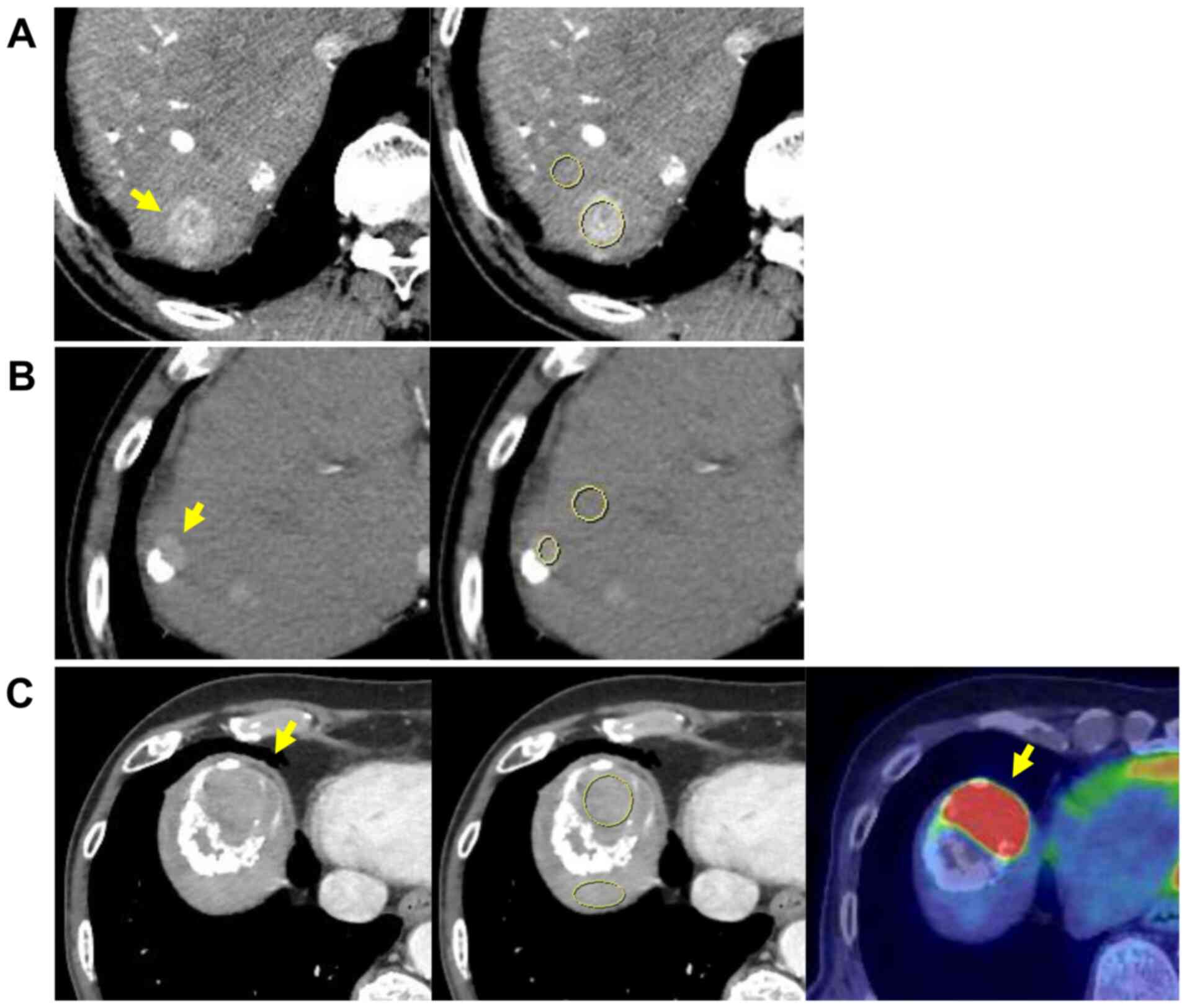
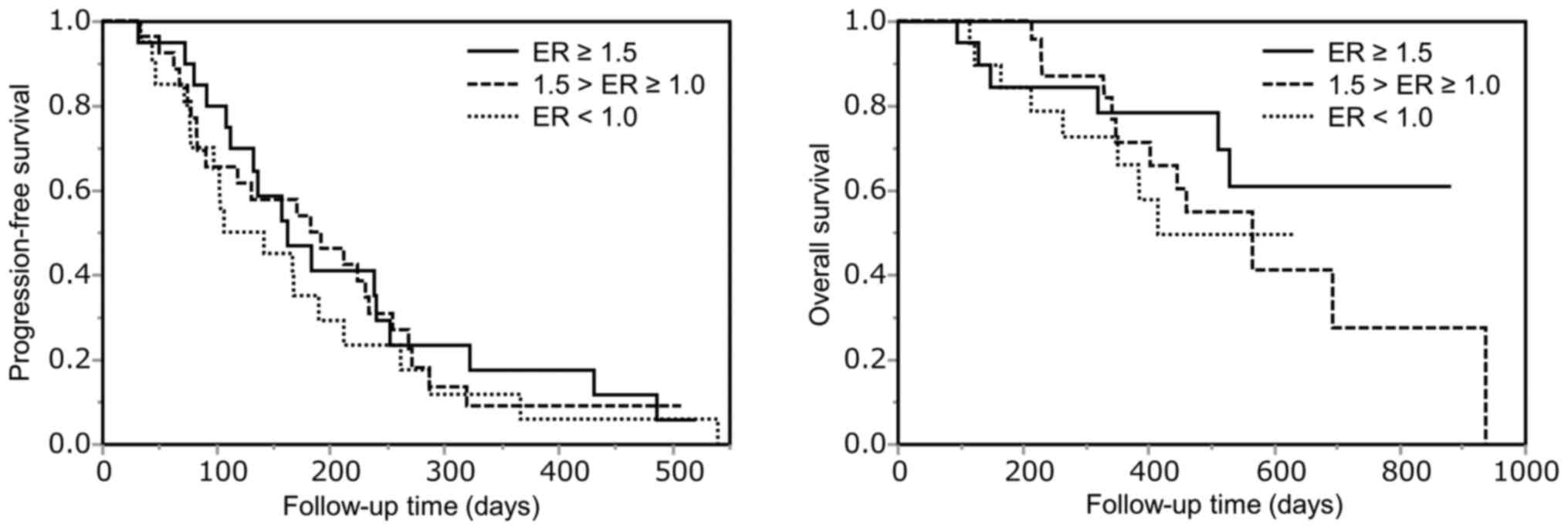
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Galle PR, Forner A, Llovet JM, Mazzaferro
V, Piscaglia F, Raoul JL, Schirmacher P and Vilgrain V: European
Association for the Study of the Liver. Electronic address:
simpleeasloffice@easloffice.eu;
European Association for the Study of the Liver. EASL Clinical
Practice Guidelines: Management of hepatocellular carcinoma. J
Hepatol. 69:182–236. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Moawad AW, Szklaruk J, Lall C, Blair KJ,
Kaseb AO, Kamath A, Rohren SA and Elsayes KM: Angiogenesis in
hepatocellular carcinoma; pathophysiology, targeted therapy, and
role of imaging. J Hepatocell Carcinoma. 7:77–89. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Heimbach JK, Kulik LM, Finn RS, Sirlin CB,
Abecassis MM, Roberts LR, Zhu AX, Murad MH and Marrero JA: AASLD
guidelines for the treatment of hepatocellular carcinoma.
Hepatology. 67:358–380. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kudo M, Finn RS, Qin S, Han KH, Ikeda K,
Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al: Lenvatinib
versus sorafenib in first-line treatment of patients with
unresectable hepatocellular carcinoma: A randomised phase 3
non-inferiority trial. Lancet. 391:1163–1173. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yamamoto Y, Matsui J, Matsushima T,
Obaishi H, Miyazaki K, Nakamura K, Tohyama O, Semba T, Yamaguchi A,
Hoshi SS, et al: Lenvatinib, an angiogenesis inhibitor targeting
VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft
models associated with microvessel density and pericyte coverage.
Vasc Cell. 6(18)2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kunimoto H, Shakado S, Tanaka T, Takata K,
Yamauchi R, Fukuda H, Tsuchiya N, Yokoyama K, Morihara D, Takeyama
Y, et al: Reduction in tumor stain at 2 weeks after treatment
initiation is a predictor of the efficacy of lenvatinib in patients
with unresectable hepatocellular carcinoma. Oncology. 98:779–786.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kuorda H, Abe T, Fujiwara Y, Okamoto T,
Yonezawa M, Sato H, Endo K, Oikawa T, Sawara K and Takikawa Y:
Change in arterial tumor perfusion is an early biomarker of
lenvatinib efficacy in patients with unresectable hepatocellular
carcinoma. World J Gastroenterol. 25:2365–2372. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kawamura Y, Kobayashi M, Shindoh J,
Kobayashi Y, Kasuya K, Sano T, Fujiyama S, Hosaka T, Saitoh S,
Sezaki H, et al: Pretreatment heterogeneous enhancement pattern of
hepatocellular carcinoma may be a useful new predictor of early
response to lenvatinib and overall prognosis. Liver Cancer.
9:275–292. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kawamura Y, Kobayashi M, Shindoh J,
Kobayashi Y, Kasuya K, Sano T, Fujiyama S, Hosaka T, Saitoh S,
Sezaki H, et al: 18F-fluorodeoxyglucose uptake in
hepatocellular carcinoma as a useful predictor of an extremely
rapid response to lenvatinib. Liver Cancer. 9:84–92.
2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Takahashi A, Moriguchi M, Seko Y, Ishikawa
H, Yo T, Kimura H, Fujii H, Shima T, Mitsumoto Y, Ishiba H, et al:
Impact of relative dose intensity of early-phase lenvatinib
treatment on therapeutic response in hepatocellular carcinoma.
Anticancer Res. 39:5149–5156. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lencioni R and Llovet JM: Modified RECIST
(mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis.
30:52–60. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shimose S, Iwamoto H, Tanaka M, Niizeki T,
Shirono T, Noda Y, Kamachi N, Okamura S, Nakano M, Suga H, et al:
Alternating lenvatinib and trans-arterial therapy prolongs overall
survival in patients with inter-mediate stage hepatocellular
carcinoma: A propensity score matching study. Cancers (Basel).
13(160)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Shimose S, Kawaguchi T, Iwamoto H, Niizeki
T, Shirono T, Tanaka M, Koga H and Torimura T: Indication of
suitable transarterial chemoembolization and multikinase inhibitors
for intermediate stage hepatocellular carcinoma. Oncol Lett.
19:2667–2676. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Nagamatsu H, Sumie S, Niizeki T, Tajiri N,
Iwamoto H, Aino H, Nakano M, Shimose S, Satani M, Okamura S, et al:
Hepatic arterial infusion chemoembolization therapy for advanced
hepatocellular carcinoma: Multicenter phase II study. Cancer
Chemother Pharmacol. 77:243–250. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Llovet JM, Di Bisceglie AM, Bruix J,
Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M,
Talwalkar J, et al: Panel of Experts in HCC-Design Clinical Trials:
Design and endpoints of clinical trials in hepatocellular
carcinoma. J Natl Cancer Inst. 100:698–711. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Asayama Y, Yoshimitsu K, Nishihara Y, Irie
H, Aishima S, Taketomi A and Honda H: Arterial blood supply of
hepatocellular carcinoma and histologic grading:
Radiologic-pathologic correlation. AJR Am J Roentgenol.
190:W28–W34. 2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lee JH, Lee JM, Kim SJ, Baek JH, Yun SH,
Kim KW, Han JK and Choi BI: Enhancement patterns of hepatocellular
carcinomas on multiphasicmultidetector row CT: Comparison with
pathological differentiation. Br J Radiol. 85:e573–e583.
2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gu KW, Kim YK, Min JH, Ha SY and Jeong WK:
Imaging features of hepatic sarcomatous carcinoma on computed
tomography and gadoxetic acid-enhanced magnetic resonance imaging.
Abdom Radiol (NY). 42:1424–1433. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Oishi K, Itamoto T, Amano H, Fukuda S,
Ohdan H, Tashiro H, Shimamoto F and Asahara T: Clinicopathologic
features of poorly differentiated hepatocellular carcinoma. J Surg
Oncol. 95:311–316. 2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lu J, Xiong XZ, Li FY, Ye H, Lin YX, Zhou
RX, Cai YL, Jin YW and Cheng NS: Prognostic significance of
sarcomatous change in patients with hepatocellular carcinoma after
surgical resection. Ann Surg Oncol. 22 (Suppl 3):S1048–S1056.
2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kawamura Y, Ikeda K, Hirakawa M, Yatsuji
H, Sezaki H, Hosaka T, Akuta N, Kobayashi M, Saitoh S, Suzuki F, et
al: New classification of dynamic computed tomography images
predictive of malignant characteristics of hepatocellular
carcinoma. Hepatol Res. 40:1006–1014. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Calderaro J, Couchy G, Imbeaud S, Amaddeo
G, Letouzé E, Blanc JF, Laurent C, Hajji Y, Azoulay D, Bioulac-Sage
P, et al: Histological subtypes of hepatocellular carcinoma are
related to gene mutations and molecular tumour classification. J
Hepatol. 67:727–738. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Calderaro J, Ziol M, Paradis V and
Zucman-Rossi J: Molecular and histological correlations in liver
cancer. J Hepatol. 71:616–630. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Rodríguez-Hernández MA, Chapresto-Garzón
R, Cadenas M, Navarro-Villarán E, Negrete M, Gómez-Bravo MA, Victor
VM, Padillo FJ and Muntané J: Differential effectiveness of
tyrosine kinase inhibitors in 2D/3D culture according to cell
differentiation, p53 status and mitochondrial respiration in liver
cancer cells. Cell Death Dis. 11(339)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang YY, Zhong JH, Su ZY, Huang JF, Lu SD,
Xiang BD, Ma L, Qi LN, Ou BN and Li LQ: Albumin-bilirubin versus
Child-Pugh score as a predictor of outcome after liver resection
for hepatocellular carcinoma. Br J Surg. 103:725–734.
2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Oh IS, Sinn DH, Kang TW, Lee MW, Kang W,
Gwak GY, Paik YH, Choi MS, Lee JH, Koh KC, et al: Liver function
assessment using albumin-bilirubin grade for patients with very
early-stage hepatocellular carcinoma treated with radiofrequency
ablation. Dig Dis Sci. 62:3235–3242. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lee IC, Hung YW, Liu CA, Lee RC, Su CW,
Huo TI, Li CP, Chao Y, Lin HC, Hou MC, et al: A new ALBI-based
model to predict survival after transarterial chemoembolization for
BCLC stage B hepatocellular carcinoma. Liver Int. 39:1704–1712.
2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ueshima K, Nishida N, Hagiwara S, Aoki T,
Minami T, Chishina H, Takita M, Minami Y, Ida H, Takenaka M, et al:
Impact of baseline ALBI grade on the outcomes of hepatocellular
carcinoma patients treated with lenvatinib: A multicenter study.
Cancers (Basel). 11(952)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hiraoka A, Kumada T, Atsukawa M, Hirooka
M, Tsuji K, Ishikawa T, Takaguchi K, Kariyama K, Itobayashi E,
Tajiri K, et al: Real-life Practice Experts for HCC (RELPEC) Study
Group, HCC 48 Group (hepatocellular carcinoma experts from 48
clinics in Japan): Prognostic factor of lenvatinib for unresectable
hepatocellular carcinoma in real-world conditions-Multicenter
analysis. Cancer Med. 8:3719–3728. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kudo M, Ueshima K, Ikeda M, Torimura T,
Tanabe N, Aikata H, Izumi N, Yamasaki T, Nojiri S, Hino K, et al:
TACTICS study group: Randomised, multicentre prospective trial of
transarterial chemoembolisation (TACE) plus sorafenib as compared
with TACE alone in patients with hepatocellular carcinoma: TACTICS
trial. Gut. 69:1492–1501. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Jain RK: Normalization of tumor
vasculature: An emerging concept in antiangiogenic therapy.
Science. 307:58–62. 2005.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Shen H, Agarwal D, Qi R, Chalasani N,
Liangpunsakul S, Lumeng L, Yoo H and Kwo P: Predictors of outcome
in patients with unresectable hepatocellular carcinoma receiving
transcatheter arterial chemoembolization. Aliment Pharmacol Ther.
26:393–400. 2007.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sergio A, Cristofori C, Cardin R, Pivetta
G, Ragazzi R, Baldan A, Girardi L, Cillo U, Burra P, Giacomin A, et
al: Transcatheter arterial chemoembolization (TACE) in
hepatocellular carcinoma (HCC): The role of angiogenesis and
invasiveness. Am J Gastroenterol. 103:914–921. 2008.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yang Y, Zhang Y, Iwamoto H, Hosaka K, Seki
T, Andersson P, Lim S, Fischer C, Nakamura M, Abe M, et al:
Discontinuation of anti-VEGF cancer therapy promotes metastasis
through a liver revascularization mechanism. Nat Commun.
7(12680)2016.PubMed/NCBI View Article : Google Scholar
|