Introduction
Preoperative evaluation of mediastinal lymph nodes
(LNs) in patients with lung cancer is essential for determining
optimal treatment strategies in the management of primary lung
cancer. On the one hand, the status of mediastinal LNs metastasis
can not only affects the formulation of perioperative diagnosis and
treatment strategies (1-3),
but also affects the survival time of patients after surgery
(4-6).
Therefore, the current National Comprehensive Cancer Network
guidelines for non-small cell lung cancer (NSCLC) suggests that N1
(ipsilateral peribronchial and/or ipsilateral hilar lymph nodes and
intrapulmonary nodes) and N2 (ipsilateral mediastinal and/or
subcarinal lymph node(s)) node resection and mapping should be a
routine component of lung cancer resections with a minimum of three
N2 stations sampled or complete lymph node dissection (7). On the other hand, mediastinal
calcified LNs (CLNs) is generally considered to be benign and are
often caused by granulomatous disease, sarcoidosis, silicosis and
Pneumocystis jirovecii infection; however, they can also be
due to metastases from ovarian cancer, colonic adenocarcinoma,
osteosarcoma or papillary thyroid carcinoma (8,9).
Previous case reports (10-12)
and a small sample study (13) have
confirmed that primary lung cancer could metastasize to CLNs (14/72
patients; 19.4%). In addition, several previous studies have
confirmed that CLN was an important predictive factor of conversion
from a video-assisted thoracoscopic surgery (VATS) to thoracotomy
(conversion rate, 29.41-40.6%), which was due to adhesion between
CLN stations (CLNS) and hilar structures, and the subsequent
difficulty of their dissection (14-17).
However, the clinicopathological characteristics of CLNS in the
preoperative computed tomography (CT) scans of patients with NSCLC
have not been fully investigated.
Therefore, in the present study, the difference in
metastatic ratio between CLNS and non-CLNS (NCLNS) was investigated
based on preoperative thin-slice CT scans of patients with NSCLC
who underwent uniportal VATS lobectomy and systemic mediastinal
nodal dissection. Furthermore, the impact of CLNS on surgical
outcomes was explored.
Materials and methods
Clinical data collection
Following approval by the Ethics Committee of The
Affiliated Hospital of Guizhou Medical University (approval no.
2020-244), consecutive patients with NSCLC scheduled to receive
surgical treatment between June and December 2020 at The Affiliated
Cancer Hospital of Guizhou Medical University were included in this
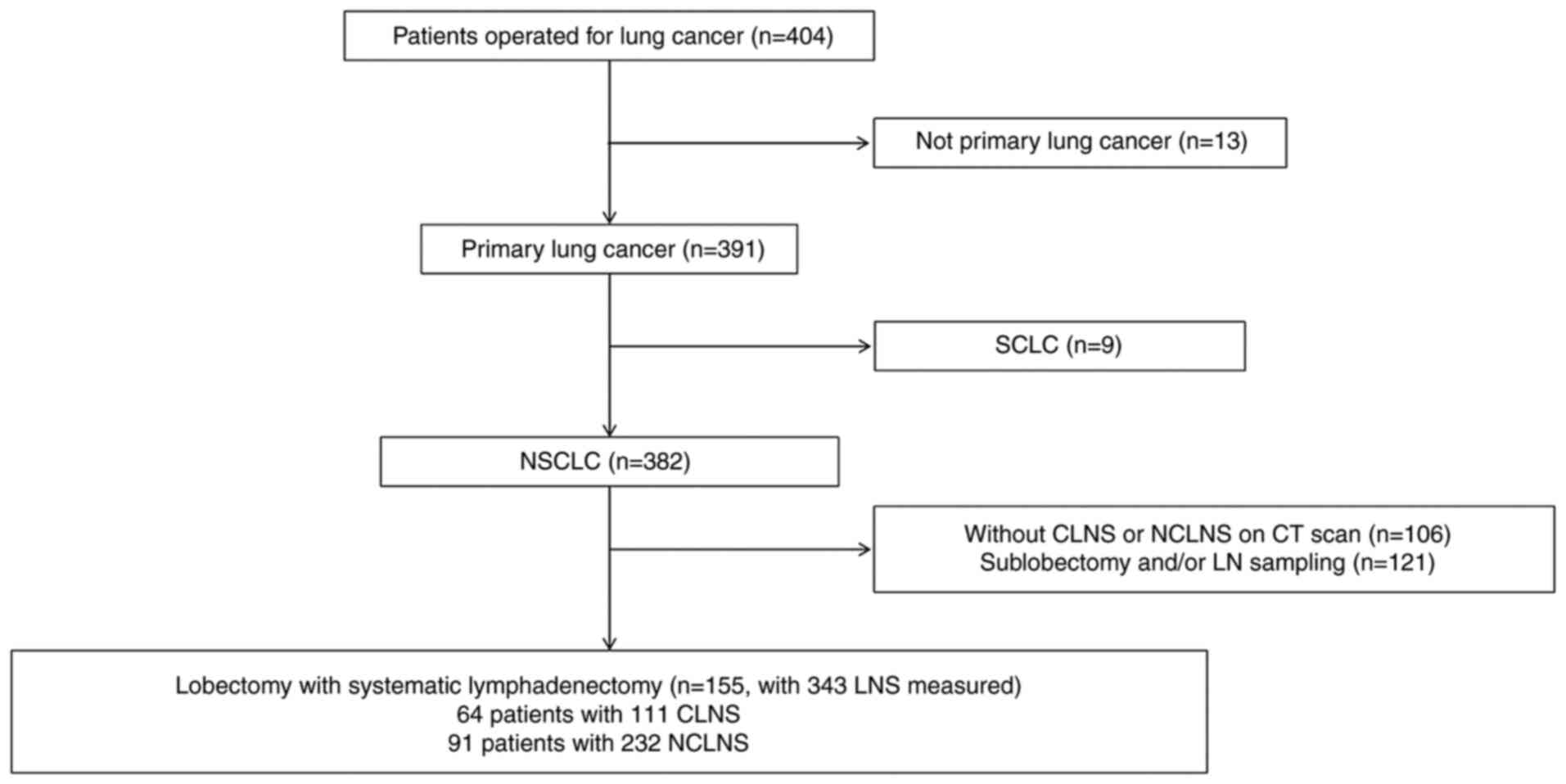
study. Fig. 1 includes a flowchart
describing patient selection. The clinical and radiological data of
patients were prospectively collected and included sex, age,
surgical lobes, number of LNS removed, duration of surgery, blood
loss, status of LNS on CT scan, tumor specimen size and pathology,
as well as T, N and M based on the International Association for
the Study of Lung Cancer TNM classification (18). Medical record review was performed
in accordance with the institutional ethics review bpard guidelines
(19).
Determination of LNS status
The preoperative CT scans without contrast were
performed on a LightSpeed VCT 64-detector scanner (Cytiva) with
subjects holding their breath at end inspiration and using the
following parameters: Detector configuration, 64x0.625 mm; pitch,
0.969; tube energy, 120 kVp; tube current, 250 mA; gantry rotation,
0.4 sec (or 100 mAs). The CT scan images were saved in DICOM
format. The DICOM data were then loaded onto the syngo.plaza
software (version VB10B, Siemens AG) to determine the LNS status. A
CLNS was defined as an LNS with calcifications of any size.
Calcification was determined by visual identification of contiguous
5- and 1-mm sections on mediastinal window setting thin-slice CT
scans (window width, 350 HU; level, 40-60 HU). NCLNS were defined
as LNS without calcification and with a minimum diameter of ≥10
mm.
Surgical procedure
Patients with a tumor size of >7 cm or CLNS
involving the pulmonary artery or vein were scheduled to receive a
thoracotomy from the beginning. For other patients, uniportal VATS
lobectomy and systemic mediastinal nodal dissection were selected
based on their advantages over thoracotomy, assuming that no
intractable technical challenge would arise during the procedure.
Uniportal VATS lobectomy and systemic mediastinal nodal dissection
were performed as previously described (20). The surgeons decided to switch from
VATS to thoracotomy if differentiating layers around the pulmonary
vessels or bronchus were deemed to be extremely dangerous, or in
the event of uncontrolled bleeding, chest wall invasion or limited
pulmonary collapse.
Statistical analysis
All data were manually entered to a excel by the
same researcher. Descriptive statistics were used to describe the
demographic characteristics. Continuous variables are presented as
the mean and standard deviation, and categorical variables as
numbers and percentages. When variances were equal, two-sample
unpaired t-test with equal variances was used for continuous
variables. For unequal variances, two-sample Wilcoxon rank-sum
(Mann-Whitney) test was used. χ2 or Fisher's exact test
was used for binary categorical data with results are presented as
odds ratios (OR) and 95% confidence interval (CI). Ordered logistic
regression (ologit) was used for ordered categorical data with
results are presented as coefficient (Coef.) and 95% CI.
Statistical analysis was performed using Stata 15.0 (StataCorp LP).
All statistical tests were two-sided and P<0.05 was considered
to indicate a statistically significant difference.
Results
Patients and LNS
A total of 155 patients with NSCLC who received
surgery were included in the present study. The clinical
characteristics of the included patients are presented in Table I. A total of 111 (32.36%) CLNS were
identified in 64 (41.29%) patients and 143 (67.64%) NCLNS in 91
(58.71%) patients. A total of 48 patients with CLNS were found to
simultaneously have 89 NCLNS. Of them, 4 patients with CLNS were
confirmed to have 5 solely metastasized NCLNS and 6 patients with
CLNS were confirmed to have 15 simultaneously metastasized NCLNS.
Among patients with NCLNS, 19 had 1 solely metastasized NCLNS and 6
patients had 2 simultaneously metastasized CLNS. In patients with
CLNS, 8 patients had 1 solely metastasized CLNS and 6 patients had
2 simultaneously metastasized CLNS. Moreover, 5 patients had 1
solely metastasized NCLNS, and there 2 patients had 2, 1 patient
had 3 and 2 patients had 4 simultaneously metastasized NCLNS. All
patients were diagnosed as M0. The differences in
clinicopathological characteristics, including age, sex, surgical
lobes, pathology, tumor size, T and N, were not significant between
patients with CLNS and NCLNS. No patient died intraoperatively or
within 30 days after surgery.
 | Table IDifferences in clinicopathologic
characteristics between patients with CLNS and NCLNS. |
Table I
Differences in clinicopathologic
characteristics between patients with CLNS and NCLNS.
| Characteristics | NCLNS | CLNS | P-value |
|---|
| Patients, n (%) | 91 (58.71) | 64 (41.29) | - |
| LN measured, n | 232.00 | 111.00 | - |
| Age, years (mean ±
SD) | 58.24±10.68 | 58.95±8.35 | 0.72a |
| Sex, n (%) | | | 0.87b |
|
Female | 31 (34.07) | 21 (32.81) | |
|
Male | 60 (65.93) | 43 (67.19) | |
| Lobes, n (%) | | | 0.54b |
|
Left
upper | 17 (18.68) | 9 (14.06) | |
|
Left
lower | 21 (23.08) | 12 (18.75) | |
|
Right
upper | 23 (25.27) | 18 (28.12) | |
|
Right
middle | 6 (6.59) | 9 (14.06) | |
|
Right
lower | 24 (26.37) | 16 (25.00) | |
| Pathology, n (%) | | | 0.60b |
|
Squamous
cell carcinoma | 43 (47.25) | 33 (51.56) | |
|
Adenocarcinoma | 48 (52.75) | 31 (48.44) | |
| Size, mm (mean ±
SD) | 39.64±15.50 | 41.61±17.11 | 0.74a |
| T stage, n (%) | | | 0.36c |
|
1b | 10 (10.99) | 4 (6.25) | |
|
1c | 17 (18.68) | 14 (21.88) | |
|
2a | 27 (29.67) | 14 (21.88) | |
|
2b | 15 (16.48) | 18 (28.12) | |
|
3 | 20 (21.98) | 11 (17.19) | |
|
4 | 2 (2.20) | 3 (4.69) | |
| N stage, n (%) | | | 0.34b |
|
0 | 52 (57.14) | 44 (68.75) | |
|
1 | 14 (15.38) | 7 (10.94) | |
|
2 | 25 (27.47) | 13 (20.31) | |
Metastatic ratio of patients and
LNS
The metastatic ratio of patients and LNS between the
CLNS and non-CLNS groups is presented in Table II. On a per-patient basis, the
differences in metastatic ratios were not significant between
patients with CNLS and NCLNS, neither in the all-patient group
(20.313 vs. 27.473%; P=0.308) nor in the patient group with solely
CLNS or NCLNS metastasized (15.000 vs. 27.473%; P=0.073). However,
on a per-nodal station basis, the metastatic ratios of patients
with CLNS were all lower than those with NCLNS, not only in the
all-LNS group (9.009 vs. 21.983%, P=0.004) but also in the LNS
group which in patients with solely CLNS or NCLNS (9.009 vs.
21.678%; P=0.009) and in the patients with CLNS (9.009 vs. 22.472%;
P=0.010).
 | Table IIComparison of metastatic ratio of
patients and LNS between the CLNS and NCLNS groups. |
Table II
Comparison of metastatic ratio of
patients and LNS between the CLNS and NCLNS groups.
|
Characteristics | Group | Metastasis, (no. of
patients or nodes) | Without metastasis
(no. of patients or nodes) | Total (no. of
patients or nodes) | Metastatic ratio,
% | P-value |
|---|
| All patients | C | 13 | 51 | 64 | 20.313 | 0.308a |
| | N | 25 | 66 | 91 | 27.473 | |
| Patients with
solely C or N metastasis | C | 9 | 51 | 60 | 15.000 | 0.073a |
| | N | 25 | 66 | 91 | 27.473 | |
| All LNS | C | 10 | 101 | 111 | 9.009 | 0.004b |
| | N | 51 | 181 | 232 | 21.983 | |
| LNS in patients
with solely C or N metastasis | C | 10 | 101 | 111 | 9.009 | 0.009b |
| | N | 31 | 112 | 143 | 21.678 | |
| LNS in patients
with CLNS | C | 10 | 101 | 111 | 9.009 | 0.010b |
| | N | 20 | 69 | 89 | 22.472 | |
Predictive factors of LNS
metastasis
On a per-patient basis, pathology (odds ratio;
OR=4.170; P<0.001) and calcification (OR=0.432; P=0.043) was
identified as a predictive factor of metastasis by single-factor
logistic analysis. Pathology (OR=7.467; P=0.001), T (OR=1.601;
P=0.014) and calcification (OR=0.392; P=0.042) were identified as
independent prognostic factors by multi-factor logistic analysis.
On a per-station basis, pathology (Coefficient; Coef.=1.308;
P=0.017) and calcification (Coef.=-0.862; P=0.037) remained a
prognostic factor following single-factor ologit analysis.
Pathology (Coef.=1.766; P=0.002), T (Coef.=0.421; P=0.021) and
calcification (Coef.=-0.905; P=0.044) were identified as
independent prognostic factors by multi-factor ologit analysis
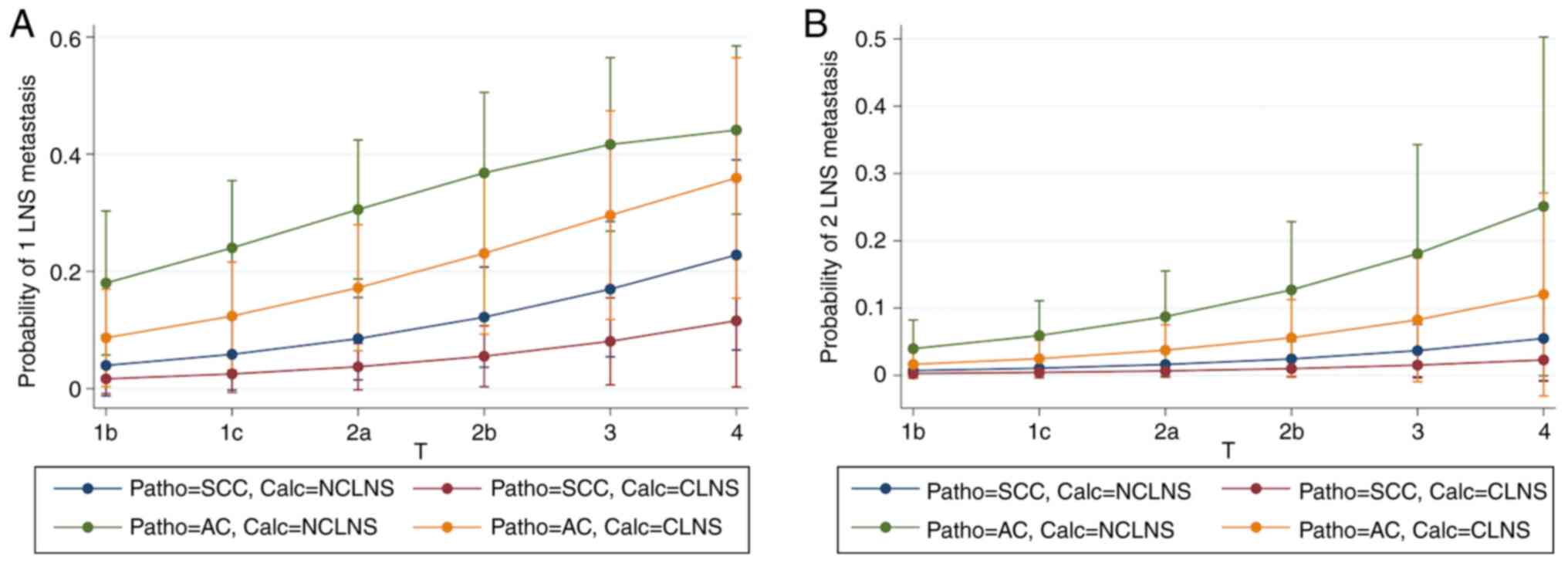
(Table III). Fig. 2 demonstrates the ability of T,
pathology and calcification to predict 1 (Fig. 2A) and 2 (Fig. 2B) LNS metastasis.
 | Table IIIEffects of clinicopathological
characteristics on lymph node stations metastasis in patients with
calcified lymph node stations or non-calcified lymph node
stations. |
Table III
Effects of clinicopathological
characteristics on lymph node stations metastasis in patients with
calcified lymph node stations or non-calcified lymph node
stations.
| | Single-factor
logistic | Multi-factor
logistic | Single-factor
ologit | Multi-factor
ologit |
|---|
|
Characteristics | OR | 95% CI | P-value | OR | 95% CI | P-value | Coef. | 95% CI | P-value | Coef. | 95% CI | P-value |
|---|
| Age | 0.982 | 0.945-1.021 | 0.358 | 0.979 | 0.939-1.021 | 0.327 | -0.017 | -0.055-2.486 | 0.377 | -0.021 | -0.060-0.019 | 0.313 |
| Sex | 0.476 | 0.219-1.037 | 0.064 | 0.872 | 0.337-2.255 | 0.778 | -0.644 | -1.414-0.125 | 0.102 | -0.105 | -1.025-0.814 | 0.822 |
| Lobes | 0.960 | 0.733-1.256 | 0.764 | 0.922 | 0.684-1.241 | 0.591 | -0.053 | -0.321-0.215 | 0.698 | -0.089 | -0.379-0.201 | 0.547 |
| Pathology | 4.170 | 1.747-9.953 | <0.001 | 7.467 | 2.333-23.901 | 0.001 | 1.308 | 0.441-2.175 | 0.017 | 1.766 | 0.661-2.871 | 0.002 |
| T stage | 1.040 | 0.780-1.386 | 0.791 | 1.601 | 1.099-2.333 | 0.014 | 0.060 | -0.228-0.347 | 0.685 | 0.421 | 0.065-0.778 | 0.021 |
| Calcification | 0.432 | 0.186-1.002 | 0.043 | 0.392 | 0.159-0.966 | 0.042 | -0.862 | -1.701-0.023 | 0.037 | -0.905 | -17.840-0.026 | 0.044 |
Impacts of CLNS on surgical
outcomes
Although the number of LNs resected in the patients
with CLNS was the same as that in the patients with NCLNS
(P=0.446), a significantly higher number of patients with CLNS
(P=0.006) than with NCLNS received the originally scheduled
thoracotomy (51.56 vs. 37.6%) or conversion from VATS to
thoracotomy (14.06 vs. 4.40%); these patients were also associated
with a higher time cost (235.516±47.685 vs. 185.330±30.256 min;
P<0.001) and blood loss (334.063±359.655 vs. 159.341±113.752 ml;
P<0.001; Table IV).
 | Table IVDifferences in surgical outcomes
between patients with CLNS and NCLNS. |
Table IV
Differences in surgical outcomes
between patients with CLNS and NCLNS.
| Surgical
outcomes | NCLNS | CLNS | P-value |
|---|
| Surgery, n (%) | | | 0.006a |
|
VATS | 53 (58.24) | 22 (34.38) | |
|
Conversion | 4 (4.40) | 9 (14.06) | |
|
Thoracotomy | 34 (37.36) | 33 (51.56) | |
| Operating time, min
(mean ± SD) | 185.330±30.256 | 235.516±47.685 |
<0.001b |
| Blood loss, ml
(mean ± SD) |
159.341±113.752 |
334.063±359.655 |
<0.001b |
| Number of LN
dissected, n (mean ± SD) | 22.242±6.664 | 21.688±6.434 | 0.446b |
Discussion
It is well known that systemic mediastinal nodal
dissection is an integral part of radical surgery for lung cancer
and is associated not only with pathological N staging and the
selection of subsequent treatment strategies, but also with patient
prognosis. In a previous study, mediastinal CLNs were commonly
observed in the preoperative CT scans of patients with NSCLC, since
they resided in an area with a high prevalence of tuberculosis
(21). A total of 64 patients
(41.29%) were found to have 111 CLNS. Although CLNs are generally
thought to be benign (8,9), a number of case reports (10,11)
have confirmed that primary lung cancer could metastasize to CLNS.
In a small sample size study, Nakanishi et al (13) evaluated 72 consecutive patients with
CLNS detected on preoperative CT who underwent pulmonary resection
for primary lung cancer. A total of 354 LNs, including 101 CLNs,
were evaluated. The frequency of metastasis to CLNS was 19.4%
(14/72 patients) on a per-patient basis and 18.8% (19/101 CLNS) on
a per-nodal station basis. In the present study, the metastatic
ratio of CLNS was 15.000% (9/60 patients) on a per-patient basis
and 9.009% (10/111 CLNS) on a per-nodal station basis, suggesting
that metastasis to CLNS was not rare in patients with NSCLC. To the
best of our knowledge, the mechanisms of metastasis to CLNS remain
unclear. Nakanishi et al (13) hypothesized that cancer secreting
calcium metastasized to previously existing CLNS and NCLNS
(11). From the pathological
findings of previous studies (3-5),
it can be concluded that cancer metastasizes to tissues surrounding
the CLN but not to the calcified body itself, since the reported
metastatic LNs were all psammomatous (10,11),
and no metastases occurred in single and large CLNS which was
single and with major size (26/101) (13). However, the precise mechanisms of
metastasis to CLNS should be investigated by future studies.
Although, on a per-station basis, the metastatic
ratios of patients with CLNS were lower than those of patients with
NCLNS [not only in the all-LNS group (9.009 vs. 21.983%; P=0.004)
but also in the LNS groups which in patients with solely CLNS or
NCLNS (9.009 vs. 21.678%; P=0.009) and in the patients with CLNS
(9.009 vs. 22.472%; P=0.010)] and CLNS was an independent risk
reduction factor on both a per-patient (OR=0.392; P=0.0402) and a
per-nodal station basis (Coef.=-0.905; P=0.044), the metastatic
ratios were comparable on a per-patient basis [both in the
all-patient group (20.313 vs. 27.473%; P=0.308) and in the patient
group with solely CLNS or NCLNS (15.000 vs. 27.473%; P=0.073)].
These results suggested that CLNS should be dissected in addition
to NCLNS to achieve radical dissection, although the results of the
present and a previous study (13)
found that CLNS is less likely to metastasize than NCLNS.
Furthermore, as stated in a previous study (22), it was also confirmed that T stage
and adenocarcinoma were independent risk factors for LNS
metastasis.
Previous studies have confirmed that dissecting CLNS
is challenging and time-consuming (14,15).
However, utmost efforts were made for their thorough removal. Thus,
the number of LNs resected was not significantly different between
patients with NCLNS and those with CLNS (22.242±6.664 vs.
21.688±6.434; P=0.446). However, more time was spent
(235.516±47.685 vs. 185.330±30.256 min; P<0.001) and more blood
was lost (334.063±359.655 vs. 159.341±113.752 ml; P<0.001)
during the surgery of patients with CLNS. Furthermore, since
several studies had demonstrated that CLNS could predict
intraoperative conversion from VATS to thoracotomy during lobectomy
due to adhesion between CLNS and hilar structures (14,15,17,23),
it was found that a significantly higher number of patients with
CLNS than with NCLNS received the originally scheduled thoracotomy
(51.56 vs. 37.36%) or underwent conversion from VATS to thoracotomy
(14.06 vs. 4.405%). These results were consistent with those of the
study by Byun et al (17),
in which 69/276 patients (25.00%) received conversion from VATS to
thoracotomy, of whom 40.58% (28/69) had CLNS. CLNS was also
confirmed in that study to be an independent risk factor for
conversion (OR=2.67; P=0.020).
The present study was not without its limitations.
It failed to further explore the associations between the
characteristics of CLNS and metastasis, such as calcification type
(focal or diffuse calcification) and LN size, which were not
classified due to the small sample size. Further investigation in a
study with a larger sample size is encouraged to clarify these
associations.
In conclusion, although CLNS are a risk reduction
factor for metastasis and their dissection is time- and
blood-consuming in patients with NSCLC, their thorough removal is
advised, since metastases were identified in ~15% patients and 9%
CLNS.
Acknowledgements
Not applicable.
Funding
This study was partly funded by the Wu Jieping Medical
Foundation (grant no. 320.6750.18470) and the Science and
Technology Fund Project of Guizhou Health Committee (grant no.
Gzwjkj2020-1-111).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LL, XW, MZ, SY, YW, HX and XD analyzed and
interpreted the data. LL, XW, MZ and HX were major contributors in
writing the manuscript. LL, SY, YW and XD confirm the authenticity
of all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by our hospital's review
board (approval no. 2020-244, the Affiliated Hospital of Guizhou
Medical University, Guiyang, Guizhou, China). Written informed
consent was obtained from all individual participants included in
the study.
Patient consent for publication
Patients signed the informed consent form regarding
the publication of their data and images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bille A, Woo KM, Ahmad U, Rizk NP and
Jones DR: Incidence of occult pN2 disease following resection and
mediastinal lymph node dissection in clinical stage I lung cancer
patients. Eur J Cardiothorac Surg. 51:674–679. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Smeltzer MP, Faris NR, Ray MA and
Osarogiagbon RU: Association of pathologic nodal staging quality
with survival among patients with non-small cell lung cancer after
resection with curative intent. JAMA Oncol. 4:80–87.
2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhong W, Yang X, Bai J, Yang J, Manegold C
and Wu Y: Complete mediastinal lymphadenectomy: The core component
of the multidisciplinary therapy in resectable non-small cell lung
cancer. Eur J Cardiothorac Surg. 34:187–195. 2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chiappetta M, Lococo F, Leuzzi G, Sperduti
I, Bria E, Petracca Ciavarella L, Mucilli F, Filosso PL, Ratto G,
Spaggiari L, et al: Survival analysis in single N2 station lung
adenocarcinoma: The prognostic role of involved lymph nodes and
adjuvant therapy. Cancers (Basel). 13(1326)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Guo ZY, Ren JH, Xu YY, Liu RJ, Tao H,
Huang J and Tan Q: The significance of systematic lymph node
dissection in surgery for early-stage non-small cell lung cancer
patients aged ≤40 years. J Thorac Dis. 13:1196–1204.
2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Osarogiagbon RU, Decker PA, Ballman K,
Wigle D, Allen MS and Darling GE: Survival implications of
variation in the thoroughness of pathologic lymph node examination
in American college of surgeons oncology group Z0030 (Alliance).
Ann Thorac Surg. 102:363–369. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ettinger DS, Wood DE, Aisner D, et al:
NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung
Cancer. Version 5. 2021. Published June 15, 2021. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1450.
Accessed June 16, 2021.
|
|
8
|
Suwatanapongched T and Gierada DS: CT of
thoracic lymph nodes. Part II: Diseases and pitfalls. Br J Radiol.
79:999–1000. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Brown K, Mund DF, Aberle DR, Batra P and
Young DA: Intrathoracic calcifications: Radiographic features and
differential diagnoses. Radiographics. 14:1247–1261.
1994.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mallens WM, Nijhuis-Heddes JM and Bakker
W: Calcified lymph node metastases in bronchioloalveolar carcinoma.
Radiology. 161:103–104. 1986.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Austin JHM, Grimes MM and Carberry D: CT
detection of calcified nodal metastases of lung adenocarcinoma. J
Comput Assist Tomogr. 12:314–316. 1988.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Murai T, Hara M, Ozawa Y, Shibamoto Y,
Shimizu S and Yano M: Mucinous colloid adenocarcinoma of the lung
with lymph node metastasis showing numerous punctate
calcifications. Clin Imaging. 35:151–155. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nakanishi K, Nakagawa K, Asakura K,
Yoshida Y, Watanabe H and Watanabe SI: Is calcification in the
regional lymph nodes a benign feature in patients with lung.
cancer? World J Surg. 43:1850–1856. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Park JS, Kim HK, Choi YS, Kim J, Shim YM
and Kim K: Unplanned conversion to thoracotomy during
video-assisted thoracic surgery lobectomy does not compromise the
surgical outcome. World J Surg. 35:590–595. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Samson P, Guitron J, Reed MF, Hanseman DJ
and Starnes SL: Predictors of conversion to thoracotomy for
video-assisted thoracoscopic lobectomy: A retrospective analysis
and the influence of computed tomography-based calcification
assessment. J Thorac Cardiovasc Surg. 145:1512–1518.
2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jin KN, Moon HJ, Sung YW, Lee Y and Wi JY:
Preoperative computed tomography of the chest in lung cancer
patients: The predictive value of calcified lymph nodes for the
perioperative outcomes of video-assisted thoracoscopic surgery
lobectomy. Eur Radiol. 23:3278–3286. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Byun CS, Lee S, Kim DJ, Lee JG, Lee CY,
Jung I and Chung KY: Analysis of unexpected conversion to
thoracotomy during thoracoscopic lobectomy in lung cancer. Ann
Thorac Surg. 100:968–973. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Goldstraw P, Chansky K, Crowley J,
Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P,
Mitchell A, Bolejack V, et al: The IASLC lung cancer staging
project: Proposals for revision of the TNM stage groupings in the
forthcoming (Eighth) edition of the TNM classification for lung
cancer. J Thorac Oncol. 11:39–51. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ippoliti R: Institutional review board.
In: Encyclopedia of Law and Economics. Backhaus J (ed). Springer,
New York, NY, pp1-4, 2015.
|
|
20
|
Sihoe ADL: The evolution of minimally
invasive thoracic surgery: Implications for the practice of
uniportal thoracoscopic surgery. J Thorac Dis. 6 (Suppl
6):S604–S617. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chen W, Zhang H, Du X, Li T and Zhao Y:
Characteristics and morbidity of the tuberculosis epidemic-China,
2019. China CDC Wkly. 2:181–184. 2020.
|
|
22
|
Cho S, Song IH, Yang HC, Kim K and Jheon
S: Predictive factors for node metastasis in patients with clinical
stage I non-small cell lung cancer. Ann Thorac Surg. 96:239–245.
2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lim CG, Shin KM, Lim JS, Lim JK, Kim HJ,
Kim WH, Cho SH, Cha SI, Lee EB, Seock Y and Jeong SY: Predictors of
conversion to thoracotomy during video-assisted thoracoscopic
surgery lobectomy in lung cancer: Additional predictive value of
FDG-PET/CT in a tuberculosis endemic region. J Thorac Dis.
9:2427–2436. 2017.PubMed/NCBI View Article : Google Scholar
|