Introduction
Among pilomatrical tumors, pilomatricoma (calcifying
epithelioma) is a well-known benign tumor type, whereas
pilomatrical carcinoma and pilomatrical carcinosarcoma are
considered malignant counterparts of pilomatricoma. Both of these
malignant tumor types are rare. In particular, pilomatrical
carcinosarcoma is a very rare tumor and, to the best of our
knowledge, it has been reported in only nine patients thus far.
Pilomatricoma is usually observed in young individuals and is
predominantly occurs in females. By contrast, pilomatrical
carcinoma is primarily observed in middle-elderly males (1,2). It
usually occurs between the 5th and 7th decade of life (2). It has been reported that the origin
of pilomatrical carcinoma may be a malignant transformation of
pilomatricoma or it may be a de novo development altogether
(1,3). Furthermore, pilomatrical tumors have
been associated with mutations in exon 3 of the β-catenin gene
(CTNNB1) and the mutation is reflected in the expression of
β-catenin in the tumors (4,5).
The origin of pilomatrical carcinosarcoma has
remained largely elusive. Histologically, it consists of
pilomatrical epithelial and sarcomatous lesions. The same genetic
abnormalities and abnormal expression of β-catenin are observed in
both epithelial and sarcomatous lesions of pilomatrical
carcinosarcoma, suggesting that both lesion types are derived from
the same precursor or that the sarcomatous lesion
transdifferentiates from the epithelial lesion (5-9).
Certain cases of pilomatrical carcinosarcoma have been observed to
exhibit slow growth in the early stages and have had a long
observation period prior to resection, which suggests malignant
transformation from a benign tumor (7-10).
For pilomatrical carcinosarcoma to be diagnosed, it
is necessary to confirm both the pilomatrical epithelial lesion
(pilomaricoma or pilomatrical carcinoma) and sarcomatous lesion in
a histological examination. Immunohistochemically, the epithelial
lesion expresses cytokeratin and it does not express vimentin,
while the mesenchymal lesion expresses vimentin and the sarcomatous
lesion or a part of it expresses cytokeratin in certain cases. It
has been reported that both epithelial and sarcomatous lesions
abnormally express β-catenin and express P53 (5-9).
Surgical resection of the tumor has been performed as a therapy for
both types of pilomatrical carcinosarcoma.
The present study reported on a case of pilomatrical
carcinosarcoma observed in a 100-old-year male. The carcinoma
component of the tumor was pilomatrical carcinoma, whereas the
sarcoma component was undifferentiated spindle cell sarcoma, with a
smooth transition between the two components.
Case report
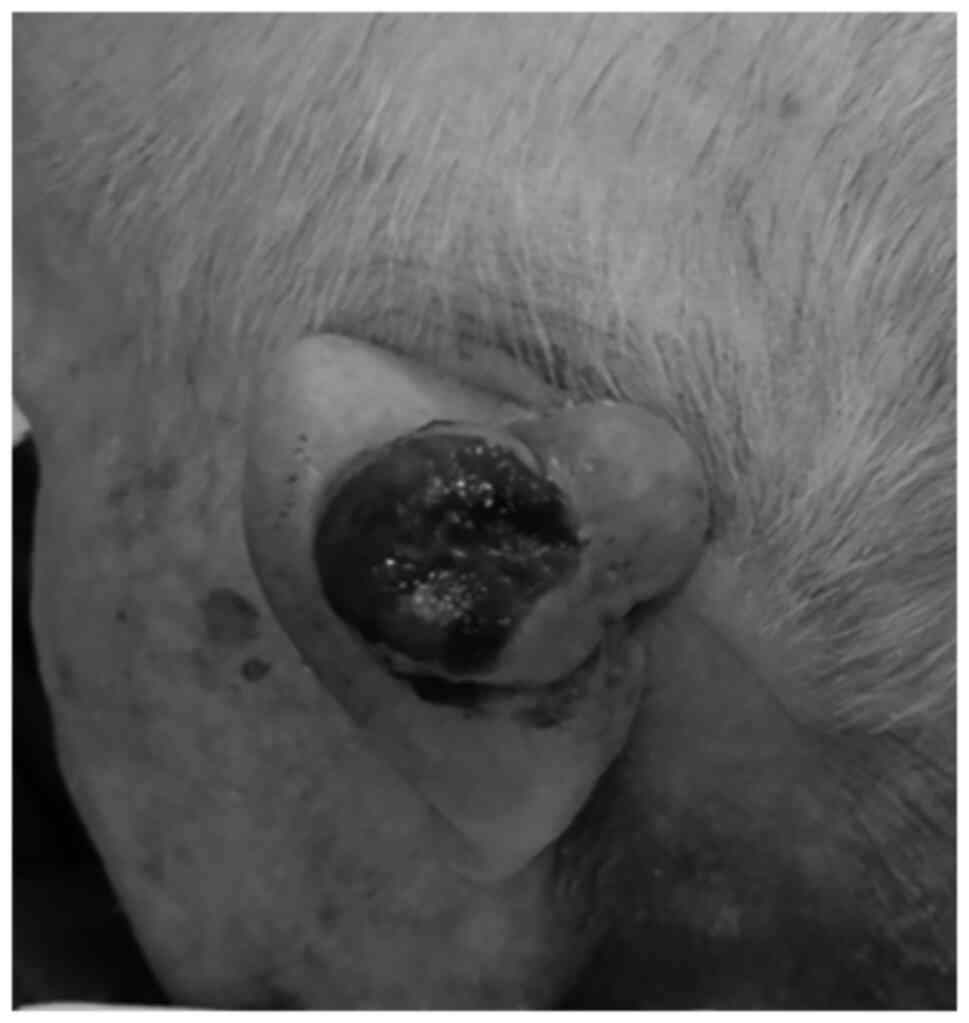
A 100-year-old male patient consulted a general
practitioner due to a 45x30 mm tumor in the posterior part of his
left auricle. It was observed to be a cutaneous tumor. The surface
of the lesion was ulcerated with bleeding (Fig. 1). The size of the ulcer was 29x23
mm. As underlying conditions, the patient had cryptogenic
organizing pneumonia, chronic kidney disease and hypothyroidism.
The tumor was noted to be ~1 cm in diameter in the posterior part
of his left auricle ~1 year prior to the consultation. The tumor
rapidly developed and erosion with bleeding was observed on the
surface of the tumor. The patient was referred to the Department of
Dermatology of Toyooka Hospital (Toyooka, Japan) in November 2019
and a needle biopsy of the tumor was performed. Histological
examination of the biopsy sample suggested that the tumor was
malignant and fibrosarcoma was suspected. Therefore, the tumor was
resected by a plastic and reconstructive surgeon.
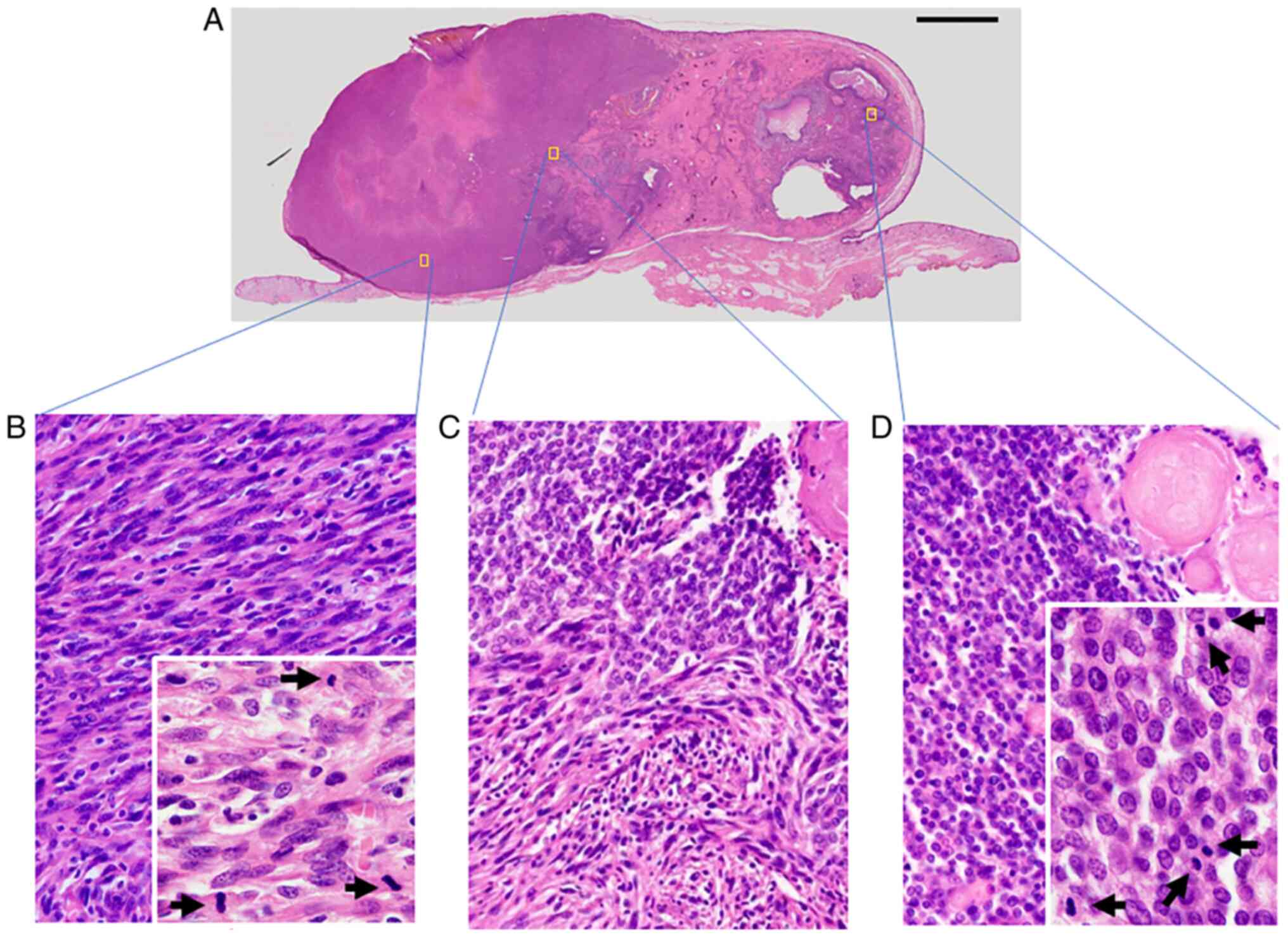
The size of the resected tumor was 45x30x16 mm with
exophytic growth. Histological examination of the tumor revealed
that the tumor had been primarily located in the dermis while
infiltrating the deeper portion of the dermis and that the tumor
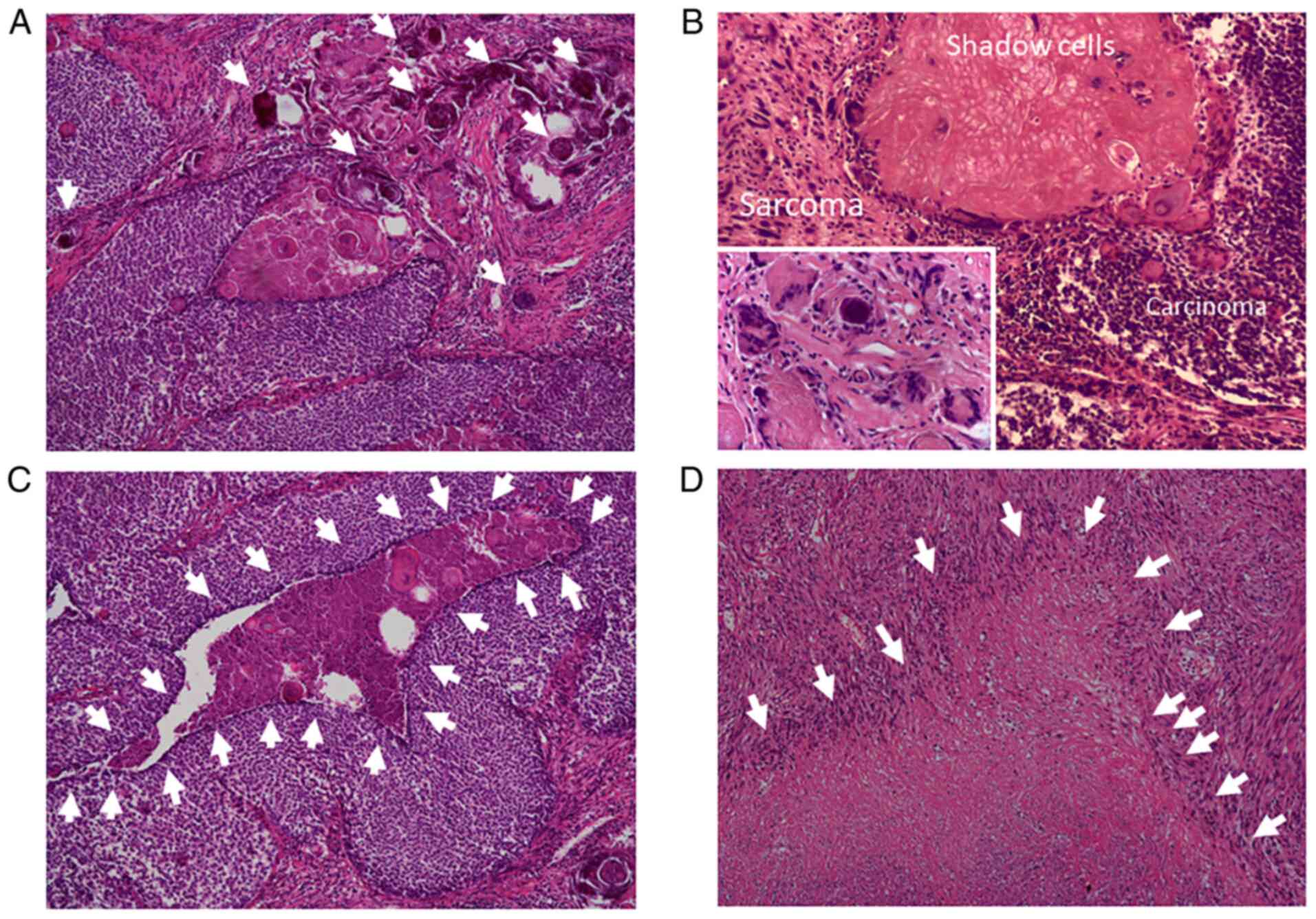
comprised two components (Fig. 2).
The first component presented as a pilomatricoma-like area (right
side of Fig. 2A and D) and the second consisted of spindle
cells (left side of Fig. 2A and
B). A transition area was present
between these two components (Fig.
2A and C). The
pilomatricoma-like area was comprised of basophilic atypical
basaloid cells, shadow cells and multinuclear giant cells with
necrosis and calcification (Figs.
2D and 3A-C). The basaloid
cells had basophilic cytoplasm, clear nucleoli and deeply stained
nuclear chromatin. The spindle cells in the second component
exhibited plump atypical nuclei (Figs.
2B and 3B and D). Necrosis and fibrosis were also
observed in the spindle cell area (Fig. 3D). In the boundary area, the two
components gradually transitioned into each other (Figs. 2C and 3B). Both components contained numerous
mitotic cells. The ratio of epithelial lesion to the spindle cell
lesion was 2:3. There was no vascular invasion and the surgical
margin was negative.
Immunohistochemistry was also performed using the
fully automated immunohistochemical stainer BOND-III (Leica
Biosystems), which uses the antigen retrieval reagent Bond epitope
retrieval (pH 9; Leica Biosystems) and a staining kit containing
second antibodies (BOND Polymer Refine Detection; cat. no. DS9800;
Leica Biosystems). The following prediluted primary antibodies were
used: Anti-Ki-67 (cat. no. IS629), P53 (cat. no. IS616),
cytokeratin (CK) AE1/AE3 (cat. no. IS053), CK 5/6 (cat. no. M7273),
epithelial membrane antigen (EMA) (cat. no. IS625), vimentin (cat.
no. IS630), CD31 (cat. no. IS610), CD34 (cat. no. IS632), CD56
(cat. no. IS628), desmin (cat. no. IS606) and α-smooth muscle actin
(α-SMA) (cat. no. IS611) antibodies (Agilent Technologies, Inc.);
anti-factor XIIIa antibody (cat. no. PA0449; Leica Biosystems); and
anti-S100 protein (cat. no. 422091), synaptophysin (cat. no. 43831)
and chromogranin A (cat. no. 412751) antibodies (Nichrei
Biosciences, Inc.). Immunohistochemically, both the components were
negative for CD34, CD56, chromogranin A, synaptophysin, S100,
desmin and factor XIIIa and positive for P53. Staining for CK
AE1/AE3 was positive in the basaloid cell component and
weakly-moderately positive in a small number of scattered cells in
the spindle cell component (Fig.
4A). Furthermore, staining for CK 5/6, P40 and EMA was positive
in the basaloid cells, but negative in the spindle cell component
(results not shown). Staining for CK AE1/AE3 and CK 5/6 was
positive in the cytoplasm and staining for EMA was positive on the
cell membrane with or without the cytoplasm. The nuclei were
positive for P40. Staining for vimentin was positive in the
cytoplasm of the spindle cell component and in a small part of the
basaloid cells (Fig. 4B). Most of
the basaloid cells were negative for vimentin. CD31 was partially
weakly-moderately positive in the cytoplasm and cell membrane of
the spindle cell component and negative in the basaloid cells
(Fig. 4C). Staining for α-SMA was
partially weakly-moderately positive in the cytoplasm of the
spindle cell component and negative in the basaloid cell component
(Fig. 4D). Furthermore, staining
for β-catenin was clearly positive in the nuclei and cytoplasm of
the basaloid cells (Fig. 4E). In
the spindle cell area, β-catenin expression was lower than that in
the basaloid cell area, but part of the spindle cells expressed
β-catenin in the cytoplasm (Fig.
4E). Staining for P53 was positive in the nuclei of the tumor
and 91.3% of the tumor cells in the carcinoma area were positive
for P53, whereas 51.0% of the tumor cells in the sarcoma area were
positive for P53 (Fig. 4F). The
Ki-67 labeling index was 36.4% in the hot-spot of the basaloid
cells (Fig. 4G). These results
suggested that the pilomatricoma-like area may be considered as a
pilomatrical carcinoma. The Ki-67 labeling index in the hot-spot of
the spindle cell component was 35.8%, suggesting that the tumor was
a pilomatrical carcinosarcoma (Fig.
4G).
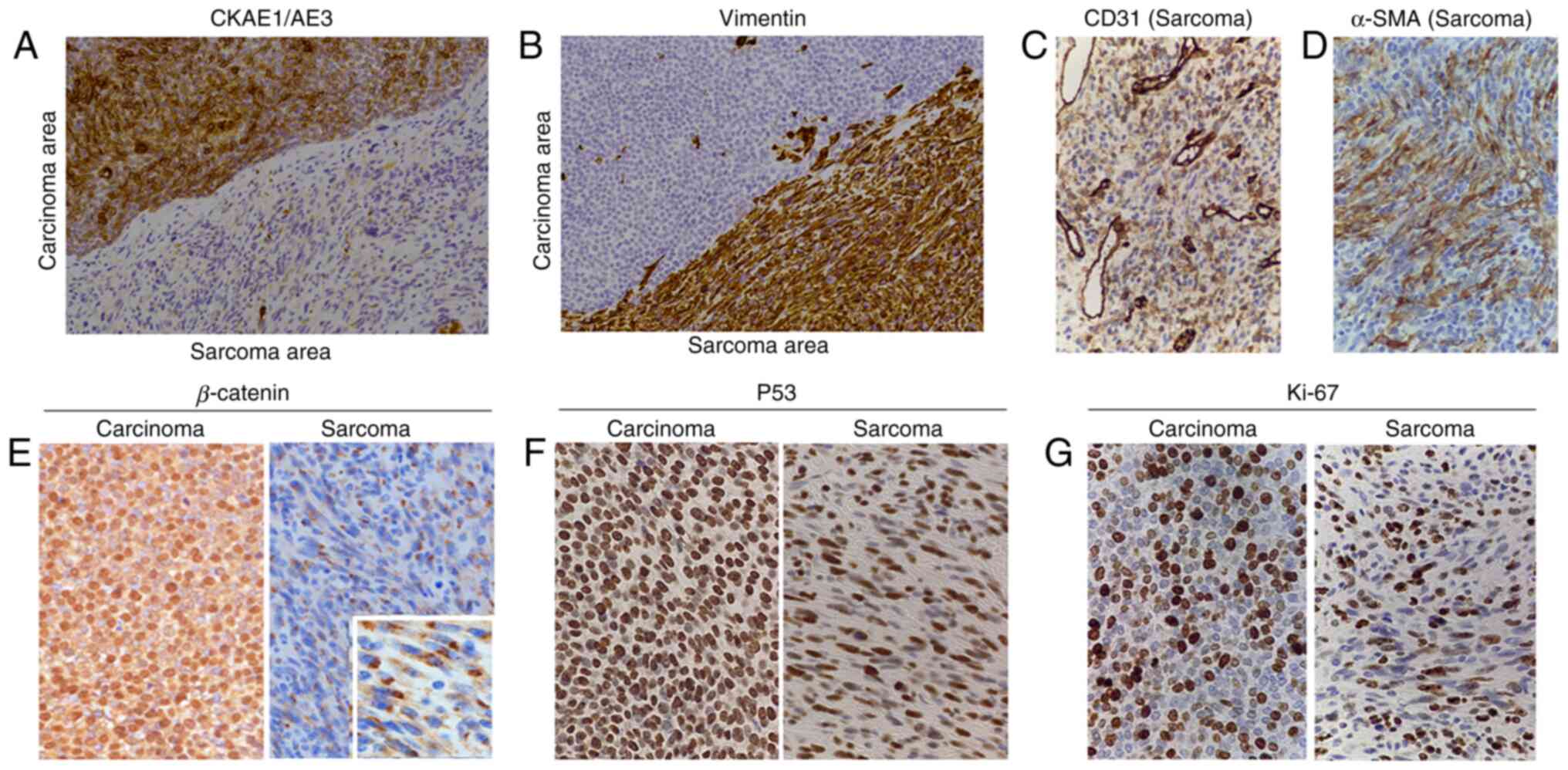 | Figure 4Immunohistochemical staining of the
tumor. Representative images of immunohistochemical staining for
(A) CK AE1/AE3, (B) Vimentin, (C) CD31, (D) α-SMA, (E) β-catenin,
(F) P53 and (G) Ki-67 in the tumor are presented. CK AE1/AE3 was
positive in the basaloid cells. A small number of cells were
weakly-moderately positive and most of the cells were negative for
CK AE1/AE3 in the spindle cell area. The spindle cell area was
positive for vimentin, but only a small number of cells in the
epithelial lesion were positive for vimentin. CD31 was partially
weakly-moderately positive in the spindle cell area. In the small
area of the spindle cells, α-SMA was positive. β-catenin was
positive in the nuclei and cytoplasm of the basaloid cells and
positive in the cytoplasm of certain spindle cells. Numerous
P53-positive cells and Ki-67-positive cells were observed among the
basaloid cells and the spindle cells (total magnification, x200 in
A-G and x500 in the inset of E). SMA, smooth muscle actin; CK,
cytokeratin. |
The patient suddenly died 75 days after the
resection of the tumor, possibly due to acute cardiac failure.
After the surgery, the patient did not exhibit any symptoms
suggesting tumor recurrence and/or metastasis. The patients came to
the hospital 6 times to receive follow-up examinations after the
surgery. There were no physical findings suggesting the recurrence
and/or metastasis of the tumor. When the patient died, there was no
tumor at the resected site. Therefore, it was suggested that the
patient did not experience any recurrence or develop any metastatic
lesion until his death.
Discussion
The present study reported on a case of pilomatrical
carcinosarcoma of the left auricle in a 100-year-old male
patient.
A total of 10 cases of pilomatrical carcinosarcoma,
including the present case, have been reported (Tables I and II). Of these, 6 patients were male and
four were female. The male-to-female ratio was 3:2. The age of the
patients at the time-point of diagnosis ranged from 9 to 100 years
and their mean and median ages were 65.7 and 75.5 years,
respectively. These results may infer that pilomatrical
carcinosarcoma typically develops in elderly males. The results are
contrary to pilomatricoma, since the latter usually develops in
young individuals and is predominantly observed in females
(6). However, due to the small
number of patients with pilomatrical carcinosarcoma, further
analysis requires to be performed after the accumulation of
cases.
 | Table ILiterature review and present case of
pilomatrical carcinosarcoma. |
Table I
Literature review and present case of
pilomatrical carcinosarcoma.
| | Histology | |
|---|
| First author
(year) | Case no. | Age, years | Sex | Site of original
tumor | Sizea, mm | Epithelial
lesion | Sarcoma | Treatment | Prognosis (OP) | (Refs.) |
|---|
| Hanly (1994) | 1 | 36 | M | Cheek | 40x40 | P (benign) | MFH | R + Ly + Ra | PMb | (11) |
| Scholl (2010) | 2 | 23 | M | Preauricular | ND | P (benign) | ND (suggesting
fibrosarcoma) | R |
Recurrencec | (12) |
| Clark (2017) | 3 | 77 | M | Cheek | 50 | PC, BCC | USCS | R | NRM (2 months) | (10) |
| Suyama, (2017) | 4 | 73 | M | Cheek | 20x20 | PC | ND | R | NRM (8 months) | (5) |
| Fernandez-Flires,
(2018) | 5 | 78 | M | Hand | 6 | PC | ND | R | NRM (8 months) | (6) |
| Leecy, (2018) | 6 | 87 | F | Hand | 16 | PC | US | R | NRM (6 months) | (7) |
| Mori (2019) | 7 | 100 | F | Temple | 15x12 | PC | US | R | ND | (8) |
| Gates, (2020) | 8 | 0 | F | Neck | 48x40 | PC | ND | R | ND | (13) |
| Luong, (2020) | 9 | 74 | F | Posterior scalp | 25 | PC, SCC | UPS | R | NRM (45 months) | (9) |
| Present case | 10 | 100 | M | Auricle | 45x30 | PC | USCS | R | NRM (2.5
months) | - |
 | Table IIImmunohistochemistry of epithelial
and sarcomatous areas in pilomatrical carcinosarcoma. |
Table II
Immunohistochemistry of epithelial
and sarcomatous areas in pilomatrical carcinosarcoma.
| First author
(year) | Case no. | Epithelial
area | Sarcomatous
area | (Refs.) |
|---|
| Hanly (1994) | 1 | HMW CK (+), EMA
(-), CEA (-), actin (-), chromogranin (-), NSE (-), HMB45 (-), ulex
(-), α1-antitrypsin (-), S100 (-), vimentin (-), CAM5.2
(-) | Vimentin (+),
CAM5.2+, HMW CK (-), EMA (-), CEA (-), actin (-), chromogranin (-),
HMB45 (-), ulex (-), NSE (-), α1-antitrypsin (-), S100
(-) | (11) |
| Scholl (2010) | 2 | CK AE1/AE3 (+) | Factor XIIIa (+),
vimentin (+) | (12) |
| Clark, (2017) | 3 | BerEP4 (+), CK5/6
(+) | Vimentin (+) | (10) |
| Suyama (2017) | 4 | CK AE1/AE3 (+),
CK5/6 (+), EMA (+), CEA (-), CAM5.2 (-), vimentin (-), α-SMA (-),
desmin (-), CD31 (-), CD34 (-), NSE (-), S100 (-), Ki-67 labeling
index 40%, β-catenin (+Cy,N) | Vimentin (+), α-SMA
(+), NSE (partially+), CK AE1/AE3 (-), CEA (-), CK5/6 (-), CAM5.2
(-), desmin (-), CD31 (-), CD34 (-), S100 (-), Ki-67 labeling index
20%, β-catenin (partially+N) | (5) |
| Fernandez-Flires
(2018) | 5 | CK AE1/AE3 (+), EMA
(+), CK5/6 (+), CAM5.2 (+), CK7 (-), CK20 (-), S100 (-), TTF-1 (-),
BerEP4 (-), CD34 (-), β-catenin (+Cy) | CK AE1/AE3
(focal+), CAM5.2 (focal+), CK7 (-), CK20 (-), S100 (-), TTF-1 (-),
BerEP4 (-), CD34(-), β-catenin (+Cy) | (6) |
| Leecy (2018) | 6 | CK AE1/AE3 (+), CK7
(-), CK20 (-), CAM5.2 (-), BerEP4 (-), CD10 (-), P63 (+), P53 (+),
β-catenin (+Cy), MIB-1 labeling index 20% | CK AE1/AE3 (-),
CK5/6 (-), CK7 (-), CK20 (-), CK[MNF116] (-), Desmin (-), CD10 (+),
P53 (+), Sox10 (-), β-catenin (+Cy), MIB-1 labeling index 5% | (7) |
| Mori (2019) | 7 | CK AE1/AE3+,
β-catenin (+Cy,N) | α-SMA+, β-catenin
(+Cy,N) | (8) |
| Gates (2020) | 8 | ND | ND | (13) |
| Luong (2020) | 9 | CK AE1/AE3 (+),
CK5/6 (+), P40 (+), P16 (+), P63 (+), P53, vimentin (-), SMA (-),
CD34 (-), TTF-1 (-), PSA (-), β-catenin (+Cy,N) | CK AE1/AE3
(scattered+), CK5/6 (-), P40 (-), P53 (+), CD34 (-), vimentin (+),
SMA (focal+), β-catenin (+Cy,N) | (9) |
| Present case | 10 | CK AE1/AE3 (+),
CK5/6 (+), P40 (+), EMA (+), S100 (-), vimentin (mostly negative,
few positive cells), CD31 (-), CD56 (-), chromogranin A (-),
synaptophysin (-), desmin (-), CA9 (-), α-SMA (-), β-catenin
(+Cy,N), Ki-67 labeling index 36.4% | CK AE1/AE3 (mostly
negative, few positive cells), CK5/6 (-), P40 (-), EMA (-), S100
(-), vimentin (+), CD31 (focally moderate-weak+), CD56 (-),
chromogranin A (-), synaptophysin (-), desmin (-), CA9 (-), α-SMA
(focally+), β-catenin (+Cy), Ki-67 labeling index 35.8% | - |
Among the 10 known cases of pilomatrical
carcinosarcoma, there was one case of distal metastasis to the
lungs and one of local recurrence (11,12).
In the remaining eight cases, including the present case, neither
metastasis nor recurrence has been reported. Regarding therapy in
the case of recurrence, tumor resection, lymph node dissection,
radiotherapy and chemotherapy were performed (12). In the case of pulmonary metastasis,
resection of the original tumor, lymph node dissection and
radiotherapy were performed initially (11). Subsequently, pulmonary metastasis
of the tumor was detected; hence, resection of the metastatic
lesions in the lung was performed (11). In the present case, resection of
the tumor without radiation or chemotherapy was performed as the
patient was very old.
Regarding the epithelial lesions of the 10
pilomatrical carcinosarcoma cases, two cases were benign
pilomatricoma, whereas the remaining eight cases were carcinomas
(Table I). Specifically, six cases
were pilomatrical carcinoma, one case was pilomatrical carcinoma
with basal cell carcinoma and the remaining case was pilomatrical
carcinoma with squamous cell carcinoma (10,13).
In the sarcomatous lesion, atypical spindle cells or pleomorphic
cells were observed. Of the 10 cases, 9 had atypical spindle cells
and one had pleomorphic cells, suggesting that atypical spindle
cells mainly grow in the sarcomatous lesions in pilomatrical
carcinosarcomas. In the present case, the ratio of the epithelial
to sarcomatous lesions was 2:3. Only for one out of the previously
reported nine cases, this ratio was determined to be 1:1(5). The spindle cells in Case 5 strongly
but focally expressed CK AE1/AE3 and CAM 5.2(6). The spindle cells of Case 1 also
expressed an epithelial marker, CAM 5.2, but were negative for
high-molecular-weight keratin and EMA. The sarcomatous areas in
Cases 4 and 6 were negative for CK AE1/AE3 and CK 5/6 (5,7). The
sarcomatous areas in Cases 9 and 10 were sparsely positive for CK
AE1/AE3 but negative for CK 5/6 and EMA (9). In Cases 2, 3, 7 and 8, epithelial
markers in the sarcomatous lesions were not reported (8,10,12,13).
The sarcomatous areas were positive for vimentin in Cases 1-4, 9
and 10. There was no mention of immunohistochemical detection of
vimentin for Cases 5-8.
In the present case, most of the atypical spindle
cells were negative for CK AE1/AE3 but positive for vimentin. A
large amount of P53-positive and Ki-67-positive cells were
observed. Therefore, the spindle cell area of the tumor was
diagnosed as a malignant mesenchymal tumor (sarcoma). Staining for
CD31 was partially weakly-moderately positive and CD34 was
completely negative in the spindle cell area. On the basis of the
findings of negative CD34 and severely atypical spindle cells,
vascular tumor and solitary fibrous tumor were unlikely diagnoses
in the present case. Negativity for CD56, synaptophysin and
chromogranin A suggested that neuroendocrine tumors were
improbable, whereas negativity for desmin and a small number of
cells positive for α-SMA suggested that myogenic tumors were also
unlikely. Negativity for S100 suggested that the tumor was not
neural. Hence, the spindle cell area was diagnosed as
undifferentiated spindle cell sarcoma.
Although α-SMA was expressed in the sarcomatous
area, suggesting differentiation to smooth muscle in Case 7, it was
diagnosed as undifferentiated sarcoma (8). The sarcomatous areas of other
pilomatrical carcinosarcomas did not express specific
differentiation markers, suggesting that these were
undifferentiated sarcomas. It is noteworthy that most sarcomatous
areas were undifferentiated sarcomas in the cases of pilomatrical
carcinosarcoma.
It has been reported that β-catenin is a useful
marker that may be utilized to establish the diagnosis of
pilomatricoma and pilomatrical carcinoma (4). Expression of β-catenin was examined
in six of the 10 pilomatrical carcinosarcoma cases. In these cases,
both the epithelioid and sarcomatous areas expressed β-catenin in
the cytoplasm. Certain cells also expressed β-catenin in the
nucleus. These results suggested an association of β-catenin
expression in the cytoplasm and/or nucleus with pilomatrical
tumors. It has been reported that the expression of β-catenin in
pilomatricoma and pilomatrical carcinoma was able to be induced by
mutations in exon 3 of CTNNB1, suggesting that these mutations may
be contributory to pilomatrical tumors (4). However, a study by Suyama et
al (5) indicated no mutation
in exon 3 of CTNNB1 and Luong et al (9) also demonstrated no mutation in exons
3, 4 and 5 of the CTNNB1 gene in their case of pilomatrical
carcinosarcoma. Further examination is required to clarify the role
of abnormal expression of β-catenin in pilomatrical
carcinosarcomas.
There are several theories regarding the development
of carcinosarcoma: A collision of two independent tumors,
development from a common precursor to carcinoma and sarcoma, or
pre-existence of carcinoma followed by mesenchymal transition
(10,14,15).
Leecy et al (7) performed a
comparative genomic hybridization analysis of both carcinoma and
sarcoma components in pilomatrical carcinosarcoma wherein a common
clonal origin was determined, similar to the abnormal expression of
β-catenin.
In six out of the previously reported nine cases of
pilomatrical carcinosarcomas, boundaries of the carcinomas and
sarcomas were described (5,7-9,11,12).
In the six cases along with the present case, the epithelial and
sarcomatous areas closely intermingled and gradually transitioned.
The morphological, immunohistochemical and genetic examination
results suggested two possibilities: Derivation of both components
from the same progenitor cells and transition of the epithelial
tumor into a mesenchymal tumor through epithelial-mesenchymal
transition (5). In Case 5, CK
AE1/AE3 and CAM 5.2 were expressed focally and strongly, suggesting
that the sarcomatous area was able to undergo
epithelial-mesenchymal transition (6).
In conclusion, the present study reported a rare
case of pilomatrical carcinosarcoma that developed in a
100-year-old male patient. Careful observation of histological
morphology and immunohistochemical staining is considered important
in establishing the diagnosis of pilomatrical carcinosarcoma.
Acknowledgements
The authors thank Ms. Hitomi Ogaki, Mr. Katsuya
Nagaoka and Mr. Hiroaki Takenaka (Department of Laboratory, Toyooka
Hospital, Toyooka, Japan) for the preparation of histopathological
specimen sections and their staining.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
KS, YA, MT and SI designed the study. KS, YA, TT and
HA analyzed and interpreted the patient data. KS, YA and SI were
major contributors in writing the manuscript. KS, YA, MT and SI
performed the histological examination of the tumor and performed
histological diagnosis. All authors except for Professor SI, who
died in June 2021, read and agreed to the final version of the
study. KS and YA checked and approved the authenticity of the raw
data.
Ethics approval and consent to
participate
The Ethics Committee of Toyooka Hospital (Toyooka,
Japan) approved the present study.
Patient consent for publication
The family of the patient provided written informed
consent for the patient information and images to be published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jones C, Twoon M, Ho W, Portelli M,
Robertson B and Anderson W: Pilomatrix carcinoma: 12-year
experience and review of the literature. J Cutan Pathol. 45:33–38.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Nogal P, Bartkowiak E, Iwanik K and
Wierzbicka M: Common sense and tumor treatment. A case of
pilomatrical carcinoma in a 21-year-old patient with surprisingly
rapid tumor progression. Oral Oncol. 112(105007)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Xing L, Marzolf SA, Vandergriff T and
Nijhawan RI: Facial pilomatrix carcinomas treated with Mohs
micrographic surgery. JAAD Case Rep. 4:253–255. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lazar AJ, Calonje E, Grayson W, Dei Tos
AP, Mihm MC Jr, Redston M and McKee PH: Pilomatrix carcinomas
contain mutations in CTNNB1, the gene encoding beta-catenin. J
Cutan Pathol. 32:148–157. 2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Suyama T, Momose S, Yokoyama M, Kikuchi J,
Izaki S, Arai E and Tamaru J: Pilomatrical carcinosarcoma of the
cheek: Immunohistochemical and molecular analysis of beta-catenin.
Pathol Int. 67:324–326. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Fernandez-Flires A and Cassarino DS:
Sarcomatoid pilomatrix carcinoma. J Cutan Pathol. 45:508–514.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Leecy T, Ardakani NM, Harvey NT and Wood
BA: Pilomatrical carcinosarcoma: Report of a case with comparative
genomic hybridization analysis. Pathology. 50:571–573.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mori D: Pilomatrical carcinosarcoma of the
temple: A case report. J Cutan Pathol. 46:267–270. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Luong TMH, Akazawa Y, Mussazhanova Z,
Matsuda K, Ueki N, Miura S, Hara T, Yokoyama H and Nakashima M:
Cutaneous pilomatrical carcinosarcoma: A case report with molecular
analysis and literature review. Diagn Pathol. 15(7)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Clark JJ, Bowen AR, Bowen GM, Hyngstrom
JR, Hadley ML, Duffy K, Florell SR and Wada DA: Cutaneous
carcinosarcoma: A series of six cases and a review of the
literature. J Cutan Pathol. 44:34–44. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hanly MG, Allsbrook WC, Pantazis CG, Lane
R, Porubsky ES and Mann ES: Pilomatrical carcinosarcoma of the
cheek with subsequent pulmonary metastases. A case report. Am J
Dermatopathol. 16:196–200. 1994.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Scholl P, Snyder N, Patt D and Talbott B:
Pilomatrical carcinosarcoma. Otolaryngol Head Neck Surg. 143 (Suppl
3):S36–S37. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gates GA, Nguyen J and Binder SW: Rare
case report of pilomatrical carcinosarcoma in a pediatric patient.
Am J Dermatopathol. 42:208–210. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zbacnik AP, Rawal A, Lee B, Werling R,
Knapp D and Mesa H: Cutaneous basal cell carcinomsarcoma: Case
report and literature review. J Cutaneous Pathol. 42:903–910.
2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bigby SM, Charlton A, Miller MY, Zwi LJ
and Oliver GF: Biphasic sarcomatoid basal cell carcinoma
(carcinosarcoma): Four cases with immunohistochemistry and review
of the literature. J Cutan Pathol. 32:141–147. 2005.PubMed/NCBI View Article : Google Scholar
|