Introduction
Although significant advances in chemotherapy for
childhood acute myeloid leukemia (AML) have been made in recent
years, 50-70% of all pediatric patients relapse after achieving the
first complete remission (CR) (1-4).
In addition, the long-term disease-free survival rate is only ~40%
(5). It is generally believed that
hematopoietic stem cell transplantation (HSCT) is currently the
only effective treatment for pediatric relapsed/refractory (R/R)
AML (6). However, whether the
patient has achieved remission prior to the transplantation
procedure markedly affects the efficacy of the treatment and
patient survival after transplantation; therefore, induction
therapy prior to HSCT is very important for pediatric patients with
R/R AML.
The clinical CR rate of treatment with
homoharringtonine (HHT) + cytarabine (Ara-C) + aclarubicin (Acla)
(HAA regimen) in adults with R/R AML has been previously reported
to be as high as 80% (7).
Therefore, the present retrospective study was conducted to
investigate whether treatment with HAA combined with
5-aza-2-deoxycytidine (DAC) can improve the clinical remission rate
in pediatric patients with R/R AML.
Patients and methods
Patients
To retrospectively evaluate the clinical efficacy,
prognosis and safety of DAC combined with HAA in the treatment of
R/R AML in pediatric patients, a total of 53 children with R/R AML
(except M3 type) admitted to the Department of Hematology of Anhui
Provincial Cancer Hospital (Hefei, China) between May 2010 and May
2020 were included in the present study. All patients were
diagnosed based on the morphology, immunophenotype, cytogenetics
and molecular biology classification criteria (8). All parents/legal guardians of the
patients provided written informed consent forms, and the study was
approved by the Ethics Committee of the Anhui Provincial Cancer
Hospital (no. 2021-EXK-02) and complied with the ethical guidelines
outlined in the 1975 Helsinki Declaration. The standard of relapse
was defined as follows: After CR, leukemic cells reappearing in the
peripheral blood, or >5.0% blast cells in the bone marrow (after
excluding other reasons, such as bone marrow regeneration after
consolidation chemotherapy), or extramedullary leukemic cell
infiltration.
Treatment
All patients were treated with DAC-HAA according to
disease conditions and the patients' compliance. The detailed
DAC-HAA regimen was as follows: DAC (20 mg/m2, qd, days
1-3), HHT (2 mg/m2, qd, days 4-10), Ara-C (100
mg/m2, q12h, days 4-10) and Acla (12 mg/m2,
qd, days 4-10).
Statistical analysis
Fisher's exact test was used to detect the factors
that influenced the CR rate. Kaplan Meier curve analysis was used
to detect the factors that influenced the overall survival rate.
The statistical analysis was performed using SPSS software, version
19 (SPSS, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
The characteristics of the patients are summarized
in Table I. A total of 53 patients
were enrolled in the present study, including 23 female and 30 male
patients. The median age of the patients was 7 years (range, 1-14
years). A total of 15 patients were diagnosed with relapsed AML,
and 38 patients were diagnosed with refractory AML. According to
the WHO classification (8), 6
patients had a diagnosis of M1, 23 patients had a diagnosis of M2,
4 patients had a diagnosis of M4, 13 patients had a diagnosis of
M5, 2 patients had a diagnosis of M6, and 5 patients had a
diagnosis of M7. A total of 2 patients had FMS-like tyrosine kinase
3-internal tandem duplication mutations, 16 patients had t(8:21)
mutations, 16 patients had AML1-ETO mutations, and 2 patients had
mixed lineage leukemia-AF9 mutations. Furthermore, 42 of these
patients had undergone HSCT.
 | Table IPatient characteristics (n=53). |
Table I
Patient characteristics (n=53).
| Characteristics | No. |
|---|
| Sex
(male/female) | 30/23 |
| Median age, years
(range) | 7 (1-14) |
| WHO classification
subtype | |
|
M1 | 6 |
|
M2 | 23 |
|
M3 | 0 |
|
M4 | 4 |
|
M5 | 13 |
|
M6 | 2 |
|
M7 | 5 |
| Genetic anomaly at
initial diagnosis | |
|
Normal
karyotype without any molecular abnormality | 10 |
|
FMS-like
tyrosine kinase 3-internal tandem duplication | 2 |
|
t(8:21) | 16 |
|
AML1-ETO | 16 |
|
Mixed
lineage leukemia-AF9 | 2 |
| Disease status | |
|
Relapsed | 15 |
|
Refractory | 38 |
| White blood cell
count, x109/l | |
|
<10 | 13 |
|
≥10 | 40 |
| Platelet count,
x109/l | |
|
≤50 | 31 |
|
>50 | 22 |
| Hemoglobin
concentration, g/l | |
|
≤100 | 33 |
|
>100 | 20 |
| Hematopoietic stem
cell transplantation | |
|
Yes | 42 |
|
No | 11 |
Response and treatment outcome
A total of 41/53 patients (77.4%) achieved CR after
the first course of induction treatment, and PR was observed in 3
patients (5.7%), with an overall response rate of 83.1%. Patients
were divided into different subgroups to analyze the association of
CR with various factors. As shown in Table II, t(8:21) had a slight favorable
effect on CR rate, as patients with t(8:21) had a CR rate of 88 vs.
73% in patients without this mutation. The analysis demonstrated
that age, sex, white blood cell count, platelet count, hemoglobin
concentration and disease status exerted no significant effect on
CR rate. Clinically, the prognosis of patients with refractory AML
is often worse compared with that of patients with recurrent AML
(9). The present study
demonstrated that the CR rate of refractory patients is similar to
that of recurrent patients, indicating that DAC-HAA also has an
excellent therapeutic effect in patients with refractory disease.
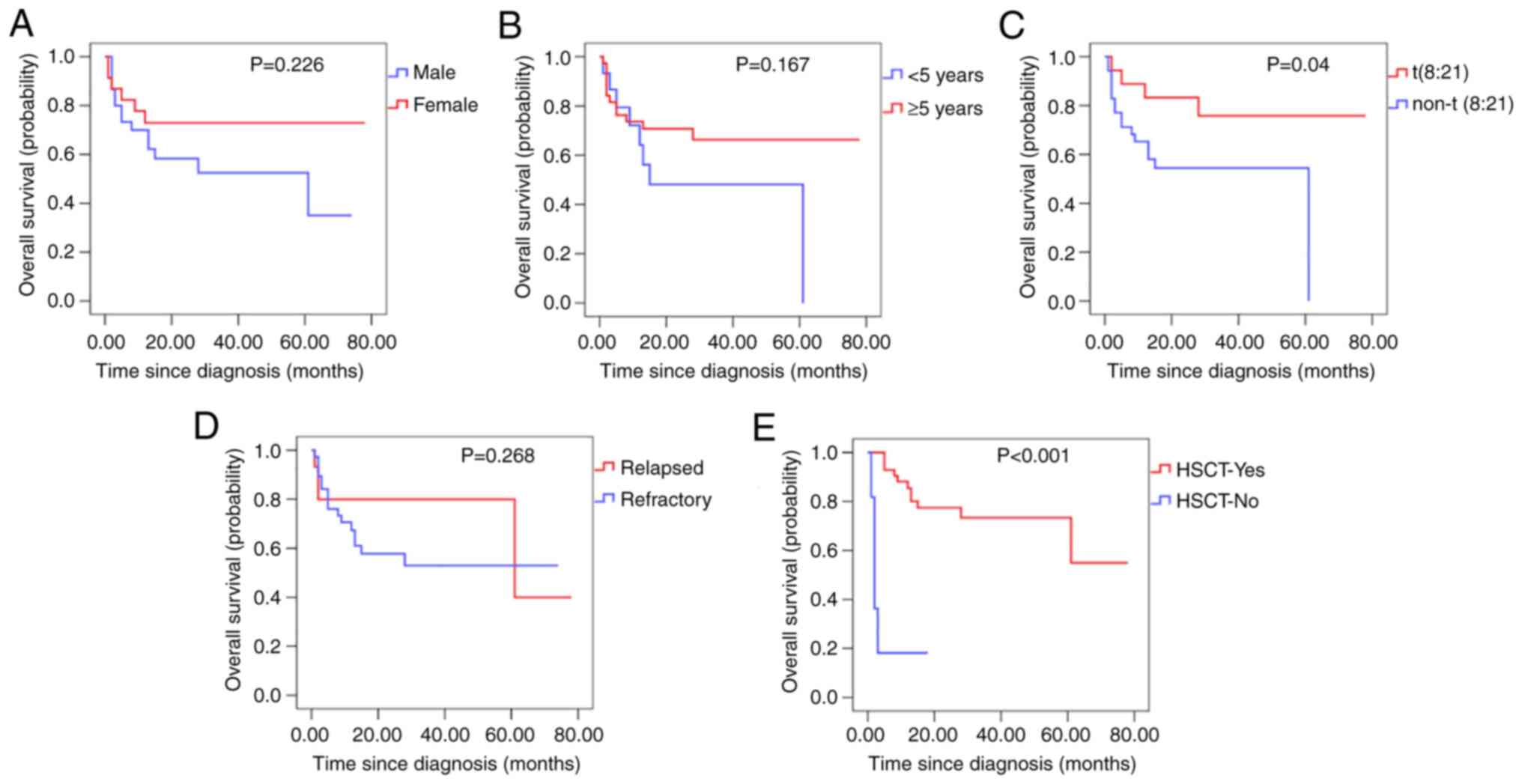
Kaplan-Meier curve analysis of the factors that influenced overall
survival indicated that t(8:21) and HSCT had a positive impact on
survival. On the other hand, sex, age, and disease status did not
significantly affect survival (Fig.
1).
 | Table IICR analysis. |
Table II
CR analysis.
| Characteristics | CR/total, n | CR rate (%) | P-value |
|---|
| Sex | | | 0.323 |
|
Male | 22/30 | 73 | |
|
Female | 19/23 | 83 | |
| Age, years | | | 0.542 |
|
<5 | 12/15 | 80 | |
|
≥5 | 29/38 | 76 | |
| WHO classification
subtype | | | |
|
M1-M4 | 27/33 | 82 | 0.253 |
|
M5-M7 | 14/20 | 70 | |
| Genetic anomaly at
initial diagnosis | | | 0.215 |
|
t(8:21) | 14/16 | 88 | |
|
Normal | 27/37 | 73 | |
| White blood cell
count, x109/l | | | 0.619 |
|
<10 | 10/13 | 77 | |
|
≥10 | 31/40 | 78 | |
| Platelet count,
x109/l | | | 0.362 |
|
≤50 | 25/31 | 81 | |
|
>50 | 16/22 | 73 | |
| Hemoglobin
concentration, g/l | | | 0.499 |
|
≤100 | 25/33 | 76 | |
|
>100 | 16/20 | 80 | |
| Disease status | | | 0.542 |
|
Relapsed | 12/15 | 80 | |
|
Refractory | 29/38 | 76 | |
Toxicity
Bone marrow suppression was evident in all children
after chemotherapy. The mean time between the end of chemotherapy
and the beginning of bone marrow hematopoiesis (neutrophil count
≥0.5x109/l) was 18.8±4.32 days; during the period from
the end of chemotherapy until the neutrophil count reached
≥0.5x109/l, the patients received red blood cell and
platelet transfusions. The ratio of red blood cell and platelet
volume to body weight was 19.02±5.81 ml/kg and 0.156±0.065 U/kg,
respectively.
A total of 11 patients in this study developed grade
4 bone marrow suppression after treatment. The median time for
granulocyte recovery (neutrophil count 0.5x109/l) in 4
patients with CR was 14 days (range, 7-25 days), and the median
time for platelet recovery (platelet count 20x109/l) was
23 days (range, 7-37 days). Fever occurred in 11 patients during
granulocytopenia, including pulmonary infection in 5 patients,
septicemia in 4 patients, skin and soft tissue infection in 1
patient, herpes zoster infection in 1 patient and acute otitis
media in 1 patient. In 1 patient, pulmonary infection combined with
alveolar hemorrhage resulted in a fatal outcome following failure
of anti-infection treatment, while the symptoms in the remaining
patients disappeared after successful anti-infection treatment.
Idarubicin (IDA) combined with high-dose Ara-C (IA)
and HHT are known to be associated with cardiotoxicity (10). Therefore, all patients in the
present study also received cardioprotective treatment with vitamin
C and coenzyme Q10 at the same time as chemotherapy. No obvious
cardiotoxicity was found during or after chemotherapy, and no
obvious abnormalities were observed in the electrocardiogram. A
total of 66.0% of the patients developed infections of different
severity, mainly manifesting as pneumonia (80%), of which 67.9% of
the cases were caused by bacteria, 17.8% were caused by fungi and
14.3% had a mixed etiology.
Other non-hematological adverse reactions included
nausea (n=10; 19%), vomiting (n=14; 26%), diarrhea (n=7; 13%),
mucositis (n=4; 7%) and constipation (n=14; 26%), which improved
with symptomatic treatment. A total of 4 patients developed
reversible liver function abnormalities during chemotherapy, and no
patient developed kidney damage.
Discussion
Children with R/R AML have poor outcome and
unfavorable response to chemotherapy, whereas the currently
accepted radical therapy is HSCT (11). Whether the proportion of blasts in
the bone marrow of the patient is <5% prior to transplantation
plays a key role in the efficacy of transplantation and long-term
survival; however, most conventional chemotherapy regimens cannot
achieve bone marrow remission in children with R/R AML. Effective
chemotherapy regimens mainly include the following: High-dose
Ara-C, mitoxantrone + etoposide + Ara-C (MEC), fludarabine + Ara-C
+ granulocyte colony-stimulating factor (G-CSF) + IDA (FLAG-IDA)
and Ara-C + aclarubicin + G-CSF (CAG), among others (12-14).
The response rate with high-dose Ara-C chemotherapy is ~20% in R/R
AML (15). The CR rate with
FLAG-Ida is 52.1% (16). MEC is
also occasionally used as the primary regimen in patients with
R/R-AML, with CR rates of 18-66% (17).
In China, the treatment of children with R/R AML
mostly includes IA, CAG or FLAG. Daunorubicin is an antitumor drug,
which can inhibit the synthesis of RNA and DNA, has a wide
antitumor spectrum and is mainly suitable for AML (18). The methoxy group is removed from
the C4 position of the daunorubicin glycosidic group to form IDA.
The structural change increases the lipophilicity of IDA, making it
easier for the drug to penetrate cell membranes. In cells, IDA is
metabolized to alcohol 4-demethoxydaunorubicin (IDAol). Compared
with IDA, IDAol has the same antitumor activity with a markedly
longer clearance time from the body, and it can penetrate through
the blood-brain barrier and placenta. Compared to other
anthracycline drugs, IDA has higher antitumor activity and can
effectively reduce tumor recurrence rates (19-21).
The application of IA chemotherapy in children with R/R AML is
associated with a higher rate of bone marrow remission (22). However, when our team applied the
IA regimen in the clinical setting, the total remission rate was
found to be suboptimal, with a long bone marrow suppression period,
high infection rate (100%) and high infection-related mortality
rate.
In recent years, it has been found that abnormal DNA
methylation plays an important role in the occurrence and
development of AML. Demethylating agents, such as DAC and
azacitidine, have been used in the treatment of adult R/R AML with
good clinical efficacy (23-26).
DAC is a highly effective inhibitor of DNA methyltransferase
interfering with DNA methylation, which can reverse the DNA
methylation process and activate silent tumor suppressor genes to
inhibit the proliferation of tumor cells (27). Qin et al (28) found that, when combined with Ara-C,
DAC can enhance its cytotoxicity. HHT is an alkaloid antitumor drug
extracted from the Cephalotaxus plant (29), and is able to inhibit the synthesis
of DNA and protein in tumor cells with no cross-resistance with
Ara-C. In addition, Zhou et al (30) reported that HHT and Ara-C also
exert a synergistic effect; in particular, HHT combined with Acla
and Ara-C (DAC-HAA) was able to achieve a high remission rate in
adult R/R AML in previous reports (31,32).
To the best of our knowledge, there are yet no
reports of this method applied as clinical treatment of children
with R/R AML. The present study was undertaken to explore the
efficacy, safety and prognostic factors of DAC-HAA treatment in
Chinese pediatric patients with R/R AML. The results demonstrated
that DAC-HAA achieved higher bone marrow remission rate, shorter
bone marrow suppression period and higher disease-free survival
rate, compared with DAC or HAA alone.
However, due to the small number of patients in the
present study, and due to the fact that there were only 3 cases of
patients with partial remission, it is impossible to systematically
analyze the factors that may affect partial remission, which is a
limitation of the present study. We hope that there will be a
larger sample of clinical studies in the future, which enable the
evaluation of the clinical efficacy of DAC-HAA in a more systematic
and comprehensive manner.
In summary, the present results suggest that the
DAC-HAA chemotherapy regimen is associated with a high bone marrow
remission rate and a good safety profile in the treatment of
pediatric patients with R/R AML, it may represent a good bridging
treatment for HSCT, and is worthy of clinical consideration.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Wu Jieping Medical
Foundation (grant no. 320.6750.18391).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
Funding acquisition: LW; investigation: LW;
resources: LW, BZ, CLiao; data curation: LW, FX, FC, SW, LC, NL;
formal analysis: CLi; methodology: HL, CLi; project administration:
HL, CLi; writing-review and editing: JW; supervision: HL. HL and
CLi confirm the authenticity of the raw data. All the authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
All parents/legal guardians of the patients provided
written informed consent forms, and the study was approved by the
Ethics Committee of the Anhui Provincial Cancer Hospital (no.
2021-EXK-02) and complied with the ethical guidelines of the 1975
Helsinki Declaration.
Patient consent for publication
Not applicable.
Competing interests
The authors that they have no competing
interests.
References
|
1
|
Radhakrishnan V, Thampy C, Ganesan P,
Rajendranath R, Ganesan TS, Rajalekshmy KR and Sagar TG: Acute
myeloid leukemia in children: Experience from Tertiary cancer
Centre in India. Indian J Hematol Blood Transfus. 32:257–261.
2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kulkarni KP and Marwaha RK: Childhood
acute myeloid leukemia: An Indian perspective. Pediatr Hematol
Oncol. 28:257–268. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kavcic M, Fisher BT, Li Y, Seif AE, Torp
K, Walker DM, Huang YS, Lee GE, Tasian SK, Vujkovic M, et al:
Induction mortality and resource utilization in children treated
for acute myeloid leukemia at free-standing pediatric hospitals in
the United States. Cancer. 119:1916–1923. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Taga T, Tomizawa D, Takahashi H and Adachi
S: Acute myeloid leukemia in children: Current status and future
directions. Pediatr Int. 58:71–80. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tomizawa D, Tabuchi K, Kinoshita A, Hanada
R, Kigasawa H, Tsukimoto I and Tsuchida M: Tokyo Children's Cancer
Study Group. Repetitive cycles of high-dose cytarabine are
effective for childhood acute myeloid leukemia: Long-term outcome
of the children with AML treated on two consecutive trials of Tokyo
Children's Cancer Study Group. Pediatr Blood Cancer. 49:127–132.
2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Okamoto Y, Kudo K, Tabuchi K, Tomizawa D,
Taga T, Goto H, Yabe H, Nakazawa Y, Koh K, Ikegame K, et al:
Hematopoietic stem-cell transplantation in children with refractory
acute myeloid leukemia. Bone Marrow Transplant. 54:1489–1498.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bose P, Vachhani P and Cortes JE:
Treatment of Relapsed/Refractory acute myeloid leukemia. Curr Treat
Options Oncol. 18(17)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Vardiman JW, Harris NL and Brunning RD:
The World Health Organization (WHO) classification of the myeloid
neoplasms. Blood. 100:2292–2302. 2002.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Thol F, Schlenk RF, Heuser M and Ganser A:
How I treat refractory and early relapsed acute myeloid leukemia.
Blood. 126:319–327. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bezwoda WR and Dansey RD: Idarubicin plus
Cytarabine versus doxorubicin plus cytarabine in induction therapy
for acute non-lymphoid leukaemia: A randomized trial. Leuk
Lymphoma. 1:221–225. 1990.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sander A, Zimmermann M, Dworzak M,
Fleischhack G, von Neuhoff C, Reinhardt D, Kaspers GJ and Creutzig
U: Consequent and intensified relapse therapy improved survival in
pediatric AML: Results of relapse treatment in 379 patients of
three consecutive AML-BFM trials. Leukemia. 24:1422–1428.
2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chen F, Wang J, Hou M, Zhao H, Yang E, Ran
X, Wang M, Yu W, Xu R, Wang Z, et al: Prospective multicentre study
of chemotherapeutic regimen containing pirarubicin on the treatment
of relapsed or refractory acute myeloid leukemia in adults.
Zhonghua Xue Ye Xue Za Zhi. 35:388–392. 2014.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
13
|
Liedtke M, Dunn T, Dinner S, Coutré SE,
Berube C, Gotlib J, Patel S and Medeiros B: Salvage therapy with
mitoxantrone, etoposide and cytarabine in relapsed or refractory
acute lymphoblastic leukemia. Leuk Res. 38:1441–1445.
2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jackson G, Taylor P, Smith GM, Marcus R,
Smith A, Chu P, Littlewood TJ, Duncombe A, Hutchinson M, Mehta AB,
et al: Amulticentre, open, Non comparative phase II study of a
combination of fludarabine phosphate, cytarabine and granulocyte
colony-stimulating factor in relapsed and refractory acute myeloid
leukemia and de novo refractory anemia with excess of blasts in
transformation. Br J Hematol. 112:127–137. 2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zeidner JF, Vincent BG, Esparza S, Ivanova
A, Moore DT, Foster MC, Coombs CC, Jamieson K, Van Deventer HW,
Blanchard L, et al: Final clinical results of a phase II study of
high dose cytarabine followed by pembrolizumab in
relapsed/refractory AML. Blood. 134 (Suppl 1)(S831)2019.
|
|
16
|
Pastore D, Specchia G, Carluccio P, Liso
A, Mestice A, Rizzi R, Greco G, Buquicchio C and Liso V: FLAG-IDA
in the treatment of refractory/relapsed acute myeloid leukemia:
Single-center experience. Ann Hematol. 82:231–235. 2003.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ruan M, Liu LP, Zhang AL, Quan Qi B, Liu
F, Liu TF, Liu XM, Chen XJ, Yang WY, Guo Y, et al: Improved outcome
of children with relapsed/refractory acute myeloid leukemia by
addition of cladribine to re-induction chemotherapy. Cancer Med.
10:956–964. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Luskin MR, Lee JW, Fernandez HF,
Abdel-Wahab O, Bennett JM, Ketterling RP, Lazarus HM, Levine RL,
Litzow MR, Paietta EM, et al: Benefit of high-dose daunorubicin in
AML induction extends across cytogenetic and molecular groups.
Blood. 127:1551–1558. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bozoglan H, Ergene U and Yoleri L: Use of
cytarabine and idarubicin in a newly diagnosed AML patient with a
severe wound. Transfus Apher Sci. 45:17–20. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ziogas DC, Voulgarelis M and Zintzaras E:
A Network Meta-analysis of randomized controlled trials of
induction treatments in acute myeloid leukemia in the elderly. Clin
Ther. 33:254–279. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mandelli F, Vignetti M, Suciu S, Stasi R,
Petti MC, Meloni G, Muus P, Marmont F, Marie JP, Labar B, et al:
Daunorubicin versus mitoxantrone versus idarubicin as induction and
consolidation chemotherapy for adults with acute myeloid leukemia:
The EORTC and GIMEMA Groups Study AML-10. J Clin Oncol.
27:5397–5403. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Murphy T and Yee KWL: Cytarabine and
daunorubicin for the treatment of acute myeloid leukemia. Expert
Opin Pharmacother. 18:1765–1780. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Fenaux P, Mufti GJ, Hellström-Lindberg E,
Santini V, Gattermann N, Germing U, Sanz G, List AF, Gore S,
Seymour JF, et al: Azacitidine prolongs overall survival compared
with conventional care regimens in elderly patients with low bone
marrow blast count acute myeloid leukemia. J Clin Oncol.
28:562–569. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lübbert M, Rüter BH, Claus R, Schmoor C,
Schmid M, Germing U, Kuendgen A, Rethwisch V, Ganser A, Platzbecker
U, et al: A multicenter phase II trial of decitabine as first-line
treatment for older patients with acute myeloid leukemia judged
unfit for induction chemotherapy. Hematologica. 97:393–401.
2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ritchie EK, Feldman EJ, Christos PJ, Rohan
SD, Lagassa CB, Ippoliti C, Scandura JM, Carlson K and Roboz GJ:
Decitabine in patients with newly diagnosed and relapsed acute
myeloid leukemia. Leuk Lymphoma. 54:2003–2007. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tawfik B, Sliesoraitis S, Lyerly S, Klepin
HD, Lawrence J, Isom S, Ellis LR, Manuel M, Dralle S, Berenzon D,
et al: Efficacy of the hypomethylating agents as frontline,
salvage, or consolidation therapy in adults with acute myeloid
leukemia (AML). Ann Hematol. 93:47–55. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Herman JG and BayIin SB: Gene silencing in
cancer in association with promoter hyper methylation. N Engl J
Med. 349:2042–2054. 2003.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Qin T, Youssef EM, Jelinek J, Chen R, Yang
AS, Garcia-Manero G and Issa JP: Effect of cytarabine and
decitabine in combination in human leukemic cell lines. Clin Cancer
Res. 13:4225–4232. 2007.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Huang MT: Harringtonine, an inhibitor of
initiation of protein biosynthesis. Mol Pharmacol. 11:511–519.
1975.PubMed/NCBI
|
|
30
|
Zhou DC, Zittoun R and Marie JP:
Homoharringtonine: An effective new natural product in cancer
chemotherapy. Bull Cancer. 82:987–995. 1995.PubMed/NCBI
|
|
31
|
Bose P, Verstovsek S, Gasior Y, Jain N,
Jabbour EJ, Estrov Z, Alvarado Y, DiNardo CD, Pemmaraju N, Kornblau
SM, et al: Phase I/II study of Ruxolitinib (RUX) with decitabine
(DAC) in patients with post-myeloproliferative neoplasm acute
myeloid leukemia (post-MPN AML): Phase I results. Blood.
128(4262)2016.
|
|
32
|
Zhu HH, Jiang H, Jiang Q, Jia JS, Qin YZ
and Huang XJ: Homoharringtonine, aclarubicin and cytarabine (HAA)
regimen as the first course of induction therapy is highly
effective for acute myeloid leukemia with t(8;21). Leuk Res.
44:40–44. 2016.PubMed/NCBI View Article : Google Scholar
|