Introduction
Medullary thyroid carcinoma (MTC), a type of
malignant tumor arising from C-cells, is characterized by the
production of calcitonin. As only 0.01-0.1% of total cells in the
human thyroid gland are C-cells, MTC is a rare tumor type that
accounts for 2-4% of all malignant thyroid neoplasms. MTC has a
more aggressive clinical course than differentiated thyroid cancers
and a poorer prognosis. Calcitonin is produced by both normal and
neoplastic C-cells and is a specific and highly sensitive
biomarker. Neoplastic C-cells also produce carcinoembryonic antigen
(CEA). These molecules are widely used markers for diagnosis,
prognosis and follow-up of patients with MTC.
MTC may exhibit several, although infrequent,
cytoarchitectural variants such as the pseudo-papillary,
follicular, spindle-cell, giant-cell, clear-cell, oncocytic,
squamous, amphicrine, paraganglioma-like, angiosarcoma-like and
small-cell forms (1). In rare
cases, MTC may be composed of carcinoma cells containing variable
amounts of melanin pigment, being referred to as the melanotic
variant of MTC (2) or
melanin-producing MTC (3). At our
clinic, a case of melanin-producing MTC that had components of
melanoma was encountered. To the best of our knowledge, only one
such case has been reported previously (4). The second case was reported in the
present study and compared with the previous one. Although the
previously reported case had no detectable recurrence or distant
metastasis up to 11 years after surgery (4), the patient of the present study died
due to brain metastasis three years after tumor resection. The
present case had a similar morphology to the previously reported
one but the outcome was poorer.
Case report
Ultrasound
Ultrasonographic imaging was performed using an
SSD-3500 (Hitachi-Aloka) with a 7.5-10 MHz linear probe. Imaging
was performed in B-mode and the focus was set to 2 cm. B-mode gain
was adjusted by the auto-optimizer. Both transverse and
longitudinal sectional images were obtained.
Computed tomography (CT)
Both plain and contrast-enhanced CT were performed
using an ECLOS 16S (Hitachi Medico). Iohexol (Omunipaque; GE
Healthcare Pharma; iodine concentration, 300 mg/ml) was used as the
contrast medium and enhanced CT was recorded 80 sec after injection
of the contrast medium. CT scans were performed with a helical type
under the following conditions: Voltage, 120 kV; current, 250 mA;
slice thickness, 5 mm; slice interval, 5 mm; imaging time, 0.8
sec.
Fine-needle aspiration cytology
(FNAC)
FNAC of the nodule was performed with a 22G needle
under ultrasound guidance. After Papanicolaou staining (Muto Pure
Chemicals Co.) (5) of the
specimen, the diagnosis was made according to the Bethesda
reporting system (6).
Pathology and immunohistochemical
(IHC) analysis
The resected whole thyroid organ was fixed for ~24 h
using 10% formalin neutral buffered solution. Subsequently, it was
embedded in paraffin according to the usual method after
dehydration using ethanol. Paraffin sections (3 µm) were prepared
for IHC analysis using primary antibodies for the detection of
melan-A (cat. no. A103; diluted 1:100), HMB-45 (cat. no. M0634;
diluted 1:50), S-100 protein (cat. no. A5114; polyclonal; diluted
1:1,500), thyroid transcription factor 1 (TTF-1; cat. no. 8G7G3/1;
diluted 1:100), calcitonin (polyclonal; cat. no. IR515; diluted
1:10), Ki-67 (MIB-1; cat. no. M7240; diluted 1:200), chromogranin A
(DAK-A3; cat. no. M0869; diluted 1:400; all from Dako; Agilent
Technologies, Inc.), CEA (COL-1; cat. no. 413212; diluted 1:4;
Histofine) and BRAF V600E (cat. no. 31-1042-00-S, RM8; diluted
1:100; RevMAb Biosciences). Epitope retrieval was performed
according to the manufacturer's recommended procedure for each
primary antibody. Prior to staining of the sections, endogenous
peroxidase activity was blocked using 3%
H2O2. After incubation with the primary
antibody at room temperature for 2 h, visualization was performed
using a polymer IHC detection system and diaminobenzidine chromogen
as a substrate (Envision Kit; Dako; Agilent Technologies, Inc.).
Visualization of BRAF V600E was performed using 3-amino-9-ethyl
carbazole. Sections were counterstained with hematoxylin.
Clinical findings
An 86-year-old male patient presented with a right
anterior neck mass that had been increasing in size (August 2012;
Kanaji Thyroid Hospital, Tokyo, Japan). The patient had first
noticed the mass 3 years previously and undergone ultrasound
examination and FNAC of the thyroid, but no treatments had been
received. The patient had no family history of multiple endocrine
neoplasia, neither type 2A or type 2B. Physical examination
revealed an elastic hard and mobile mass 5 cm in diameter in the
right lobe of the thyroid. No enlarged lymph nodes were palpable in
the neck region. Presurgical physiological data (blood parameters)
are presented in Table I. Thyroid
function tests yielded normal values (Table I). The thyroglobulin level was 37.9
ng/ml (Table I) and the levels of
serum calcitonin and CEA were markedly elevated (Table I). Ultrasound revealed a nodule
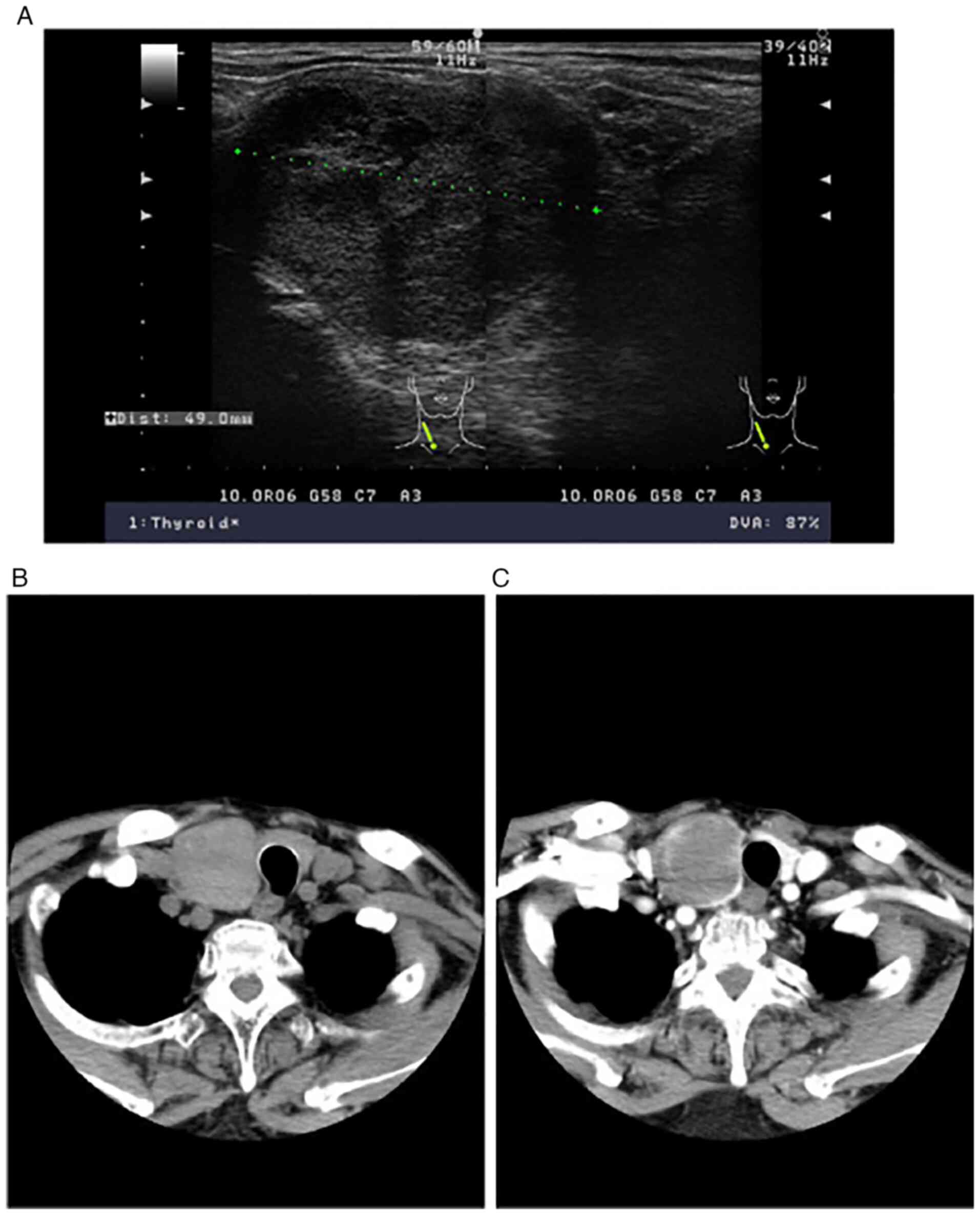
measuring 49x48x40 mm in the right lobe of the thyroid. It was
well-defined, hypoechoic and heterogeneous (Fig. 1A). On CT scans, the nodule appeared
homogeneous with peripheral enhancement (Fig. 1B and C). The imaging findings were interpreted
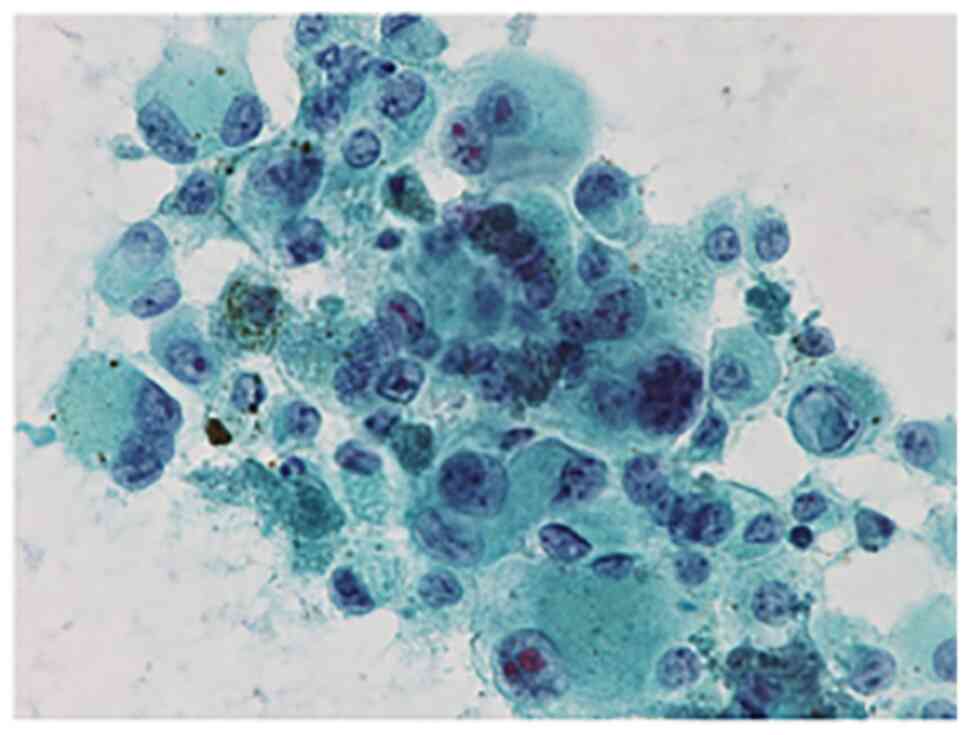
as suspicious for follicular tumor. FNAC performed on the nodule
revealed numerous large pleomorphic cells without specific
structure, and therefore, anaplastic thyroid carcinoma (ATC) was
suspected (Fig. 2). The patient
underwent total thyroidectomy without neck nodal dissection at
Kanaji Thyroid Hospital (Tokyo, Japan). As the pathological
diagnosis was melanoma, thyroid metastasis of melanoma was
considered and an extensive examination at the dermatology
department of another hospital was performed after the operation,
but no primary site of the melanoma was detected. Subsequently, the
serum calcitonin and CEA levels returned to normal. However, a
brain tumor, which was assumed to be metastasis of the melanoma,
had occurred and the patient died three years after the operation.
Although no definitive diagnosis was obtained regarding whether the
brain tumor was metastasis of the melanoma or a primary tumor such
as glioma because no autopsy was performed, the clinical course led
to the assumption that the cause of death was brain metastasis of
the melanoma.
 | Table IClinical findings and data of
preoperative biochemical tests. |
Table I
Clinical findings and data of
preoperative biochemical tests.
| Item | Present case | Previous
casea | Normal range |
|---|
| Age, years | 86 | 66 | - |
| Sex | Male | Female | - |
| Prognosis after
surgery, years | 3 | 11 | - |
| Biochemical
tests | | | |
|
Thyroid-stimulating
hormone, µIU/ml | 2.84 | - | 0.35-3.80 |
|
Free
thyroxine, ng/dl | 1.2 | - | 0.7-1.7 |
|
Free
triiodothyronine, pg/ml | 3.1 | - | 2.2-4.1 |
|
Anti-thyroglobulin
antibody, U/ml | <15.0 | - | ≤40 |
|
Anti-thyroid
peroxidase antibody, U/ml | <28.0 | - | ≤50 |
|
Thyroglobulin,
ng/ml | 37.9 | - | ≤32.8 |
|
Calcitonin,
pg/ml | 2,298 | 960 | ≤5.15 |
|
CEA,
ng/ml | 27.0 | 6.1 | ≤5.0 |
Pathological findings
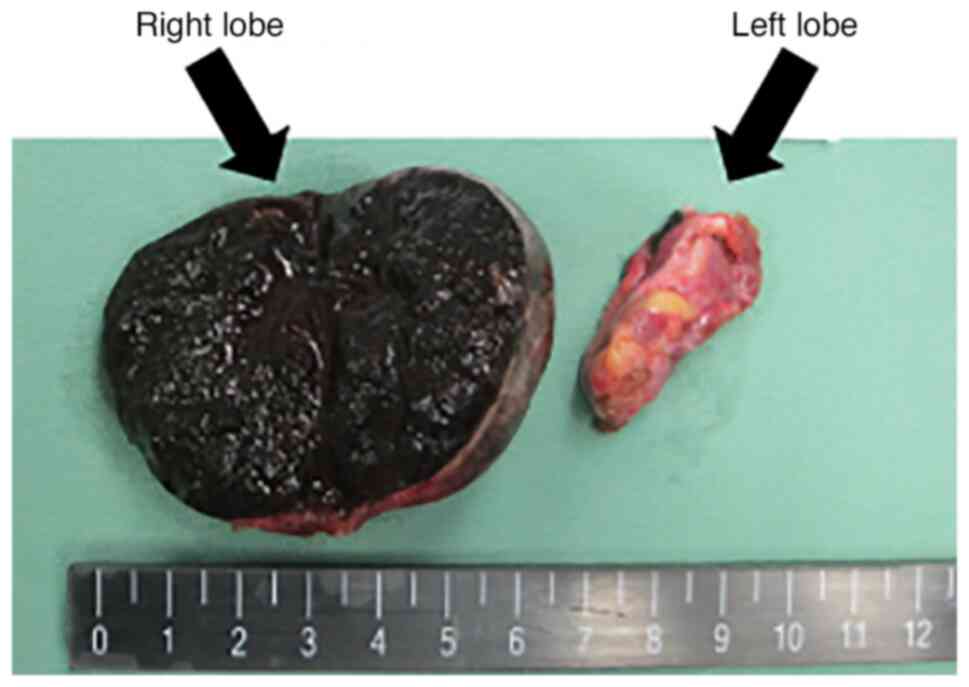
On gross examination, the nodule measured 5.0 cm in
maximum diameter. It was well encapsulated, soft and located in the
right lobe of the thyroid. The cut surface was solid and black
(Fig. 3).
Light microscopy indicated that the nodule was
composed mainly of large, occasionally huge, pleomorphic cells with
a solid or alveolar growth pattern (Fig. 4A) and necrotic areas. The nuclei of
the pleomorphic cells were large and hyperchromatic. Intranuclear
cytoplasmic inclusions, multinucleation, irregularly shaped nuclei
and mitotic figures were apparent. The nucleoli were large,
prominent and frequently had a perinucleolar halo. The cytoplasm
was abundant and eosinophilic. Most of the atypical cells contained
melanin pigment, which frequently occupied most of the cytoplasm.
The morphology of the pleomorphic cells was consistent with that of
ATC, except for the presence of melanin pigment. Aggregated
macrophages containing a large amount of melanin pigment were
present in the areas occupied by the pleomorphic cells. In the
subcapsular areas, solid or lobular growth of small spindle cells
was evident (Fig. 4B). These cells
had granular chromatin but nuclear pleomorphism was not apparent.
The cytoplasm was scant and amphophilic. A small to moderate amount
of melanin pigment was present in the cytoplasm. The large
pleomorphic cells and small spindle cells were intimately
intermingled and neither exhibited capsular or vascular invasion.
No amyloid deposition was evident throughout the tumor.
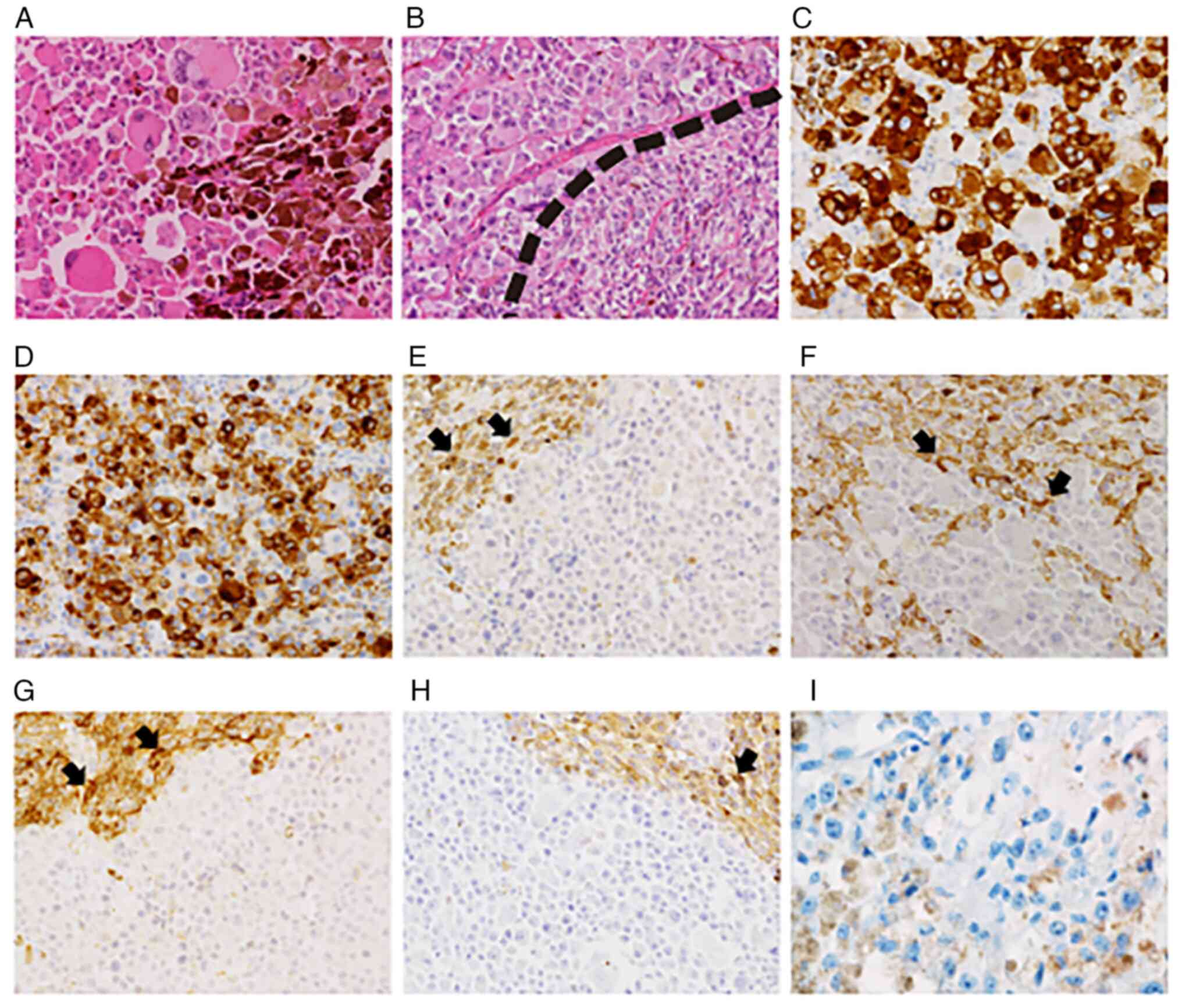 | Figure 4(A) Representative HE staining image.
Large pleomorphic melanoma cells exhibit a diffuse growth pattern
and contain melanin pigment. (B) Representative HE staining image.
Melanoma cells (upper left) and medullary carcinoma cells (lower
right) are intimately intermingled. Medullary carcinoma cells also
contained melanin pigment. (C-I) On immunohistochemistry, melanoma
cells were positive for (C) melan-A and (D) HMB-45 and negative for
(E) TTF-1, (F) calcitonin, (G) chromogranin A, (H) CEA and (I) BRAF
V600E. Visualization of BRAF V600E was performed using AEC. AEC
visualization develops a red color. Brown material in I are melanin
pigments. Medullary carcinoma cells were positive for TTF-1,
calcitonin, chromogranin A and CEA, as indicated by the arrows
(magnification, x200). CEA, carcinoembryonic antigen; TTF-1,
thyroid transcription factor 1; HMB, human melanoma black; AEC,
3-amino-9-ethyl carbazole. |
IHC revealed that the large pleomorphic cells were
positive for melan-A (Fig. 4C),
HMB-45 (Fig. 4D) and S-100
protein, and negative for TTF-1 (Fig.
4E), calcitonin (Fig. 4F),
chromogranin A (Fig. 4G), CEA
(Fig. 4H) and BRAF V600E (Fig. 4I). The small spindle cells were
positive for TTF-1 (Fig. 4E),
calcitonin (Fig. 4F), chromogranin
A (Fig. 4G) and CEA (Fig. 4H). The Ki-67 labeling indices of
the pleomorphic cells and spindle cells were >80 and <3%,
respectively. A comparison of the IHC results between the present
case and previous case (4) is
provided in Table II.
 | Table IIImmunohistochemical findings of two
cases of pigmented medullary carcinoma with malignant melanoma. |
Table II
Immunohistochemical findings of two
cases of pigmented medullary carcinoma with malignant melanoma.
| | Present case | Previous
casea |
|---|
| Antibodies | Medullary carcinoma
cells | Melanoma cells | Medullary carcinoma
cells | Melanoma cells |
|---|
| Melan-A | - | + | - | + |
| HMB-45 | - | + | - | + |
| S-100 protein | - | + | - | + |
| TTF-1 | + | - | + (focal) | - |
| Calcitonin | + | - | + | + (focal) |
| Chromogranin A | + | - | + | + (focal) |
| CEA | + | - | + | - |
| BRAF V600E | - | - | / | / |
| Ki-67 | <3% | >80% | <1% | <60% |
Discussion
The thyroid tumor of the present case was
encapsulated and composed of two types of carcinoma cells
containing melanin pigments: Large pleomorphic cells and small
spindle cells, the former accounting for the majority.
Morphologically, the large pleomorphic cells were similar to those
of ATC except for the presence of melanin pigment. On IHC analysis,
the cells were positive for melan-A and S-100 protein, being
consistent with melanoma. The small spindle cells were positive for
TTF-1, calcitonin, chromogranin and CEA, suggesting the diagnosis
of MTC. The presence of spindle cells with melanin pigment was
consistent with melanin-producing MTC.
As to the melanoma component in the present case,
either a primary or a metastatic origin may be considered.
Metastases to the thyroid gland are not unusual and frequent sites
for primary tumors include the kidney (22%), lung (22%) and head
and neck (12%) (7). Melanoma is
able to metastasize to the thyroid (8). However, in an extensive examination
of other organs, no primary melanoma lesion or other metastatic
lesions were detected. Neuroendocrine carcinoma (NEC) may exhibit
melanocytic differentiation (9-13).
MTC is a form of NEC and is well known to exhibit melanocytic
differentiation (2,14-19).
In the present case, the fact that both the MTC and melanoma
contained melanin pigment and were intermingled without the
formation of a front suggested an intimate relationship between the
two. In the only previous case of melanoma arising in MTC reported
so far, the two elements described by Hirokawa et al
(4) had a morphology and
distribution similar to those in the present case. On this basis,
it was concluded that in the present case, the MTC had undergone
transformation to melanoma.
The previous case also had a high Ki-67 labeling
index and extensive nodal metastasis. However, no detectable
recurrence or distant metastasis was evident up to 11 years after
surgery. The prognosis of the anaplastic variant of MTC composed of
large pleomorphic cells resembling ATC is better than that of ATC
(19). However, the patient of the
present study died due to brain metastasis three years after tumor
resection. BRAF mutation is present in ~50% of all melanomas
(20) and is linked to a higher
incidence of brain metastasis and shorter survival time (21). Despite the fact that the present
case had brain metastases and a poor outcome, BRAF mutation was not
detected. In the case reported by Hirokawa et al (4), BRAF mutations were not measured.
Thus, the prognosis of melanoma that has transformed from MTC still
appears to be uncertain.
In conclusion, the present case of melanoma
transformed from MTC is the second of its type to have been
reported. In comparison with the case reported previously, the
morphology of the present case was similar, but the outcome was
poorer. Therefore, it is not possible to make any definitive
conclusions regarding the prognosis of this disease until further
cases have accumulated.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data analysed during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
KY was the major contributor in the conception and
design of this case report and writing of the manuscript. KY, TT,
MH, TF, KS, SI, HO, EY, YS, SS and TY were contributors in the
acquisition of data. KY, MH, AM and TY analyzed and interpreted the
data. All authors read and approved the final manuscript. KY and MH
checked and approved the raw data.
Ethics approval and consent to
participate
All samples were collected after obtaining informed
consent from the patient and any personally identifiable
information was kept confidential in this study.
Patient consent for publication
The daughter of the patient provided written
informed consent for the publication of clinical data and
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lloyd RV, Osamura RY, Klöppel G and Rosai
J (eds): WHO Classification of Tumours of Endocrine Organs. IARC,
Lyon, pp108-113, 2017.
|
|
2
|
de Lima MA, Dias Medeiros J, Rodrigues Da
Cunha L, de Cássia Caldas Pessôa R, Silveira Tavares F, de Fátima
Borges M and Marinho EO: Cytological aspects of melanotic variant
of medullary thyroid carcinoma. Diagn Cytopathol. 24:206–208.
2001.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Eng HL and Chen WJ: Melanin-producing
medullary carcinoma of the thyroid gland. Arch Pathol Lab Med.
113:377–380. 1989.PubMed/NCBI
|
|
4
|
Hirokawa M, Miyauchi A, Otsuru M and Daa
T: Malignant melanoma arising in melanin-producing medullary
thyroid carcinoma. Int J Surg Case Rep. 20:118–122. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chantziantoniou N, Donnelly AD, Mukherjee
M, Boon ME and Austin RM: Inception and development of the
papanicolaou stain method. Acta Cytol. 61:266–280. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cibas ES and Ali SZ: The 2017 Bethesda
system for reporting thyroid cytopathology. Thyroid. 27:1341–1346.
2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hegerova L, Griebeler ML, Reynolds JP,
Henry MR and Gharib H: Metastasis to the thyroid gland: Report of a
large series from the Mayo clinic. Am J Clin Oncol. 38:338–342.
2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pusztaszeri M, Wang H, Cibas ES, Powers
CN, Bongiovanni M, Ali S, Khurana KK, Michaels PJ and Faquin WC:
Fine-needle aspiration biopsy of secondary neoplasms of the thyroid
gland: A multi-institutional study of 62 cases. Cancer Cytopathol.
123:19–29. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lack EE, Kim H and Reed K: Pigmented
(‘black’) extraadrenal paraganglioma. Am J Surg Pathol. 22:265–269.
1998.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bellezza G, Giansanti M, Cavaliere A and
Sidoni A: Pigmented ‘black’ pheochromocytoma of the adrenal gland:
A case report and review of the literature. Arch Pathl Lab Med.
128:e125–e128. 2004.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Klemm KM, Moran CA and Suster S: Pigmented
thymic carcinoids: A clinicopathological and immunohistochemical
study of two cases. Mod Pathol. 12:946–948. 1999.PubMed/NCBI
|
|
12
|
Eyden B, Pandit D and Banerjee SS:
Malignant melanoma with neuroendocrine differentiation: Clinical,
histological, immunohistochemical and ultrastructural featyres of
three cases. Histopathology. 47:402–409. 2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pilozzi E, Cacchi C, Di Napoli A, Pini B,
Duranti E, D'Andrilli A and Ruco L: Primary malignant tumour of the
lung with neuroendocrine and melanoma differentiation. Virchows
Arch. 459:239–243. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Karaarslan S, Nur Yurum F, Ebru Pala E,
Ortac R and Husnu Bugdayci M: The relationship of melanocytic
differentiation with prognostic markers in medullary thyroid
carcinomas. Pathol Res Pract. 211:356–360. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Marcus JN, Dise CA and LiVolci VA: Melanin
production in medullary thyroid carcinoma. Cancer. 49:2518–2526.
1982.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ikeda T, Satoh M, Azuma K, Sawada N and
Mori M: Medullary thyroid carcinoma with a paraganglioma-like
pattern and melanin production: A case report with ultrastructural
and immunohistochemical studies. Arch Pathol Lab Med. 122:555–558.
1998.PubMed/NCBI
|
|
17
|
Ben Romdhane K, Khattech R, Ben Othman M,
Gamoudi A, Ammar A and Cammoun M: Melanin production in medullary
thyroid carcinoma. Histpathology. 27:569–571. 1995.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mohamad I, Zainuddin N, Zawawi N and Naik
VR: Melanocytic variant of medullary thyroid carcinoma in a
previously treated papillary carcinoma patient. Ann Acad Med
Singap. 40:300–301. 2011.PubMed/NCBI
|
|
19
|
Mendelsohn G, Baylin SB, Binger SH, Wells
SA Jr and Eggleston JC: Anaplastic variants of medullary thyroid
carcinoma: A light-microscopic and immunohistochemical study. Am J
Surg Pathl. 4:333–341. 1980.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shtivelman E, Davies MA, Hwu P, Yang J,
Lotem M, Oren M, Flaherty KT and Fisher DE: Pathways and
therapeutic targets in melanoma. Oncotarget. 7:1701–1752.
2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Redmer T: Deciphering mechanisms of brain
metastasis in melanoma-the gist of the matter. Mol Cancer.
17(106)2018.PubMed/NCBI View Article : Google Scholar
|