Introduction
Hepatocellular carcinoma (HCC) is the most common
primary malignancy of the liver and is among the leading causes of
cancer-related mortality worldwide (1). Most patients with HCC are diagnosed
at an advanced stage, resulting in a poor prognosis (2). Recently, several types of molecular
targeted agents (MTAs) have been approved for the treatment of
unresectable HCC (3,4). Lenvatinib (LEN) is an MTA approved as
a first-line treatment; it targets vascular endothelial growth
factor receptors (VEGFRs) 1-3, fibroblast growth factor receptors
(FGFRs) 1-4, platelet-derived growth factor receptor-α, RET and KIT
(5). LEN exerts strong antitumour
effects by inhibiting both VEGFR- and FGFR-induced angiogenesis, as
well as FGFR- and RET-induced abnormal cancer cell proliferation
(6).
Several studies have reported therapeutic
effect-associated imaging findings of LEN (7-10).
As early imaging biomarkers, a reduction in tumour enhancement
intensity in the arterial phase on contrast-enhanced (CE)-CT at 2
weeks and a decrease in the time-intensity curve in the arterial
phase on contrast-enhanced ultrasound at 1 week are useful
predictors of LEN effectiveness (7,8). On
pretreatment imaging, the heterogeneous enhancement pattern of HCC
in the arterial phase on CE-CT and
18F-fluorodeoxyglucose (FDG) uptake on
18F-FDG-positron emission tomography (PET)/CT for HCC
may be useful predictors of early response to LEN (9,10).
MTAs exert antitumour effects mainly by inhibiting
angiogenesis. Imaging of angiogenesis is best performed using CE-CT
and MRI. The enhancement pattern of HCC depends on the changes in
the vasculature that occur during tumour angiogenesis (3). Clinically, however, it is unclear
whether strong tumour staining is associated with a high
therapeutic efficacy of MTAs, and vice versa.
To the best of our knowledge, there have been no
reports to date focusing on the association between the degree of
contrast enhancement on pretreatment imaging and LEN effectiveness
in HCC. The aim of the present study was to retrospectively
investigate the association between the precise degree of contrast
enhancement on pretreatment CE-CT and the therapeutic efficacy of
LEN in patients with HCC.
Materials and methods
Patients
Between March 2018 and December 2020, 114
consecutive patients with HCC who received LEN at Kurume University
Hospital (Kurume, Japan) were enrolled in the present study. The
study was conducted in accordance with the principles outlined in
the Declaration of Helsinki, and the protocol was approved by the
Ethics Review Committee of Kurume University (approval no. 20192).
An opt-out approach was used to obtain informed consent from the
patients, and personal information was protected during data
collection.
The inclusion criteria for this study were as
follows: i) Patients with intrahepatic tumours; ii) patients taking
LEN for >30 days; and iii) patients who underwent CE-CT at the
scheduled time prior to LEN administration. Of the 114 patients, 67
met the inclusion criteria and were subjected to further
radioclinical analysis.
Administration of LEN
LEN (Eisai Co., Ltd.) was orally administered at a
dose of 12 mg for a body weight of ≥60 kg and at 8 mg for a body
weight of <60 kg, once per day. Adverse events (AEs) were
assessed using the National Cancer Institute's Common Terminology
Criteria for Adverse Events, version 4.0 (https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm).
For patients with AEs of grade ≥3, either LEN dose reduction or
discontinuation was allowed. If required, the dose was reduced from
12 to 8 mg, or from 8 to 4 mg. In addition, alternate-day
administration or 5 days-on/2 days-off (weekends-off) were also
performed according to the patient's condition. The relative dose
intensity (RDI) was defined as the actual dose divided by the
standard dose. The 8-week RDI (8W-RDI) was calculated as the
cumulative dose within the initial 8 weeks of starting LEN
treatment divided by the standard dose (11).
Evaluation of therapeutic response and
follow-up schedule
Therapeutic response was evaluated using dynamic CT
or MRI at 4-6 weeks after the initiation of LEN according to the
Modified Response Evaluation Criteria in Solid Tumours (mRECIST)
(12), and at intervals of 2-3
months thereafter, until patient death or study completion.
CT protocol and image analysis
CT examinations were performed using multi-detector
raw CT scanners: Discovery, GE Healthcare (n=21); Revolution, GE
Healthcare (n=20); iCT, Philips Healthcare (n=18); Aquilion, Canon
Medical Systems Corporation (n=4); and SOMATOM, Siemens
Healthineers (n=4). Multiphasic dynamic CT was performed with a
delay of 30-40 sec (arterial phase), 60-70 sec (portal phase) and
150-180 sec (equilibrium phase) with intravenous administration of
non-ionic contrast material at a rate of 3-4 ml/sec using an
automated power injector. The dose of contrast medium was adjusted
according to the renal function of each patient.
If patients had multiple HCC nodules, the largest
representative nodule was examined per patient. On CE-CT, the CT
value was measured using a region of interest (ROI) in the arterial
phase with a maximum round or oval area set within the tumour in
the slice where the tumour was best visualised (almost equal to the
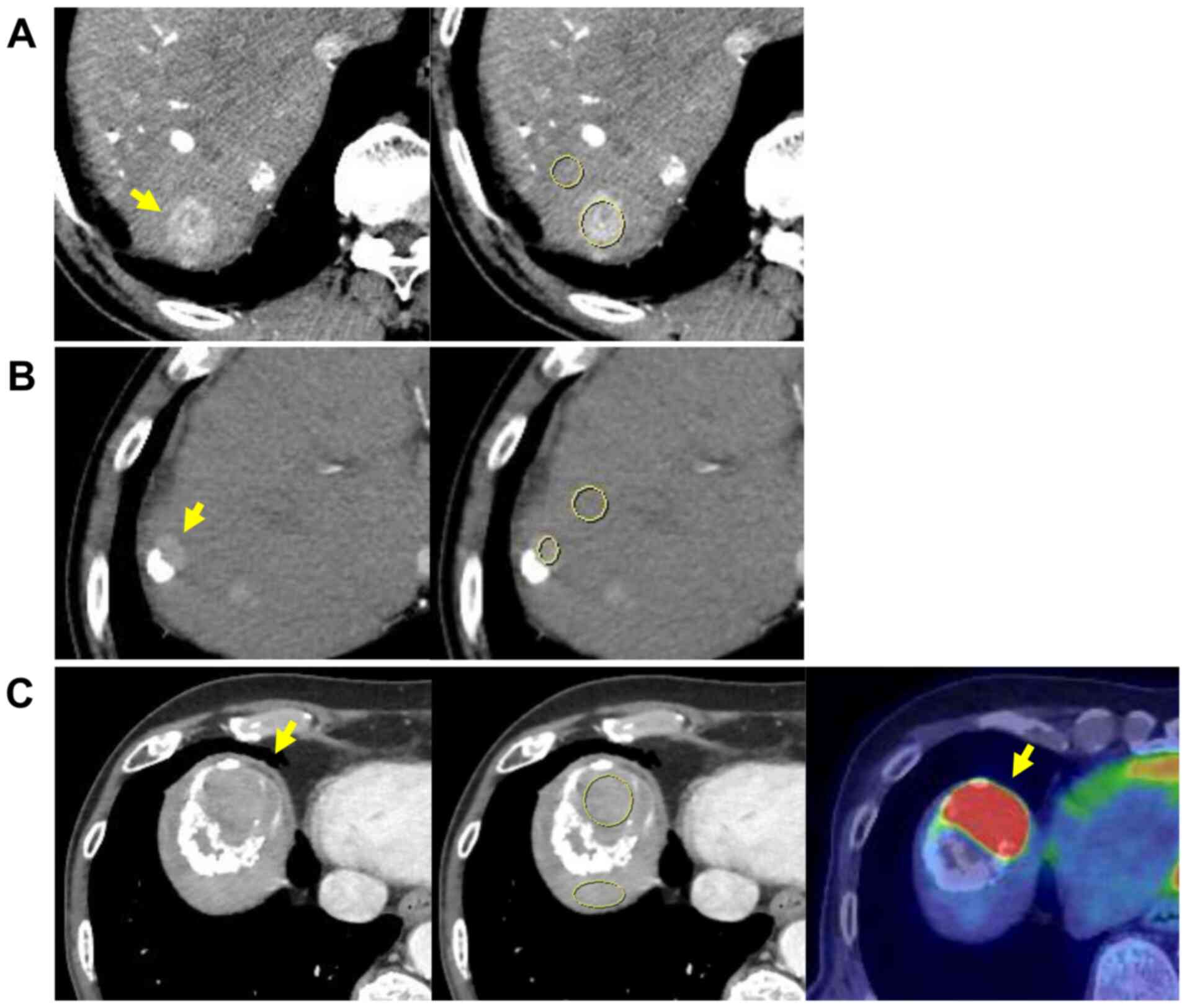
slice including the largest diameter of the tumour) using Picture
Archiving and Communication System (Fig. 1A). If the lipiodol was partially
deposited in the tumour to be evaluated, the ROI was carefully
defined to avoid the lipiodol deposition area based on plain
(non-CE) CT (Fig. 1B).
Furthermore, if the main tumour exhibited a poor contrast
enhancement, we referred to 18F-FDG-PET/CT to confirm
FDG uptake (Fig. 1C) or previous
CT or MRI scans to confirm the increase in size and to distinguish
necrotic tissue from viable lesions. The CE-CT imaging protocols
were uniform across all cases. However, the dose of contrast medium
was reduced in some cases due to renal dysfunction. This could have
influenced the contrast enhancement and, thus, the absolute CT
value of the nodule in the arterial phase could not be used.
Therefore, the background liver was set as the object. The ROI in
the liver parenchyma was placed near the nodule to be evaluated in
the same slice with a size of at least 100 mm2,
excluding major vessels and artifacts. The enhancement ratio (ER)
was calculated as follows: ER = CT value of ROI in the tumour/CT
value of ROI in the liver parenchyma. Furthermore, the ER values
were divided into three groups: ER <1.0 (low contrast
enhancement compared with the liver parenchyma), 1.0≤ ER <1.5
(moderate contrast enhancement compared with the liver parenchyma)
and ER ≥1.5 (high contrast enhancement compared with the liver
parenchyma).
Alternating LEN and transarterial
therapy (AT)
In addition, as will be described later, AT has been
employed for cases with progressive disease (PD) at our facility.
Details of AT are provided below.
AT involves the administration of treatment using a
transarterial approach, such as transcatheter arterial
chemoembolization (TACE) and hepatic arterial infusion chemotherapy
(HAIC), upon the development of PD, as indicated by the
reappearance of contrast enhancement in the tumour or appearance of
new lesions in the liver during LEN treatment.
The details of AT administration were as follows: i)
LEN was discontinued 2 days prior to transarterial therapy; ii)
TACE was performed, except in cases exhibiting multinodular or
invasive growth, in which HAIC was performed instead; iii) within 2
weeks of transarterial therapy, depending on the condition of each
patient, LEN administration was resumed at the same or half the
dose as that administered prior to transarterial therapy (13).
TACE and HAIC protocol
Angiography was performed for the celiac and the
common hepatic arteries using a 3- or 4-Fr catheter, and digital
subtraction angiography was performed using a non-ionic iodine
contrast agent. The tumour-containing segment was evaluated by
imaging techniques including cone-beam CT. Subsequently, a 1.7- or
1.9-Fr microcatheter (Piolax, Inc.) was inserted into the
subsegmental artery with the adapted microwire (Piolax, Inc.). The
catheter was advanced towards the tumour-feeding artery. Depending
on the size and number of tumours, conventional TACE (C-TACE) was
performed using 20-50 mg of epirubicin (Nippon Kayaku Co., Ltd.) or
cisplatin (Nippon Kayaku Co., Ltd.) with lipiodol (Guerbet Co.,
Ltd.), and was absorbed by gelatin sponge particles (Nippon Kayaku
Co., Ltd.) (13,14).
HAIC was conducted via the insertion of an implanted
catheter (Piolax, Inc.) An indwelling catheter (5-Fr W-Spiral
catheter; Piolax, Inc.) was inserted through the right femoral
artery, with the distal end of the catheter extending into the
hepatic or gastroduodenal artery, and the proximal end connected to
the port system (Soph-A-Port®; Sophysa). Following the
inpatient regimen of HAIC, 50 mg of fine-powder cisplatin was
suspended in 5-10 ml of lipiodol; the suspension volume was
determined according to the tumour size [the volume (in ml) was 1-2
less than the maximum diameter (in cm)]. On day 1, the
cisplatin-lipiodol suspension was injected through the implanted
angiography catheter, followed by the injection of 250 mg of
5-fluorouracil (5-FU). Then, 1,250 mg of 5-FU was continuously
infused for 5 days using an infusion balloon pump (Surefuser™+,
Nipro Pharma Corporation). This regimen was administered once per
week during the first 2 weeks of admission, and then a combination
of 20 mg cisplatin with lipiodol and 5-FU (500-1,250 mg) was
infused every 2 weeks at the outpatient department until disease
progression (13,15).
Statistical analysis
Continuous variables are expressed as median
(range). The χ2 test or Fisher's exact test were used to
analyse the association between categorical variables, and
Wilcoxon's test was used to analyse the association between
continuous variables. Progression-free survival (PFS) and overall
survival (OS) were calculated from the date of initiation of LEN
administration to tumour progression and death, respectively. A Cox
proportional hazard model was used for univariate and multivariate
analyses to identify any independent variables associated with PFS
and OS. The PFS and OS rates were evaluated using the Kaplan-Meier
method, and the log-rank test was used to compare the patient
groups. P<0.05 was considered to indicate statistically
significant differences. JMP software (version 15; SAS Institute,
Inc.) was used for all statistical analyses.
Results
Patient and tumour
characteristics
The patient and tumour characteristics are
summarised in Table I. The
majority of the patients had Child-Pugh class A liver cirrhosis,
with the exception of 2 patients with class B cirrhosis.
Albumin-bilirubin (ALBI) grade 1 and 2 were observed in 30 and 37
patients, respectively. A total of 2 patients had stage A tumours,
43 had stage B tumours and 22 had stage C tumours as per the
Barcelona Clinic Liver Cancer (BCLC) staging system (16). Only 1 patient had macrovascular
invasion; 21 of the 22 patients with BCLC stage C HCC had
extrahepatic metastasis. There were 25 patients within up-to-seven
criteria [defined as HCC with 7 as the sum of the size of the
largest tumour (in cm) and the number of intrahepatic tumours] and
42 beyond this criteria.
 | Table IPatient and tumour
characteristics. |
Table I
Patient and tumour
characteristics.
|
Characteristics | n=67 |
|---|
| Age (years) | 73 (47-90) |
| Sex
(male/female) | 54/13 |
| Aetiology
(HBV/HCV/others) | 9/30/28 |
| Albumin (g/dl) | 3.9 (2.9-4.7) |
| Total bilirubin
(mg/dl) | 0.7 (0.4-1.9) |
| Prothrombin
activity (%) | 98 (56-130) |
| Child-Pugh score
(A/B/C) | 65/2/0 |
| ALBI grade
(1/2/3) | 30/37/0 |
| Tumour number
(<5/5-10/≥10) | 30/18/19 |
| Tumour size
(mm) | 25 (10-170) |
| Macrovascular
invasion (present/absent) | 1/66 |
| Extrahepatic
metastasis (present/absent) | 21/46 |
| BCLC stage
(A/B/C) | 2/43/22 |
| Up-to-seven
criteria (within/beyond) | 25/42 |
| AFP (ng/ml) | 25.5
(1.4-118,560) |
| DCP (mAU/ml) | 396
(12-179,531) |
| TACE refractoriness
(yes/no) | 51/16 |
| ER | 1.28
(0.01-2.30) |
| ER <1.0/1.0≤ ER
<1.5/ER ≥1.5 | 20/27/20 |
| Initial dose of
lenvatinib (8/12 mg) | 46/21 |
| 8W-RDI (%) | 75 (15-100) |
Among the 67 patients, 51 had HCC that was
refractory to TACE. C-TACE was performed in 46 patients and
drug-eluting beads TACE (DEB-TACE) was performed in 5 patients as
pretreatment of LEN. Lesions treated with DEB-TACE may display
reduced enhancement and lower ER. However, since the main nodules
to be evaluated in these cases were new lesions appearing after
DEB-TACE (n=1), or previously existing lesions increasing in size
with no therapeutic efficacy (n=2), or lesions displaying tumour
angiogenesis resumption and increase in size after DEB-TACE (n=2),
it was hypothesized that DEB-TACE did not affect the evaluation of
the contrast enhancement in this study. Therefore, these cases were
included in the present analysis. The initial dose of LEN was 8 mg
for 46 patients and 12 mg for 21 patients. The RDI at 8 weeks was
75% (range, 15-100%). By the time LEN was discontinued, dose
reduction had been performed in 50 (74.6%) patients. The main
reasons for dose reduction were fatigue (n=14), decreased appetite
(n=10), proteinuria (n=4), ascites (n=4), palmar-plantar
erythrodysaesthesia syndrome (n=3), diarrhoea (n=3), elevated
aspartate aminotransferase levels (n=2), high ammonia levels (n=2),
hypertension (n=1), hoarseness (n=1), neutropenia (n=1), increased
blood bilirubin levels (n=1), hyperthyroidism (n=1), vomiting
(n=1), headache (n=1) and gingival bleeding (n=1). The contrast
medium dose was reduced in 24 patients (35.8%) due to renal
dysfunction. The CT values in the ROI of the tumour and liver
parenchyma were 100.56 Hounsfield units (HU) (range, 0.48-169.86
HU) and 80.79 HU (range, 54.79-113.41 HU), respectively. The areas
of the ROI in the tumour and liver parenchyma were 176.98
mm2 (range, 25.51-5332.46 mm2) and 256.68
mm2 (range, 112.55-947.37 mm2), respectively.
The median ER was 1.28 (range, 0.01-2.3).
Evaluation using mRECIST after the
initial treatment with LEN
Complete response, partial response, stable disease
and PD were observed in 10.4% (7/67), 46.3% (31/67), 32.8% (22/67)
and 10.4% (7/67) of the patients, respectively. The overall
objective response rate (ORR) and disease control rate (DCR) were
56.7% (38/67) and 89.6% (60/67), respectively.
Comparison of PFS and OS among the ER
groups
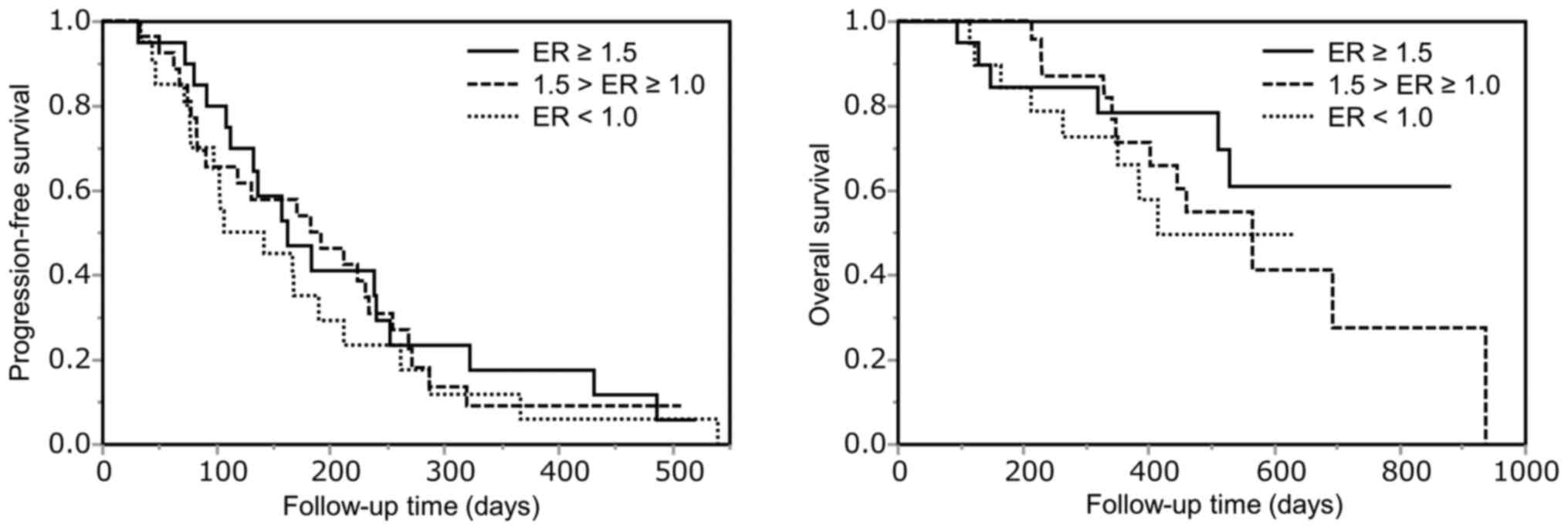
The PFS and OS curves stratified by ER in the
arterial phase on CE-CT are shown in Fig. 2. The ER ≥1.5, 1.0≤ ER <1.5 and
ER <1.0 groups comprised 20, 27 and 20 patients, respectively.
There was no significant difference among the ER groups (PFS,
P=0.63; OS, P=0.455).
Comparison of patient and tumour
characteristics between the ER ≥1.0 and <1.0 groups
Next, we focused on comparing baseline patient and
tumour characteristics between the ER ≥1.0 and ER <1.0 groups,
based on the high or low contrast enhancement compared with that of
the liver parenchyma. A comparison of patient and tumour
characteristics between the two groups is shown in Table II. The ER <1.0 group comprised
significantly more cases with larger tumour diameters (P<0.001),
BCLC stage C with extrahepatic metastases (P=0.007) and higher
des-γ-carboxy prothrombin (DCP) values (P=0.046) compared with the
ER ≥1.0 group, suggesting that ER <1.0 was characteristic of
aggressive types of HCC. There was no significant difference in the
ORR and DCR between the ER groups (ORR, P=0.072; DCR, P=0.938).
 | Table IIComparisons of patient and tumour
characteristics based on the ER. |
Table II
Comparisons of patient and tumour
characteristics based on the ER.
|
Characteristics | ER ≥1.0 | ER <1.0 | P-value |
|---|
| Age (years) | 76 (54-90) | 73 (47-88) | 0.344 |
| Sex
(male/female) | 37/10 | 16/4 | 0.906 |
| Aetiology
(HBV/HCV/others) | 5/23/19 | 4/7/9 | 0.451 |
| Albumin (g/dl) | 3.8 (2.9-4.7) | 4.0 (3.1-4.4) | 0.287 |
| Total bilirubin
(mg/dl) | 0.73 (0.4-1.9) | 0.70 (0.5-1.3) | 0.967 |
| Prothrombin
activity (%) | 96 (56-124) | 103 (76-130) | 0.415 |
| Child-Pugh score
(A/B/C) | 45/2/0 | 20/0/0 | 0.349 |
| ALBI grade
(1/2/3) | 18/29/0 | 12/8/0 | 0.102 |
| Tumour number
(<5/5-10/≥10) | 18/15/14 | 12/3/5 | 0.214 |
| Tumour size
(mm) | 22 (10-170) | 42 (17-122) | <0.001 |
| Macrovascular
invasion (present/absent) | 1/46 | 0/20 | 0.511 |
| Extrahepatic
metastasis (present/absent) | 9/38 | 12/8 | 0.001 |
| BCLC stage
(A/B/C) | 2/35/10 | 0/8/12 | 0.007 |
| Up-to-seven
criteria (within/beyond) | 17/30 | 8/12 | 0.767 |
| AFP (ng/ml) | 26.1
(1.6-40804) | 25.0
(1.4-118560) | 0.827 |
| DCP (mAU/ml) | 232 (12-15120) | 1408
(22-179531) | 0.046 |
| TACE refractoriness
(yes/no) | 40/7 | 11/9 | 0.008 |
| ER | 1.45
(1.01-2.30) | 0.84
(0.01-0.98) | <0.001 |
| Initial dose of
lenvatinib (8/12 mg) | 34/13 | 12/8 | 0.319 |
| 8W-RDI
(≥75%/<75%) | 24/23 | 10/10 | 0.937 |
| Objective response
rate | 30 (63.8%) | 8 (40%) | 0.072 |
| Disease control
rate | 42 (89.3%) | 18 (90%) | 0.938 |
Factors associated with PFS and
OS
Independent predictors of PFS (Table III) and OS (Table IV) were investigated using Cox
proportional hazard analysis in all patients. The results of the
univariate analysis showed that tumour size (≥30 mm; P=0.02),
α-foetoprotein (AFP) (≥100 ng/ml; P=0.04) and DCP (≥200 mAU/ml;
P=0.03) were significant risk factors for PFS. The multivariate
analysis revealed that tumour size (≥30 mm; HR=2.14; 95% CI:
1.18-3.85; P=0.012) and AFP (≥100 ng/ml; HR=1.98; 95% CI:
1.08-3.63; P=0.026) were independent predictors of shorter PFS. The
results of the univariate analysis showed that BCLC stage C
(P=0.019) was a significant risk factor for OS. The multivariate
analysis revealed that ALBI grade 2 (HR=2.60; 95% CI: 1.08-6.26;
P=0.034) and BCLC stage C (HR=2.86; 95% CI: 1.28-6.38; P=0.011)
were independent predictors of poor OS. The ER was confirmed to be
a non-significant predictor of both PFS and OS.
 | Table IIIUnivariate and multivariate analyses
of progression-free survival of patients with hepatocellular
carcinoma. |
Table III
Univariate and multivariate analyses
of progression-free survival of patients with hepatocellular
carcinoma.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Variables | HR (95% Cl) | P-value | HR (95% Cl) | P-value |
|---|
| Age (≥70
years) | 0.96
(0.56-1.66) | 0.883 | | |
| Sex (male) | 0.97
(0.50-1.87) | 0.916 | | |
| HCV positivity | 1.62
(0.95-2.78) | 0.073 | 1.60
(0.93-2.77) | 0.091 |
| ALBI grade 2 | 1.17
(0.69-1.96) | 0.561 | | |
| Tumour number
(≥5) | 1.04
(0.62-1.75) | 0.882 | | |
| Tumour size (≥30
mm) | 1.85
(1.10-3.12) | 0.020 | 2.14
(1.18-3.85) | 0.012 |
| BCLC stage C | 1.12
(0.64-1.96) | 0.679 | | |
| Up-to-seven
criteria (beyond) | 1.36
(0.79-2.33) | 0.272 | | |
| AFP (≥100
ng/ml) | 1.76
(1.03-3.00) | 0.040 | 1.98
(1.08-3.63) | 0.026 |
| DCP (≥200
mAU/ml) | 1.82
(1.06-3.12) | 0.030 | 1.33
(0.75-2.36) | 0.329 |
| TACE
refractoriness | 1.06
(0.58-1.95) | 0.839 | | |
| ER <1.0 | 1.30
(0.74-2.26) | 0.364 | | |
| Initial dose of
lenvatinib 8 mg | 1.55
(0.89-2.72) | 0.123 | | |
| 8W-RDI
(<75%) | 1.53
(0.91-2.58) | 0.108 | | |
 | Table IVUnivariate and multivariate analyses
of overall survival of patients with hepatocellular carcinoma. |
Table IV
Univariate and multivariate analyses
of overall survival of patients with hepatocellular carcinoma.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Variables | HR (95% Cl) | P-value | HR (95% Cl) | P-value |
|---|
| Age (≥70
years) | 0.98
(0.42-2.28) | 0.966 | | |
| Sex (male) | 1.06
(0.40-2.84) | 0.908 | | |
| HCV positivity | 1.15
(0.52-2.52) | 0.729 | | |
| ALBI grade 2 | 2.34
(0.98-5.61) | 0.057 | 2.60
(1.08-6.26) | 0.034 |
| Tumour number
(≥5) | 1.06
(0.48-2.37) | 0.884 | | |
| Tumour size (≥30
mm) | 1.71
(0.78-3.77) | 0.181 | | |
| BCLC stage C | 2.58
(1.17-5.69) | 0.019 | 2.86
(1.28-6.38) | 0.011 |
| Up-to-seven
criteria (beyond) | 1.18
(0.51-2.75) | 0.694 | | |
| AFP (≥100
ng/ml) | 1.70
(0.77-3.73) | 0.186 | | |
| DCP (≥200
mAU/ml) | 1.82
(0.79-4.19) | 0.157 | | |
| TACE
refractoriness | 1.09
(0.41-2.94) | 0.859 | | |
| ER <1.0 | 1.47
(0.63-3.46) | 0.373 | | |
Factors associated with OS in patients
with BCLC stage B HCC
As there were 43 HCC patients with BCLC B, among
whom 23 patients (53%) underwent AT as a post-PD treatment, it was
considered important that the effect of such additive AT on OS was
assessed. Therefore, the OS of 43 patients with BCLC stage B was
analysed, focusing on the presence or absence of AT (Table V). The univariate analysis revealed
that ALBI grade 2 (HR=4.77; 95% CI: 1.05-21.55; P=0.043) and non-AT
(HR=20.95; 95% CI: 2.7-162.55; P=0.004) were significant factors
affecting OS. The multivariate analysis revealed only non-AT as an
independent predictor of unfavourable OS (HR=16.42; 95% CI:
2.03-133.04; P=0.009). ER was not identified as a significant
predictor in this analysis.
 | Table VUnivariate and multivariate analyses
of overall survival of patients with BCLC stage B hepatocellular
carcinoma (n=43). |
Table V
Univariate and multivariate analyses
of overall survival of patients with BCLC stage B hepatocellular
carcinoma (n=43).
| | Univariate
analysis | Multivariate
analysis |
|---|
| Variables | HR (95% Cl) | P-value | HR (95% Cl) | P-value |
|---|
| Age (≥70
years) | 1.33
(0.36-4.86) | 0.670 | | |
| Sex (male) | 0.97
(0.27-3.53) | 0.963 | | |
| HCV positivity | 0.85
(0.28-2.53) | 0.769 | | |
| ALBI grade 2 | 4.77
(1.05-21.55) | 0.043 | 2.12
(0.45-9.99) | 0.341 |
| Tumour number
(≥5) | 1.06
(0.33-3.53) | 0.892 | | |
| Tumour size (≥30
mm) | 1.08
(0.38-3.66) | 0.770 | | |
| Up-to-seven
criteria (beyond) | 0.96
(0.29-3.11) | 0.943 | | |
| AFP (≥100
ng/ml) | 1.37
(0.46-4.07) | 0.576 | | |
| DCP (≥200
mAU/ml) | 2.16
(0.69-6.72) | 0.180 | | |
| ER <1.0 | 0.91
(0.20-4.11) | 0.901 | | |
| Non-AT | 20.95
(2.7-162.55) | 0.004 | 16.42
(2.03-133.04) | 0.009 |
Discussion
The present study evaluated the association between
the precise degree of contrast enhancement and the therapeutic
efficacy of LEN. Through various analyses, it was observed that
there was no significant difference in PFS and OS among the ER
groups; therefore, ER was not found to be a significant predictor
in LEN-treated patients with HCC.
On dynamic CT, hypoattenuation in the arterial phase
is frequently observed in both well-differentiated and poorly
differentiated HCCs (17,18). Furthermore, sarcomatous hepatic
tumours generally exhibit hypovascularity, which is seen as rim
enhancement or non-enhancement on arterial phase imaging (19). Thus, nodules with poor contrast
enhancement in the arterial phase are considered as either less or
highly malignant. The present study included two cases of BCLC
stage A (both in the ER >1.5 group); however, advanced or
unresectable HCCs, for which LEN treatment is usually recommended,
are in BCLC stage B or C (2).
Although this could not be ascertained in the present study, as
histopathological examinations were not performed immediately prior
to LEN administration, it is unlikely that well-differentiated HCCs
were included. In fact, in the present study, the number of
patients with aggressive HCC, characterized by large size,
extrahepatic metastases and high DCP values, was significantly
higher in the ER <1.0 group compared with the other groups.
Although such HCCs are refractory to various treatments and have a
poor prognosis (20,21), no significant difference in PFS has
been reported. Previously, Kawamura et al (9) examined the association between PFS
and the pretreatment enhancement patterns of HCC on CE-CT in
LEN-treated patients. The patterns were defined as homogeneous or
heterogeneous, and it was concluded that there was no difference in
PFS between the patterns, despite the heterogeneity indicating the
highly malignant nature of HCC (9,22).
Although that evaluation method was quite different from the one
used in the present study, the findings suggest that LEN exerts a
strong therapeutic effect regardless of the degree of HCC
differentiation.
In human HCC, CTNNB1 and TP53
mutations define two distinct tumour phenotypes, and histological
subtypes are associated with clinical and molecular characteristics
(23). The CTNNB1 mutation
is frequent, even in well-differentiated HCC, whereas TP53
mutations are more frequent in poorly differentiated HCCs and those
with foci of sarcomatous change (23,24).
Recently, Rodríguez-Hernández et al (25) reported that LEN was more effective
in moderately-to-poorly differentiated liver cancer cells with the
p53 mutation. Thus, these reports support our finding that
LEN is effective against moderately-to-poorly differentiated
HCC.
In the present study, the ER was not identified as a
significant predictor of PFS or OS. The multivariate analyses
demonstrated that ALBI grade 2 and BCLC stage C were independent
predictors of poor OS. ALBI grade is useful in predicting prognosis
after HCC treatment, such as liver resection, radiofrequency
ablation and TACE (26-28).
Regarding the predictive potential of the ALBI grade in LEN
treatment, Ueshima et al (29) reported that HCC patients with ALBI
grade 1 presented a higher response rate and lower frequency of
treatment discontinuation due to severe AEs. Furthermore, Hiraoka
et al (30) reported that
modified ALBI grade 2b or 3 was superior to Child-Pugh
classification in predicting poor prognosis of patients with HCC
receiving LEN treatment. Although the modified ALBI grading system
was not used in the present study, classical ALBI grade 2 was found
to be a prognostic factor in patients with HCC receiving LEN
treatment.
As there were 23 patients (53%) with BCLC stage B
HCC in whom AT was performed after PD, a sub-analysis of OS in BCLC
stage B patients was performed. Although 38 of the 43 patients
(88%) treated with LEN had TACE-refractory HCC, the additive effect
of AT significantly prolonged OS in this study. Previously, Kudo
et al (31) reported that
TACE plus sorafenib significantly improved PFS compared with TACE
alone in patients with unresectable HCC. They considered that
sorafenib-induced normalisation of tumour blood vessels leads to
increased accumulation of TACE-derived mixture of lipiodol and
anticancer agent compared with TACE alone. LEN is also an
angiogenesis inhibitor, similar to sorafenib; therefore, it was
suggested that a similar mechanism was involved in the limited
number of patients with BCLC stage B disease. Accordingly,
normalisation of the tumour vasculature may increase responsiveness
to AT in hypovascular HCCs, which are known to hardly respond to
TACE (32,33).
Recently, Shimose et al (13) reported that AT prolonged OS in
LEN-treated patients with BCLC stage B HCC in the propensity score
matching (PSM) analysis of their multicentre study. They deduced
the mechanism underlying the efficacy of AT as follows:
Transarterial therapy-induced intratumour ischaemia upregulates
hypoxia-inducible factor 1-α expression, which leads to increased
expression of its downstream angiogenic factors for tumour growth,
and LEN administration after transarterial therapy suppresses the
effects of these angiogenic factors (34,35).
Similarly, it was considered reasonable that the AT strategy
contributed to the improved OS of the LEN-treated patients with
BCLC stage B HCC in the present study.
There were certain limitations to the present study.
First, this was a retrospective study that involved a small number
of patients from a single centre; thus, there was a possibility of
selection bias. Furthermore, the ER <1.0 group with larger
tumour diameters, BCLC stage C with extrahepatic metastases and
higher DCP values, consisted of only 20 patients, which may
represent possible confounding factors and lead to underestimation
of the impact of ER on PFS and OS. This may be resolved by matching
tumour factors with PSM analysis in a larger number scale. Second,
only one case of macrovascular invasion was enrolled. Thus, PFS and
OS could not be analysed in HCC patients with macrovascular
invasion. Third, a histological examination was not performed
immediately prior to LEN administration. Therefore, the result of
the present study was only the association between the contrast
enhancement and the therapeutic effect. However, procedures like
aspiration biopsy are invasive and have drawbacks, including
sampling errors. Therefore, histological examination is often not
performed immediately prior to LEN administration in the clinical
setting. Therefore, we believe that this result may have potential
benefits for LEN treatment. Finally, the ER used in the present
study may be affected by the haemodynamic condition of organs, the
types of CT devices and the types of contrast media, regardless of
their superiority over absolute CT values in assessing the contrast
enhancement on the tumour. Further studies are needed to determine
whether pretreatment degree of contrast enhancement is important
through prospective analyses in a larger number of cases with a
unified CT device.
In conclusion, the lower pretreatment contrast
enhancement in HCC did not contribute to the prediction of the PFS
or OS in patients with LEN-treated HCC. The results of the present
study suggested that LEN exerted a strong therapeutic effect on
HCC, regardless of the degree of contrast enhancement in the
tumour. In addition, AT may prolong the OS of patients with
LEN-treated BCLC stage B HCC, regardless of the tumour
vascularity.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SO participated in the conception and design of
study, acquisition and interpretation of data, and drafting of the
manuscript. SS participated in the conception and design of the
study, acquisition and interpretation of data. SO and SS have seen
and can confirm the authenticity of the raw data. TN, NK, YN, TS,
HI, MN and RK participated in the acquisition of data. HK and TT
participated in the interpretation of data and critical revision of
the manuscript for important intellectual content. All the authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the Declaration of Helsinki, and the protocol was approved by the
Ethics Review Committee of Kurume University (approval no. 20192).
An opt-out approach was used to obtain informed consent from the
patients, and personal information was protected during data
collection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Galle PR, Forner A, Llovet JM, Mazzaferro
V, Piscaglia F, Raoul JL, Schirmacher P and Vilgrain V: European
Association for the Study of the Liver. Electronic address:
simpleeasloffice@easloffice.eu;
European Association for the Study of the Liver. EASL Clinical
Practice Guidelines: Management of hepatocellular carcinoma. J
Hepatol. 69:182–236. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Moawad AW, Szklaruk J, Lall C, Blair KJ,
Kaseb AO, Kamath A, Rohren SA and Elsayes KM: Angiogenesis in
hepatocellular carcinoma; pathophysiology, targeted therapy, and
role of imaging. J Hepatocell Carcinoma. 7:77–89. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Heimbach JK, Kulik LM, Finn RS, Sirlin CB,
Abecassis MM, Roberts LR, Zhu AX, Murad MH and Marrero JA: AASLD
guidelines for the treatment of hepatocellular carcinoma.
Hepatology. 67:358–380. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kudo M, Finn RS, Qin S, Han KH, Ikeda K,
Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al: Lenvatinib
versus sorafenib in first-line treatment of patients with
unresectable hepatocellular carcinoma: A randomised phase 3
non-inferiority trial. Lancet. 391:1163–1173. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yamamoto Y, Matsui J, Matsushima T,
Obaishi H, Miyazaki K, Nakamura K, Tohyama O, Semba T, Yamaguchi A,
Hoshi SS, et al: Lenvatinib, an angiogenesis inhibitor targeting
VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft
models associated with microvessel density and pericyte coverage.
Vasc Cell. 6(18)2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kunimoto H, Shakado S, Tanaka T, Takata K,
Yamauchi R, Fukuda H, Tsuchiya N, Yokoyama K, Morihara D, Takeyama
Y, et al: Reduction in tumor stain at 2 weeks after treatment
initiation is a predictor of the efficacy of lenvatinib in patients
with unresectable hepatocellular carcinoma. Oncology. 98:779–786.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kuorda H, Abe T, Fujiwara Y, Okamoto T,
Yonezawa M, Sato H, Endo K, Oikawa T, Sawara K and Takikawa Y:
Change in arterial tumor perfusion is an early biomarker of
lenvatinib efficacy in patients with unresectable hepatocellular
carcinoma. World J Gastroenterol. 25:2365–2372. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kawamura Y, Kobayashi M, Shindoh J,
Kobayashi Y, Kasuya K, Sano T, Fujiyama S, Hosaka T, Saitoh S,
Sezaki H, et al: Pretreatment heterogeneous enhancement pattern of
hepatocellular carcinoma may be a useful new predictor of early
response to lenvatinib and overall prognosis. Liver Cancer.
9:275–292. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kawamura Y, Kobayashi M, Shindoh J,
Kobayashi Y, Kasuya K, Sano T, Fujiyama S, Hosaka T, Saitoh S,
Sezaki H, et al: 18F-fluorodeoxyglucose uptake in
hepatocellular carcinoma as a useful predictor of an extremely
rapid response to lenvatinib. Liver Cancer. 9:84–92.
2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Takahashi A, Moriguchi M, Seko Y, Ishikawa
H, Yo T, Kimura H, Fujii H, Shima T, Mitsumoto Y, Ishiba H, et al:
Impact of relative dose intensity of early-phase lenvatinib
treatment on therapeutic response in hepatocellular carcinoma.
Anticancer Res. 39:5149–5156. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lencioni R and Llovet JM: Modified RECIST
(mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis.
30:52–60. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shimose S, Iwamoto H, Tanaka M, Niizeki T,
Shirono T, Noda Y, Kamachi N, Okamura S, Nakano M, Suga H, et al:
Alternating lenvatinib and trans-arterial therapy prolongs overall
survival in patients with inter-mediate stage hepatocellular
carcinoma: A propensity score matching study. Cancers (Basel).
13(160)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Shimose S, Kawaguchi T, Iwamoto H, Niizeki
T, Shirono T, Tanaka M, Koga H and Torimura T: Indication of
suitable transarterial chemoembolization and multikinase inhibitors
for intermediate stage hepatocellular carcinoma. Oncol Lett.
19:2667–2676. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Nagamatsu H, Sumie S, Niizeki T, Tajiri N,
Iwamoto H, Aino H, Nakano M, Shimose S, Satani M, Okamura S, et al:
Hepatic arterial infusion chemoembolization therapy for advanced
hepatocellular carcinoma: Multicenter phase II study. Cancer
Chemother Pharmacol. 77:243–250. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Llovet JM, Di Bisceglie AM, Bruix J,
Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M,
Talwalkar J, et al: Panel of Experts in HCC-Design Clinical Trials:
Design and endpoints of clinical trials in hepatocellular
carcinoma. J Natl Cancer Inst. 100:698–711. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Asayama Y, Yoshimitsu K, Nishihara Y, Irie
H, Aishima S, Taketomi A and Honda H: Arterial blood supply of
hepatocellular carcinoma and histologic grading:
Radiologic-pathologic correlation. AJR Am J Roentgenol.
190:W28–W34. 2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lee JH, Lee JM, Kim SJ, Baek JH, Yun SH,
Kim KW, Han JK and Choi BI: Enhancement patterns of hepatocellular
carcinomas on multiphasicmultidetector row CT: Comparison with
pathological differentiation. Br J Radiol. 85:e573–e583.
2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gu KW, Kim YK, Min JH, Ha SY and Jeong WK:
Imaging features of hepatic sarcomatous carcinoma on computed
tomography and gadoxetic acid-enhanced magnetic resonance imaging.
Abdom Radiol (NY). 42:1424–1433. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Oishi K, Itamoto T, Amano H, Fukuda S,
Ohdan H, Tashiro H, Shimamoto F and Asahara T: Clinicopathologic
features of poorly differentiated hepatocellular carcinoma. J Surg
Oncol. 95:311–316. 2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lu J, Xiong XZ, Li FY, Ye H, Lin YX, Zhou
RX, Cai YL, Jin YW and Cheng NS: Prognostic significance of
sarcomatous change in patients with hepatocellular carcinoma after
surgical resection. Ann Surg Oncol. 22 (Suppl 3):S1048–S1056.
2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kawamura Y, Ikeda K, Hirakawa M, Yatsuji
H, Sezaki H, Hosaka T, Akuta N, Kobayashi M, Saitoh S, Suzuki F, et
al: New classification of dynamic computed tomography images
predictive of malignant characteristics of hepatocellular
carcinoma. Hepatol Res. 40:1006–1014. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Calderaro J, Couchy G, Imbeaud S, Amaddeo
G, Letouzé E, Blanc JF, Laurent C, Hajji Y, Azoulay D, Bioulac-Sage
P, et al: Histological subtypes of hepatocellular carcinoma are
related to gene mutations and molecular tumour classification. J
Hepatol. 67:727–738. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Calderaro J, Ziol M, Paradis V and
Zucman-Rossi J: Molecular and histological correlations in liver
cancer. J Hepatol. 71:616–630. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Rodríguez-Hernández MA, Chapresto-Garzón
R, Cadenas M, Navarro-Villarán E, Negrete M, Gómez-Bravo MA, Victor
VM, Padillo FJ and Muntané J: Differential effectiveness of
tyrosine kinase inhibitors in 2D/3D culture according to cell
differentiation, p53 status and mitochondrial respiration in liver
cancer cells. Cell Death Dis. 11(339)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang YY, Zhong JH, Su ZY, Huang JF, Lu SD,
Xiang BD, Ma L, Qi LN, Ou BN and Li LQ: Albumin-bilirubin versus
Child-Pugh score as a predictor of outcome after liver resection
for hepatocellular carcinoma. Br J Surg. 103:725–734.
2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Oh IS, Sinn DH, Kang TW, Lee MW, Kang W,
Gwak GY, Paik YH, Choi MS, Lee JH, Koh KC, et al: Liver function
assessment using albumin-bilirubin grade for patients with very
early-stage hepatocellular carcinoma treated with radiofrequency
ablation. Dig Dis Sci. 62:3235–3242. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lee IC, Hung YW, Liu CA, Lee RC, Su CW,
Huo TI, Li CP, Chao Y, Lin HC, Hou MC, et al: A new ALBI-based
model to predict survival after transarterial chemoembolization for
BCLC stage B hepatocellular carcinoma. Liver Int. 39:1704–1712.
2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ueshima K, Nishida N, Hagiwara S, Aoki T,
Minami T, Chishina H, Takita M, Minami Y, Ida H, Takenaka M, et al:
Impact of baseline ALBI grade on the outcomes of hepatocellular
carcinoma patients treated with lenvatinib: A multicenter study.
Cancers (Basel). 11(952)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hiraoka A, Kumada T, Atsukawa M, Hirooka
M, Tsuji K, Ishikawa T, Takaguchi K, Kariyama K, Itobayashi E,
Tajiri K, et al: Real-life Practice Experts for HCC (RELPEC) Study
Group, HCC 48 Group (hepatocellular carcinoma experts from 48
clinics in Japan): Prognostic factor of lenvatinib for unresectable
hepatocellular carcinoma in real-world conditions-Multicenter
analysis. Cancer Med. 8:3719–3728. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kudo M, Ueshima K, Ikeda M, Torimura T,
Tanabe N, Aikata H, Izumi N, Yamasaki T, Nojiri S, Hino K, et al:
TACTICS study group: Randomised, multicentre prospective trial of
transarterial chemoembolisation (TACE) plus sorafenib as compared
with TACE alone in patients with hepatocellular carcinoma: TACTICS
trial. Gut. 69:1492–1501. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Jain RK: Normalization of tumor
vasculature: An emerging concept in antiangiogenic therapy.
Science. 307:58–62. 2005.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Shen H, Agarwal D, Qi R, Chalasani N,
Liangpunsakul S, Lumeng L, Yoo H and Kwo P: Predictors of outcome
in patients with unresectable hepatocellular carcinoma receiving
transcatheter arterial chemoembolization. Aliment Pharmacol Ther.
26:393–400. 2007.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sergio A, Cristofori C, Cardin R, Pivetta
G, Ragazzi R, Baldan A, Girardi L, Cillo U, Burra P, Giacomin A, et
al: Transcatheter arterial chemoembolization (TACE) in
hepatocellular carcinoma (HCC): The role of angiogenesis and
invasiveness. Am J Gastroenterol. 103:914–921. 2008.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yang Y, Zhang Y, Iwamoto H, Hosaka K, Seki
T, Andersson P, Lim S, Fischer C, Nakamura M, Abe M, et al:
Discontinuation of anti-VEGF cancer therapy promotes metastasis
through a liver revascularization mechanism. Nat Commun.
7(12680)2016.PubMed/NCBI View Article : Google Scholar
|