. With increasing
bladder volume, D1cc (OR, 0.012; 95% CI, 0.009-0.016; P<0.001)
and D2cc (OR, 0.012; 95% CI, 0.009-0.015; P<0.001) of bladder
wall increased; D1 (OR, 0.002; 95% CI, -0.001-0.005; P=0.150) and
D2cc (OR, 0.002; 95% CI, 0.000-0.004; P=0.084) of the rectal wall
also increased. However, the D1 (OR, -0.005; 95% CI, -0.008-0.002;
P=0.003) and D2cc (OR, -0.004; 95% CI, -0.007-0.002; P=0.001) of
sigmoid wall and HR-CTV D90 (OR, -0.005; 95% CI, -0.009-0.001;
P=0.010) and D95 (OR, -0.005; 95% CI, -0.009-0.001; P=0.006)
decreased with increased bladder volume. These data were based on
intracavitary brachytherapy. From these linear regression models,
HR-CTV volume affected D1 and D2cc of rectal (P=0.058 and 0.035,
respectively) and sigmoid wall (P=0.015 and 0.002, respectively)
and HR-CTV D90 and D95 (both P<0.001); applicator length
affected the D1 and D2cc of rectal (both P=0.012) and sigmoid wall
(both P<0.001); and BMI affected D1 and D2cc (both P=0.001) of
bladder wall; corpus angle affected D1 and D2cc of rectal (both
P<0.001) and sigmoid wall (P=0.056 and 0.015, respectively) and
HR-CTV D90 and D95 (both P<0.001).
Effect of HR-CTV cumulative dose on
local failure and OS
The median follow-up time was 28 months (range, 3-59
months); the median OS for the entire patient population was 54.0
months (95% CI, 36.3-71.7 months). Among the 99 patients who
completed all four brachytherapy insertions, 14 experienced local
failure. Patients with local failure had significantly lower
cumulative HR-CTV D90 than those without local failure (mean ± SD;
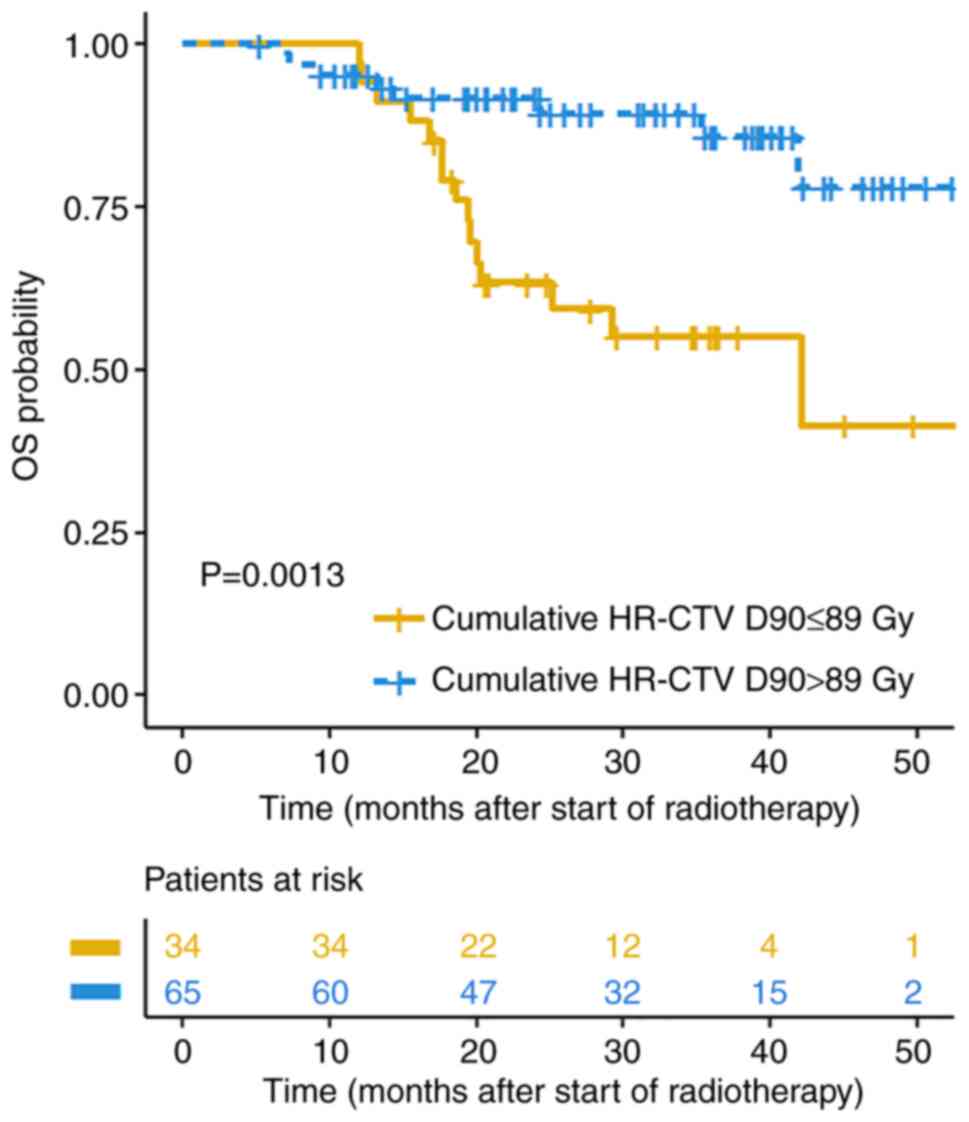
89.5±9.3 vs. 97.0±12.4 Gy; P=0.032). The cut-off value of
cumulative HR-CTV D90 to predict local control on ROC analysis was
89.6 Gy with area under curve=0.702, sensitivity=70.6% and
specificity=64.3% (P=0.016). Patients with cumulative HR-CTV
D90≤89.6 Gy had shorter OS than those with cumulative HR-CTV
D90>89.6 Gy (median OS, 42.1 months vs. not reached; P=0.001;
Fig. 3). Cox multivariate analysis
demonstrated that higher cumulative HR-CTV D90 [hazard ratio (HR),
0.940; 95% CI, 0.892-0.990; P=0.019] was associated with better OS
after adjusting age, ECOG, FIGO stage, hemoglobin before treatment
and concurrent chemotherapy cycles (Table IV).
 | Table IVCox regression analysis of factors
associated with OS. |
Table IV
Cox regression analysis of factors
associated with OS.
| | 95% CI limit | |
|---|
| Factor | HR | Lower | Upper | P-value |
|---|
| Age (<60/≥60
years) | 2.277 | 0.730 | 7.108 | 0.156 |
| ECOG (0-1/2) | 0.541 | 0.173 | 1.692 | 0.291 |
| FIGO stage
(I-II/III-IV) | 0.897 | 0.353 | 2.276 | 0.818 |
| Hemoglobin
(<110/≥110 g/l) | 2.271 | 0.933 | 5.532 | 0.071 |
| Concurrent
chemotherapy cycles (0-4/5-6) | 3.252 | 1.209 | 8.748 | 0.020 |
| Cumulative HR-CTV
D90 | 0.940 | 0.892 | 0.990 | 0.019 |
Effect of bladder volume on
treatment-associated toxicity
Toxicity during CCRT was graded according to CTCAE
4.03 criteria (19). Cumulative
bladder wall D2cc is defined as the integrated dose to the highest
irradiated 2 cc area of bladder wall, including both EBRT and
brachytherapy phase. Patients with grade 2 acute urinary toxicity
had significantly higher cumulative bladder wall D2cc than those
with acute urinary toxicity <grade 2 (mean ± SD; 86.7±3.7 vs.
78.5±7.9 Gy; P=0.001; data not shown). Only one patient experienced
grade 3 late intestinal toxicity.
Discussion
The present study analyzed the effect of bladder
volume on OARs and HR-CTV radiation dose and associated treatment
results during brachytherapy for cervical cancer and aimed to
provide recommendations for bladder volume control during
brachytherapy to decrease the dose to normal tissue while
optimizing the dose to the primary tumor. The present study found
as bladder volume increased, the dose to the bladder and rectal
walls increased and the dose to sigmoid wall and HR-CTV decreased.
A higher priority was set for OARs to avoid serious complications
and results were based on intracavitary, rather than interstitial,
brachytherapy (20). Higher doses
of HR-CTV predicted better OS throughout the course of radiotherapy
and higher dose to the bladder wall was associated with more grade
2 acute urotoxicity. From a clinical perspective, it is therefore
better to minimize bladder volume during brachytherapy for cervical
cancer.
Based on previous studies (8,12,21),
there is no consistent recommendation for bladder volume control
during brachytherapy. Sharma et al (21) showed that D2cc of the bladder
increase with bladder volume; bladder volume ≤70 cc proved better
for achieving lower radiation dose of bladder. Siavashpour et
al (8) showed that bladder
D0.1 and D2cc increase significantly with bladder volume and
concluded that bladder volume <70 cc might be a better choice
than bladder volume >70 cc. Mahantshetty et al (12) suggested that bladder filling
protocol with 50 or 100 cc was well tolerated and achieved a
reasonably reproducible bladder volume during cervical
brachytherapy. The present study showed that bladder volume was
significantly and linearly associated with D1 and D2cc of the
bladder wall and there was a 1 Gy increase in D1 or D2cc with every
83 or 90 cc increase in bladder volume, respectively. This means
that the smaller the bladder volume, the lower the bladder wall
dose.
Sharma et al (21) showed that the dose to rectum
increases with bladder volume but decreases after bladder volume
reaches 110 cc; sigmoid DVH parameters followed a similar trend as
that of the rectum. Siavashpour et al (8) showed that the rectum dose decreases
with bladder volume >140 cc and sigmoid dose decreases with
bladder volumes <75 cc; however, for bladder volume >75 cc,
the sigmoid dose increased. The D2cc of bladder and rectum were
higher for longer applicator lengths than shorter ones (8). Mahantshetty et al (12) found that rectal and sigmoid doses
are not significantly affected by bladder volume. Harmon et
al (7) showed that sigmoid D2
and D1cc are significantly decreased in full bladder plans; the
rectum shows no significant difference in D2 and D0.1cc between
different bladder volumes. The present data showed that D1 and D2cc
of rectal wall increased with bladder volume. However, D1 and D2cc
of sigmoid decreased with increased bladder volume. The different
results between these studies may be due to different methods of
bladder volume control and grouping. The present study showed
HR-CTV volume affected the D1 and D2cc of rectal and sigmoid wall
and HR-CTV D90 and D95; and applicator length affected the D1 and
D2cc of rectal and sigmoid wall. Applicator length may affect doses
of OARs through the position change of bladder and rectum. BMI
affected the D1 and D2cc of bladder wall; corpus angle affected D1
and D2cc of rectal and sigmoid wall and HR-CTV D90 and D95.
Siavashpour et al (10)
demonstrated that empty bladder status may decrease the dose to
rectum and sigmoid with tandems >4 cm, which was similar to
results of the present study. Several studies have demonstrated
significant increase in small bowel dose with decreased bladder
volume (7,9,12).
Nevertheless, the small bowel dose received may not be a true
reflection from the dose estimated by the radiotherapy plan because
of the changes in the position of the small intestine during
treatment (9,22,23).
The present study demonstrated that bladder volume
at the fourth insertion was significantly increased compared with
the first insertion. However, the Foley's catheter was kept open
during CT simulation for all patients. The present study showed
that sigmoid and rectum volume were associated with bladder volume
during brachytherapy. HR-CTV volume, corpus angle, age and BMI did
not affect bladder volume during brachytherapy. Statistical
analysis showed that BMI affected D1 and D2cc of bladder wall but
not bladder volume. This may be because obesity increases the
difficulty and affects the accuracy of application insertion and
subcutaneous fat may shorten the distance between the bladder and
tumor, resulting in an increased radiation dose to the bladder wall
without affecting the bladder volume. In addition, although the
catheter was kept open, bladder volumes varied greatly. Potential
causes include poor drainage of the catheter, decreased bladder
contraction caused by radiotherapy and general anesthesia affecting
bladder emptying. Lee et al (24) showed that interfractional
dosimetric variation for both target and OARs resulted in a change
of treatment plan in 6% of cases. Therefore, controlling bladder
volume is an important clinical issue.
The present study showed that cumulative HR-CTV D90
in patients with local failure was significantly lower than that in
patients without local failure. Local control and OS were lower
when cumulative HR-CTV D90≤89.6 Gy. Patients with grade 2 acute
urinary toxicity exhibited significantly higher cumulative bladder
wall D2cc than those with acute urinary toxicity <grade 2, which
is similar to other studies (4,6). The
present study analyzed both treatment outcomes and effect of
bladder volume, filling gaps in other studies. However, the study
was limited by the retrospective nature of the design. The catheter
remained open during brachytherapy without bladder volume control
procedures; bladder volume control will be the focus of future
research. Interstitial brachytherapy has notable dosimetric
advantages for patients with cervical cancer with asymmetrical
growth or large mass which makes it difficult to achieve ideal dose
distribution via intracavitary brachytherapy (20). Magnetic resonance image-guided
brachytherapy or interstitial brachytherapy provide more accurate
tumor and OAR delineation and dose assessment (20,25,26),
which is also a future research direction.
In conclusion, restricting bladder volume during
brachytherapy may decrease doses to the bladder and rectal wall.
Higher dose to the bladder wall was associated with more grade 2
acute urinary toxicity. Moreover, smaller bladder volume may
increase the dose to HR-CTV; patients with cumulative HR-CTV
D90>89.6 Gy have better local control and OS.
Acknowledgements
Not applicable.
Funding
The present study was supported in part by Health Commission of
Guangdong Province (grant no. B2020100); High Level-Hospital
Program, Health Commission of Guangdong Province (grant no.
HKUSZH201902031); Shenzhen Healthcare Research Project, China
(grant no. SZXJ2018003); Shenzhen Key Medical Discipline
Construction Fund (grant no. SZXK014) and Shenzhen Science and
Technology Program (grant no. KQTD20180411185028798).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LC, ZX, ATC, YZ, CZ, FMK and QL designed the study.
ZX, ATC, YZ, CZ, FMK, QL, LM and LY performed the literature
review, analyzed data and wrote the manuscript. ZX, LY, ATC, YZ,
CZ, FMK and LC guaranteed the integrity of the study. ZX, LY, YZ,
QL and CZ, LM collected data. LC, ZX, LY, QL, ATC and FMK, LM
revised the manuscript. All authors have read and approved the
final version of the manuscript. ZX and LY confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of the University of Hong Kong-Shenzhen Hospital
(approval no. hkuszh2019119).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Haie-Meder C, Pötter R, Van Limbergen E,
Briot E, De Brabandere M, Dimopoulos J, Dumas I, Hellebust TP,
Kirisits C, Lang S, et al: Recommendations from gynaecological
(GYN) GEC-ESTRO working group (I): Concepts and terms in 3D image
based 3D treatment planning in cervix cancer brachytherapy with
emphasis on MRI assessment of GTV and CTV. Radiother Oncol.
74:235–245. 2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Charra-Brunaud C, Harter V, Delannes M,
Haie-Meder C, Quetin P, Kerr C, Castelain B, Thomas L and Peiffert
D: Impact of 3D image-based PDR brachytherapy on outcome of
patients treated for cervix carcinoma in France: Results of the
French STIC prospective study. Radiother Oncol. 103:305–313.
2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Manea E, Escande A, Bockel S, Khettab M,
Dumas I, Lazarescu I, Fumagalli I, Morice P, Deutsch E, Haie-Meder
C and Chargari C: Risk of late urinary complications following
image guided adaptive brachytherapy for locally advanced cervical
cancer: Refining bladder dose-volume parameters. Int J Radiat Oncol
Biol Phys. 101:411–420. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mazeron R, Maroun P, Castelnau-Marchand P,
Dumas I, del Campo ER, Cao K, Slocker-Escarpa A, M'Bagui R,
Martinetti F, Tailleur A, et al: Pulsed-dose rate image-guided
adaptive brachytherapy in cervical cancer: Dose-volume effect
relationships for the rectum and bladder. Radiother Oncol.
116:226–232. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zakariaee R, Hamarneh G, Brown CJ, Gaudet
M, Aquino-Parsons C and Spadinger I: Bladder accumulated dose in
image-guided high-dose-rate brachytherapy for locally advanced
cervical cancer and its relation to urinary toxicity. Phys Med
Biol. 61:8408–8424. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Harmon G, Chinsky B, Surucu M, Harkenrider
M and Small W Jr: Bladder distension improves the dosimetry of
organs at risk during intracavitary cervical high-dose-rate
brachytherapy. Brachytherapy. 15:30–34. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Siavashpour Z, Aghamiri MR, Jaberi R,
Manshadi HR, Ghaderi R and Kirisits C: Optimum organ volume ranges
for organs at risk dose in cervical cancer intracavitary
brachytherapy. J Contemp Brachytherapy. 8:135–142. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Nesseler JP, Charra-Brunaud C, Salleron J,
Py JF, Huertas A, Meknaci E, Courrech F, Peiffert D and
Renard-Oldrini S: Effect of bladder distension on doses to organs
at risk in pulsed-dose-rate 3D image-guided adaptive brachytherapy
for locally advanced cervical cancer. Brachytherapy. 16:976–980.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Siavashpour Z, Aghamiri MR, Jaberi R,
ZareAkha N, Manshadi HRD, Kirisits C and Sedaghat M: A comparison
of organs at risk doses in GYN intracavitary brachytherapy for
different tandem lengths and bladder volumes. J Appl Clin Med Phys.
17:5–13. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jamema SV, Saju S, Mahantshetty U, Pallad
S, Deshpande DD, Shrivastava SK and Dinshaw KA: Dosimetric
evaluation of rectum and bladder using image-based CT planning and
orthogonal radiographs with ICRU 38 recommendations in
intracavitary brachytherapy. J Med Phys. 33:3–8. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mahantshetty U, Shetty S, Majumder D,
Adurkar P, Swamidas J, Engineer R, Chopra S and Shrivastava S:
Optimal bladder filling during high-dose-rate intracavitary
brachytherapy for cervical cancer: a dosimetric study. J Contemp
Brachytherapy. 9:112–117. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
No authors listed: Prescribing, recording,
and reporting brachytherapy for cancer of the cervix. J ICRU 13:
1-2, NP, 2013.
|
|
14
|
Pötter R, Tanderup K, Kirisits C, de Leeuw
A, Kirchheiner K, Nout R, Tan LT, Haie-Meder C, Mahantshetty U,
Segedin B, et al: The EMBRACE II study: The outcome and prospect of
two decades of evolution within the GEC-ESTRO GYN working group and
the EMBRACE studies. Clin Transl Radiat Oncol. 9:48–60.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pecorelli S: Revised FIGO staging for
carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol
Obstet. 105:103–104. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gay HA, Barthold HJ, O'Meara E, Bosch WR,
El Naqa I, Al-Lozi R, Rosenthal SA, Lawton C, Lee WR, Sandler H, et
al: Pelvic normal tissue contouring guidelines for radiation
therapy: A radiation therapy oncology group consensus panel atlas.
Int J Radiat Oncol Biol Phys. 83:e353–e362. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fowler JF: Review: Total doses in
fractionated radiotherapy-implications of new radiobiological data.
Int J Radiat Biol Relat Stud Phys Chem Med. 46:103–120.
1984.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bentzen SM and Joiner MC: The
linear-quadratic approach in clinical practice. In: Basic clinical
radiobiology. Joiner MC, van der Kogel A (eds). 4th edition. CRC
Press, Boca Raton, FL, pp120-134, 2009.
|
|
19
|
Chen AP, Setser A, Anadkat MJ, Cotliar J,
Olsen EA, Garden BC and Lacouture ME: Grading dermatologic adverse
events of cancer treatments: The common terminology criteria for
adverse events version 4.0. J Am Acad Dermatol. 67:1025–1039.
2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Otter S, Coates A, Franklin A, Cunningham
M and Stewart A: Improving dose delivery by adding interstitial
catheters to fixed geometry applicators in high-dose-rate
brachytherapy for cervical cancer. Brachytherapy. 17:580–586.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sharma AD, Poddar J, Suryanarayan KU, Shah
SP, Parikh A, Mehta V and Phys M, Kumar T and Phys M: Dosimetric
analysis of the effects of the bladder volume on organs at risk
(OAR) in high-dose-rate intracavitary brachytherapy in carcinoma
cervix-an institutional study. J Contemp Brachytherapy. 10:26–31.
2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liao Y, Dandekar V, Chu JC, Turian J,
Bernard D and Kiel K: Reporting small bowel dose in cervix cancer
high-dose-rate brachytherapy. Med Dosim. 41:28–33. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Damato AL, Buzurovic I, Bhagwat MS,
Cormack RA, Devlin PM, Friesen S, Hansen J, Lee LJ, Manuel MM, Cho
LP, et al: The value of systematic contouring of the bowel for
treatment plan optimization in image-guided cervical cancer
high-dose-rate brachytherapy. Brachytherapy. 16:579–585.
2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lee S, Rodney E, Traughber B, Biswas T,
Colussi V and Podder T: Evaluation of interfractional variation of
organs and displacement of catheters during high-dose-rate
interstitial brachytherapy for gynecologic malignancies.
Brachytherapy. 16:1192–1198. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tan LT, Potter R, Sturdza A, Fokdal L,
Haie-Meder C, Schmid M, Gregory D, Petric P, Jurgenliemk-Schulz I,
Gillham C, et al: Change in patterns of failure after image-guided
brachytherapy for cervical cancer: Analysis from the RetroEMBRACE
study. Int J Radiat Oncol Biol Phys. 104:895–902. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zaffino P, Pernelle G, Mastmeyer A,
Mehrtash A, Zhang H, Kikinis R, Kapur T and Francesca Spadea M:
Fully automatic catheter segmentation in MRI with 3D convolutional
neural networks: Application to MRI-guided gynecologic
brachytherapy. Phys Med Biol. 64(165008)2019.PubMed/NCBI View Article : Google Scholar
|