Introduction
Currently, the number of patients with pancreatic
cancer is gradually increasing both in Japan (https://ganjoho.jp/reg_stat/statistics/data/dl/en.html)
and in the United States (US) (1,2).
Pancreatic cancer accounts for ~4% of all cancer cases in Japan,
and was reported to be the fourth leading cause of cancer-related
deaths in Japan in 2019 (1,3). In
addition, the 5-year survival rate for patients with pancreatic
cancer in Japan is ~8.5% (4).
Similarly, in the US, pancreatic cancer is the third leading cause
of cancer-related mortality, with a low 5-year survival rate of
7-8% (5). For most operable
pancreatic tumors, surgery with curative intent is still the best
treatment (6). However, the
disease is usually in an advanced stage by the time of diagnosis
involving metastasis to other organs. In such cases, chemotherapy
or palliative therapy is a treatment option. Organs to which this
cancer commonly spreads include the liver, lungs, stomach, bones
and intestines (7). However,
metastasis to the bladder is very rare. The current study presents
a case of pancreatic cancer associated with gross hematuria due to
bladder metastasis. If the chief complaint is hematuria, it may be
important to perform a systemic search at an early stage,
considering the possibility of metastatic bladder cancer.
Case report
Presentation and diagnosis
A 90-year-old woman presented with a 2-month history
of gross hematuria associated with worsening of diabetes mellitus.
The patient visited a local general physician and underwent an
abdominal ultrasound in December 2015. However, no abnormal
findings were found at that time. Approximately one week later, the
patient was referred to Okayama Medical Center (Okayama, Japan) for
the assessment of hematuria. Urine cytology showed increased
chromatin and irregularly shaped epithelial cells in clusters,
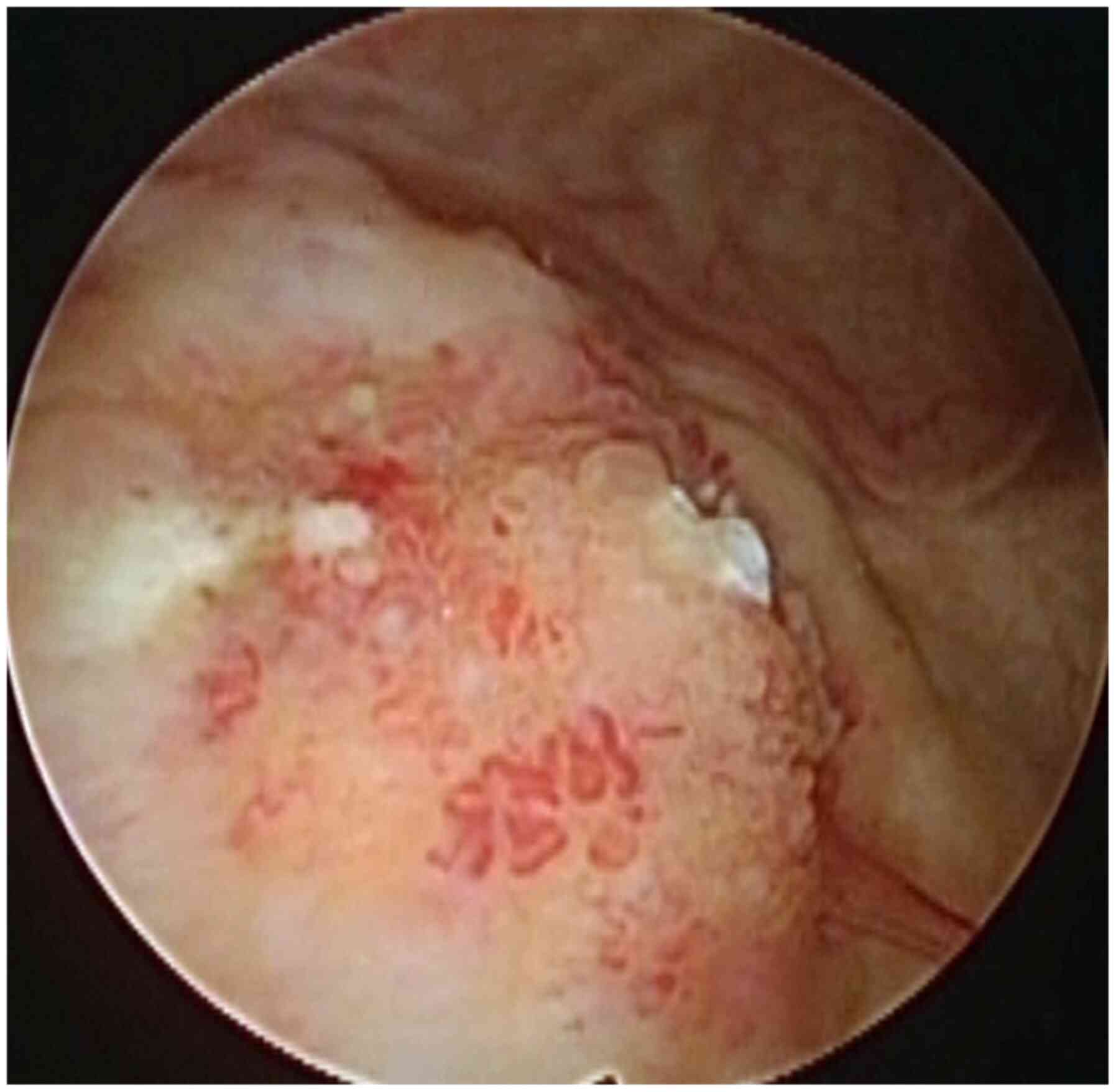
suggesting the possibility of malignancy. Cystoscopy revealed a
15-mm, non-papillary, broad-based tumor on the posterior wall of
the bladder (Fig. 1). Complete
blood cell count and other laboratory test results were within the
normal ranges, except for blood sugar levels (209 mg/dl; normal
range, 73-140 mg/dl) and hemoglobin A1c levels (9.2%; normal range,
4.9-6.0%).
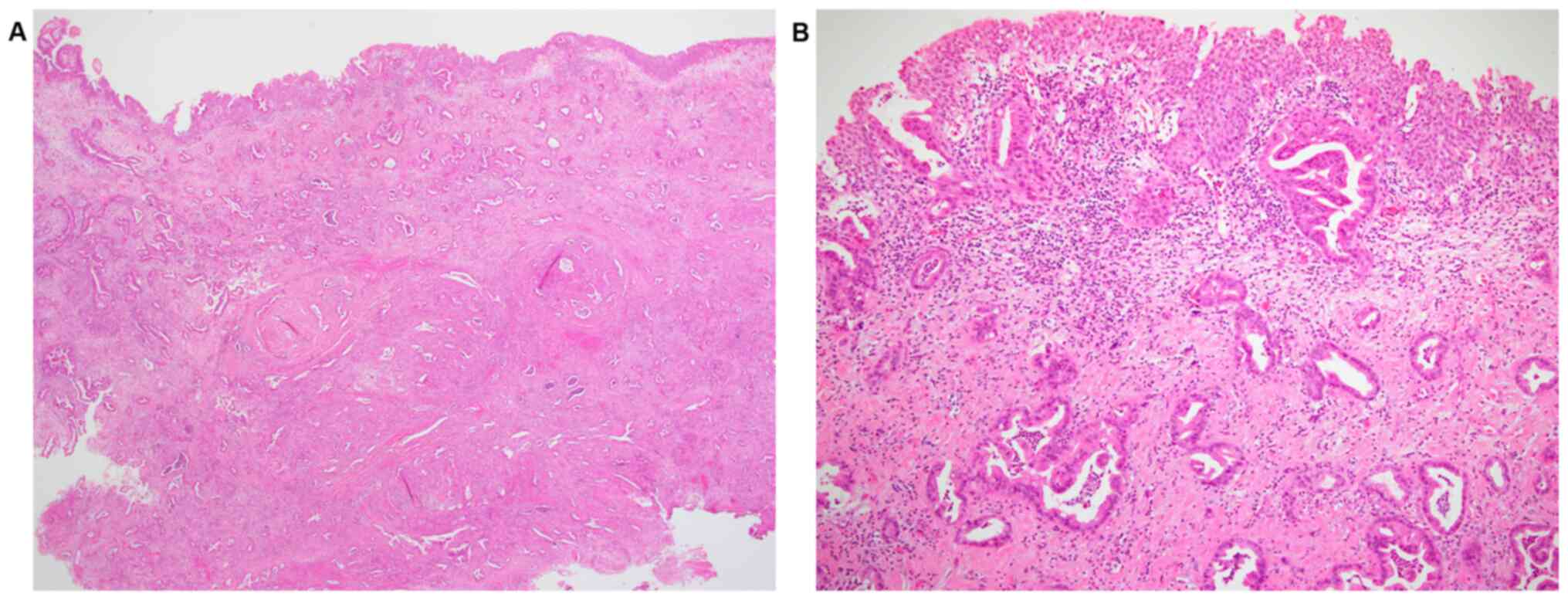
A transurethral resection of the bladder tumor
(TURBT) from the posterior wall was performed, and a
histopathological diagnosis of invasive adenocarcinoma was
established, as the main locus of the tumor was submucosal to
intramuscular. The urothelium did not show any atypia. In addition,
the adenocarcinoma appeared to grow from the muscular layer to the
surface (Fig. 2). Therefore, it
was necessary to determine whether this adenocarcinoma was primary
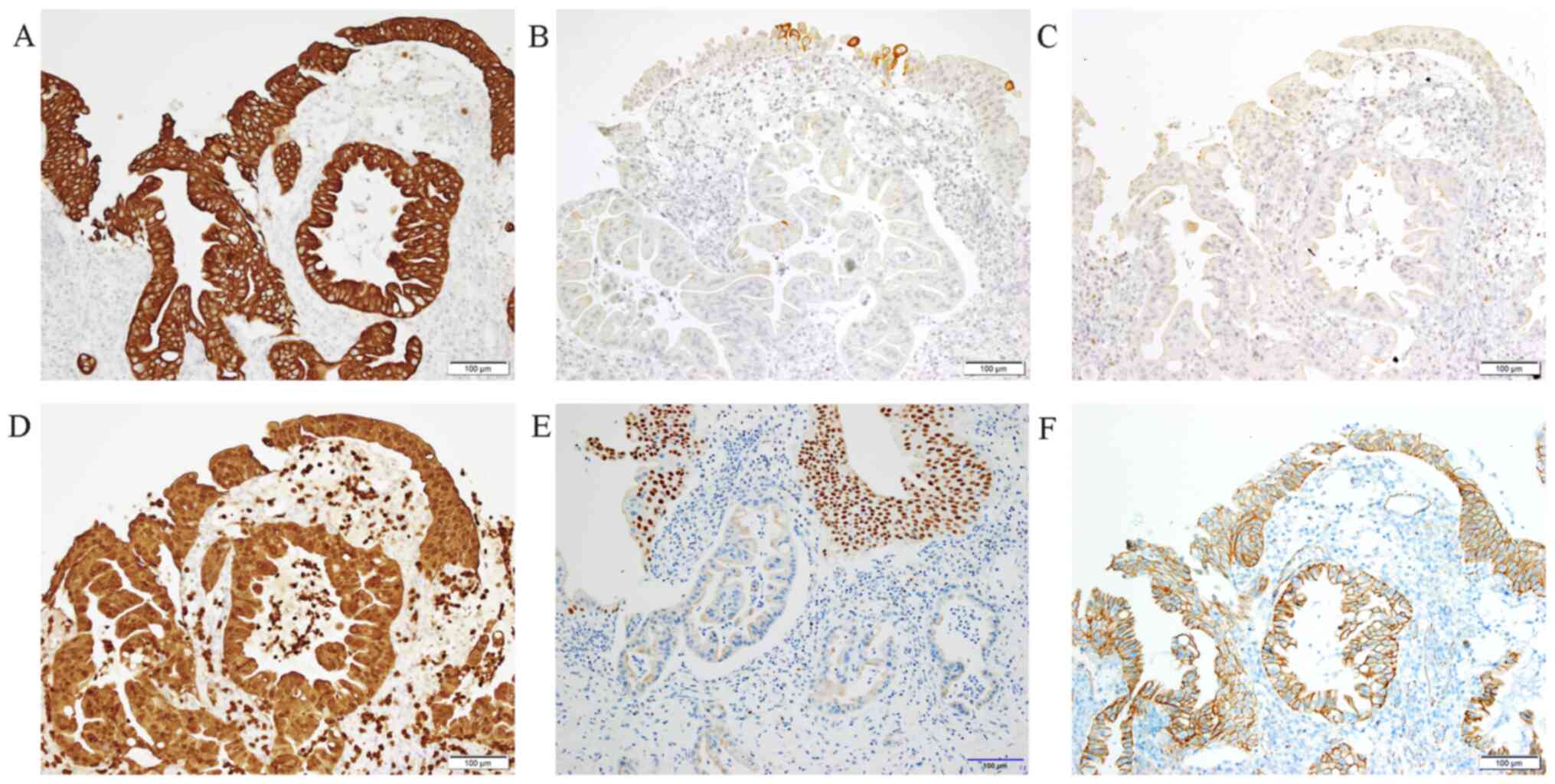
or metastatic. Immunostaining for cytokeratin (CK)7 and S100p was
positive. However, immunohistochemistry was negative for CK20,
estrogen receptor, trans-acting T-cell-specific
transcription factor GATA-3 and nuclear β-catenin. This staining
pattern suggested that the lesion was unlikely to have a
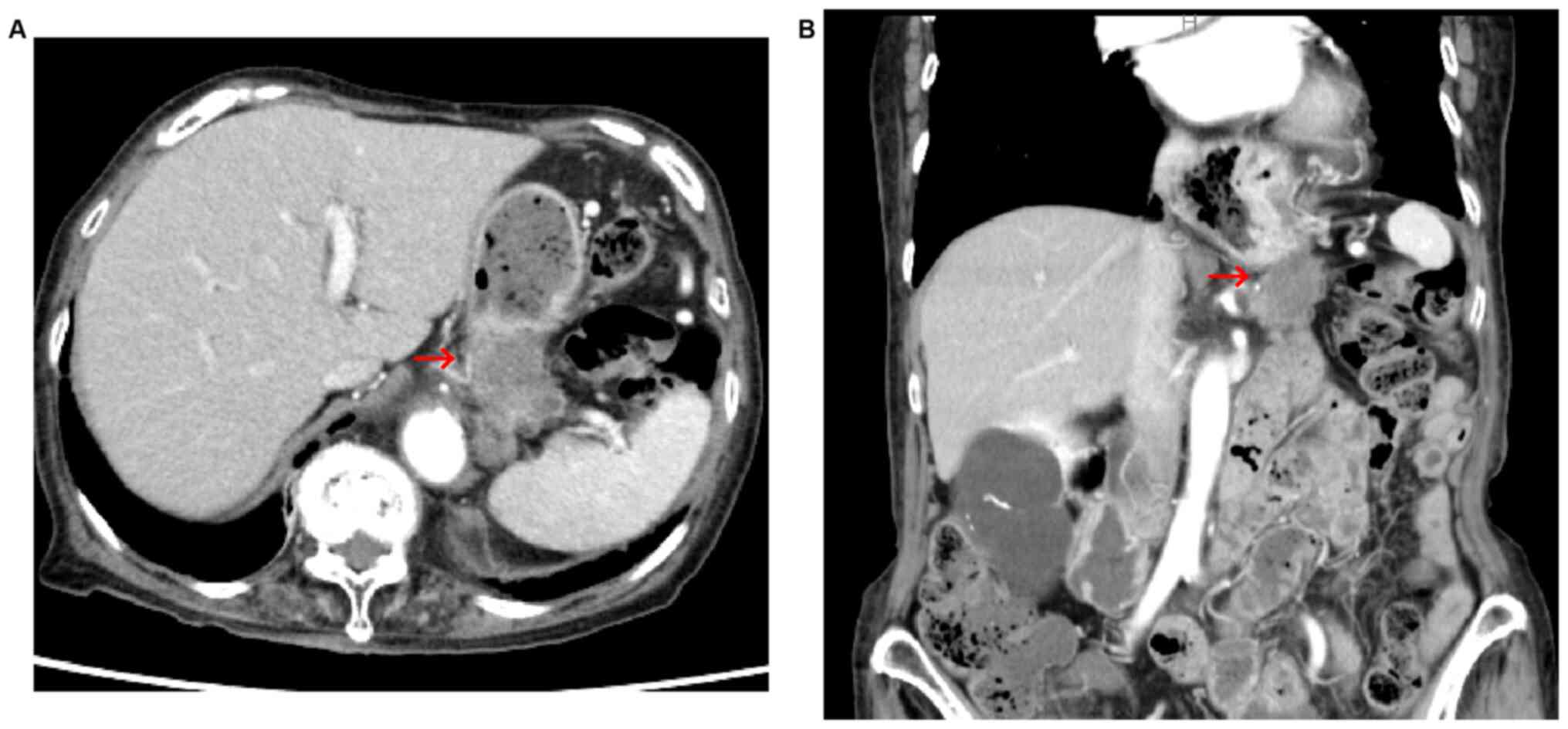
gynecological or a colorectal origin (Fig. 3). Contrast-enhanced computed
tomography scan revealed a tumor with poor contrast effect in the
pancreatic tail, which infiltrated the splenic vessels and
metastasized to the left adrenal gland (Fig. 4). Based on these findings, a
diagnosis of invasive ductal carcinoma of the pancreas, cT4N0M1,
was established according to the Union for International Cancer
Control (UICC) staging system (8),
with metastasis to the urinary bladder and left adrenal gland. This
carcinoma had not been revealed by ultrasound examination
previously performed at the general clinic; it is possible that the
doctor did not examine the pancreas region, as the chief complaint
of the patient was hematuria. The patient declined aggressive
treatment due to old age and chose to receive the best supportive
care. The patient died 6 months after the diagnosis.
Tissue analysis
For analysis of the resected sample, the tissue was
fixed with 10% neutral buffered formalin at room temperature
overnight and paraffin embedded. Immunohistochemistry was performed
using 5-µm-thick sections on an automated immunohistochemistry
system (Ventana BenchMark GX; Roche Diagnostics, Inc.) and the
iVIEWDAB universal detection kit (Roche Diagnostics, Inc.). The
primary antibodies were diluted with Antibody Diluent with
Background Reducing Components (cat. no. S3022; Dako; Agilent
Technologies, Inc.) containing carrier protein for blocking,
accordingly the blocking time was the same as the incubation time,
and the temperature was 35˚C. The antibody clone, supplier,
dilution ratio, CC1 pretreatment time, incubation time and
magnification set for each primary antibody were as follows: CK7
(OV-TL 12/30; Dako; Agilent Technologies, Inc.; 1:100; 60 min; 26
min; x200), CK20 (Ks 20.8; Dako; Agilent Technologies, Inc.; 1:100;
60 min; 20 min; x200), estrogen receptor (SP1; Ventana Medical
Systems, Inc.; ready-to-use; 60 min; 26 min; x200), S100P (rabbit
polyclonal; Atlas Antibodies AB; 1:3,000; 30 min; 26 min; x200) and
GATA-3 (L50-823; Biocare Medical LLC; 1:200; 60 min; 32 min; x200).
The counterstaining was performed with hematoxylin for 5 min at
room temperature. β-catenin (β-catenin-1; Dako; Agilent
Technologies, Inc.) was stained for at another facility, thus, the
details of the staining conditions are unknown. The specimens were
observed with a light microscope.
Discussion
The present study reports a rare case of pancreatic
cancer associated with gross hematuria due to bladder metastasis.
In Japan, the age-standardized incidence rate of pancreatic cancer
in 2018 was 14.3 per 100,000 people, which was the sixth highest in
the Japanese population (1). In
2019, the number of pancreatic cancer-related deaths was 18,124
among men and 18,232 among women, making it the fourth leading
cause of death for men and the third for women (3). The risk factors for pancreatic cancer
include family history, diabetes mellitus, chronic pancreatitis,
intraductal papillary mucinous neoplasm and poor lifestyle choices,
such as smoking (9).
Displacement of the left kidney and ureteral
obstruction are common urological involvements in pancreatic
cancer. However, metastasis to the bladder is rare. Warden et
al (10) examined 20 cases of
pancreatic cancer with urological involvement, excluding cases of
bladder metastasis. Malignant tumors resulting in bladder
metastasis are those of the pelvic organs, including urogenital,
colon and rectal cancer. Of the malignancies that may metastasize
to the bladder from distant organs, malignant melanoma, breast
cancer and stomach cancer are common. However, metastasis from
pancreatic cancer is very rare (11,12),
and has only been reported in 16 autopsy and clinical cases since
1927. Of these cases, 11 were detected in autopsies (11,13-17),
and only five were diagnosed in living patients (7,18-21).
Among the five clinical cases, gross hematuria due to bladder
metastasis was observed in 3 individuals. Chiang et al
(19) reported a case of
pancreatic adenocarcinoma that metastasized to the bladder
presenting with gross hematuria and jaundice. In the other two
cases, metastasis to the bladder was observed during the treatment
of pancreatic cancer (20,21). To the best of our knowledge, after
the study by Chiang et al in 1992(19), this is the second case of bladder
metastasis from pancreatic adenocarcinoma diagnosed by the
detection of gross hematuria.
In conclusion, when an adenocarcinoma is diagnosed
after TURBT, a differential diagnosis between primary bladder
cancer and metastatic adenocarcinoma should be made. When gross
hematuria is observed in patients with pancreatic cancer, invasion
and metastasis to the urinary tract should be considered.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
TT and RK conceived and designed this case report.
TI was in charge of the patient and decided their treatment. TT, KD
and RK acquired data from the diagnostic imaging and histological
results, and wrote the initial draft of the report. YI and RK
acquired data from the diagnostic imaging and histological results,
and all authors are also responsible for the analysis. YS performed
additional immunostaining and contributed to the histological
interpretation and discussion. TT revised the manuscript critically
for important intellectual content. All authors read and approved
the final version of the manuscript. RK and TT confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
As this is a case report and the substitute decision
maker provided written informed consent for publication, it was
granted exemption from approval by the Okayama Medical Center
Clinical Research Review Committee (Okayama, Japan).
Patient consent for publication
As the patient had died, written informed consent
was obtained from the family for the publication of the present
report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cancer Information Service, National
Cancer Center, Japan: National Cancer Registry (Ministry of Health,
Labour and Welfare). https://ganjoho.jp/reg_stat/statistics/data/dl/en.html.
Accessed September 29, 2021.
|
|
2
|
American Cancer Society: Cancer Facts and
Figures 2021. American Cancer Society, Atlanta, GA, 2021.
|
|
3
|
Cancer statistics Cancer Information
Service, National Cancer Center, Japan (Vital Statistics of Japan,
Ministry of Health, Labour and Welfare). https://ganjoho.jp/reg_stat/statistics/data/dl/en.html.
Accessed September 29, 2021.
|
|
4
|
Monitoring of Cancer Incidence in Japan -
Survival 2009-2011 Report (Center for Cancer Control and
Information Services, National Cancer Center, 2020). https://ganjoho.jp/reg_stat/statistics/data/dl/en.html.
Accessed September 29, 2021.
|
|
5
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tempero MA, Malafa MP, Al-Hawary M, Asbun
H, Bain A, Behrman SW, Benson AB III, Binder E, Cardin DB, Cha C,
et al: Pancreatic adenocarcinoma, Version 2.2017, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
15:1028–1061. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shah A, Korrapati P, Siegel J and Kasmin
F: Rare metastasis of primary pancreatic adenocarcinoma to the
bladder. ACG Case Rep J. 5(e27)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Amin MB, Edge SB, Greene FL, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: American Joint Committee on Cancer: AJCC Cancer
Staging Manual. 8th edition. Springer, New York, NY, 2017.
https://doi.org/10.1007/978-3-319-40618-3.
|
|
9
|
Yamaguchi K, Okusaka T, Shimizu K, Furuse
J, Ito Y, Hanada K, Shimosegawa T, Yamaguchi K, Okusaka T, Shimizu
K, et al: Committee for revision of clinical guidelines for
pancreatic cancer of Japan Pancreas Society: EBM-based Clinical
Guidelines for Pancreatic Cancer (2013) issued by the Japan
Pancreas Society: A synopsis. Jpn J Clin Oncol. 44:883–888.
2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Warden SS, Fiveash JG Jr, Tynes WV II and
Schellhammer PF II: Urologic aspects of pancreatic adenocarcinoma.
J Urol. 125:265–267. 1981.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Goldstein AG: Metastatic carcinoma to the
bladder. J Urol. 98:209–215. 1967.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Velcheti V and Govindan R: Metastatic
cancer involving bladder: A review. Can J Urol. 14:3443–3448.
2007.PubMed/NCBI
|
|
13
|
Klinger ME: Secondary tumors of the
genito-urinary tract. J Urol. 65:144–153. 1951.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kiefer ED: Carcinoma of the pancreas. Arch
Intern Med. 40:1–29. 1927.
|
|
15
|
Sommers SC and Meissner WA: Unusual
carcinomas of the pancreas. AMA Arch Pathol. 58:101–111.
1954.PubMed/NCBI
|
|
16
|
Bell ET: Carcinoma of the pancreas. I. A
clinical and pathologic study of 609 necropsied cases. II. The
relation of carcinoma of the pancreas to diabetes mellitus. Am J
Pathol. 33:499–523. 1957.PubMed/NCBI
|
|
17
|
Sheehan EE, Greenberg SD and Scott R Jr:
Metastatic neoplasms of the bladder. J Urol. 90:281–284.
1963.PubMed/NCBI View Article : Google Scholar
|
|
18
|
van Dyk D, Lang R, Jutrin Y, Shapira J and
Ravid M: Bizarre urologic manifestations of pancreas carcinoma.
Hepatogastroenterology. 27:62–63. 1980.PubMed/NCBI
|
|
19
|
Chiang KS, Lamki N and Athey PA:
Metastasis to the bladder from pancreatic adenocarcinoma presenting
with hematuria. Urol Radiol. 13:187–189. 1992.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lavelle RS, Williams SB and O'Leary MP: An
84-year-old female with gross hematuria. Urology. 77:533–534.
2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cellini M and Deighton DA: Radiological
case: Non-papillary bladder metastasis from pancreatic
adenocarcinoma. Appl Radiol. 43:74–76. 2014.
|