Introduction
Primary acinic cell carcinoma (AcCC) of the breast
was first identified as an entity by Roncaroli et al
(1) in 1996 and since then 51 cases
have been reported in the literature (1-30).
It is a very rare subtype of the salivary gland-like
tumour group that occurs in breast tissue. This group comprises
three sub-categories: Tumours displaying pure myoepithelial cell
differentiation, such as myoepitheliomas; tumours with mixed
epithelial and myoepithelial cell differentiation, such as
pleomorphic adenoma, adenomyoepithelioma and adenoid cystic
carcinoma; and tumours showing pure epithelial cell
differentiation, such as acinic cell carcinoma, oncocytic
carcinoma, mucoepidermoid carcinoma and polymorphous adenocarcinoma
(31).
Breast AcCC shares many classical features with
salivary gland counterpart, with frequent expression of S-100,
lysozyme, salivary-type amylase, and alpha-1-antichimotrypsin
positive (A1-ACT) and periodic acid-Schiff (PAS) positivity in
addition (32). Specific risk
factors for AcCC of breast are still unknown. However, it is
primarily observed in women after the age of 40 years (the mean age
of presentation is around 55 years). Furthermore, based on its
similarity with the salivary gland counterpart, previous radiation
exposure as well as familial history of breast cancer could
represent an important risk factor for developing this rare type of
breast malignancy.
The first early case reports and reviews suggested a
relatively favourable prognosis for patients with AcCC, even though
this variant is often of the triple negative breast cancer (TNBC)
subtype on immunohistochemistry. However, reports of AcCC
recurrence cases have been more recently published (10,13,24,25).
Based on available data, the prognosis seems to be largely driven
by the presence of poorly differentiated components in these
tumours. Furthermore, in the majority of cases, patients affected
by AcCC received chemotherapy and radiotherapy as adjuvant
treatment, as the optimal therapeutic strategy has not yet been
established for this rare variant of breast cancer.
Here, we report an unusual case of high grade,
Estrogen Receptor (ER) negative AcCC associated with poor response
to anthracycline and taxane based neo-adjuvant chemotherapy (NACT),
a rapid disease progression within a short time from NACT
completion and a prolonged progression free survival (PFS) on
combination regimen of Carboplatin and Paclitaxel.
Case report
A 59-year-old woman presented to the Western General
Hospital, Edinburgh, UK, having noticed a lump in her right breast.
Urgent mammography identified a 30x22x30 mm ill-defined solid
lesion that corresponded anatomically with the mass clinically
palpated. A further mass inferior to this measuring 15x8 mm was
also identified. Multiple simple cysts were seen in both breasts
and a mammography performed two years ago as part of a screening
programme, showed that these opacities were long-standing benign
changes.
Tissue analysis of a breast core biopsy revealed an
invasive carcinoma grade 3 exhibiting areas of necrosis focally
associated with atypical acinar structures containing brightly
eosinophilic secretions reminiscent of microglandular adenosis
(MGA) at the peripheries of the tumour.
Immunohistochemically, the specimen including
collections of glandular differentiation areas showed a lack of
myoepithelial markers such as CK14 and p63. A stain for ER was
focal and weakly positive (Allred score 2) while negative for
Progesterone receptor (PR) and Human epidermal growth factor
receptor 2 (Her2). No carcinoma in-situ or lymphovascular
invasion was observed.
The patient received six cycles of NACT with 3
cycles of FEC-100 (Epirubicin 100 mg/m2 with
5-Fluorouracil 500 mg/m2 and Cyclophosphamide 500
mg/m2 every 21 days) followed by 3 cycles of Docetaxel
100 mg/m2. At the end of the 6 cycles a repeated
mammogram and ultrasound of the breast confirmed a large reduction
in volume of the main lesion with no significant residual mass seen
on scans. No nodes were identified in the right axilla. This was
considered a good radiological response to treatment.
Three weeks later, the patient proceeded to a right
breast wide local excision (WLE) and sentinel node biopsy (SNB).
Pathology report confirmed the previous diagnosis of invasive
carcinoma showing glandular cell-like features. Sentinel lymph
nodes were negative (0/2) for invasive carcinoma with no evidence
of fibrosis suggesting no previous infiltration from cancer (TNM
stage ypT2, ypN0 (sn)). Macroscopic analysis of the 40x50x40 mm
sample identified vaguely cream-coloured tissue with yellowish
flecks within posterior tissue.
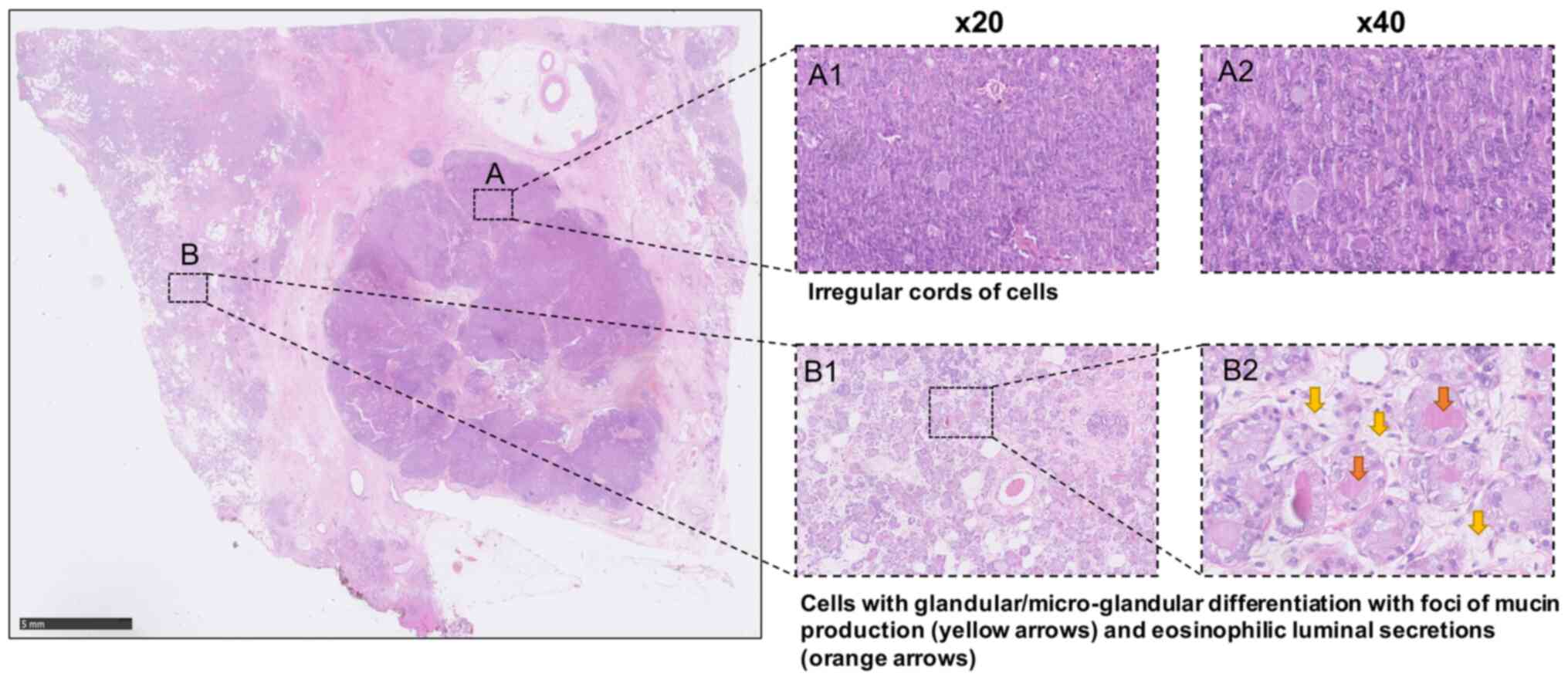
Microscopically, a circumscribed tumour focus
composed of solid and cohesive nests of cells displaying a more
spindle cell morphology in places as well as some glandular
differentiation with foci of mucin production was observed at the
medial margin. However, elsewhere, the tumour displayed a more
dispersed and infiltrative appearance and included irregular cords
of cells and some single cell infiltration and areas with
coalescence and proliferation of solid cell nests showing variable
eosinophilic granular cytoplasm. The areas with a microglandular
appearance presented densely eosinophilic luminal secretions and
formed some coalescing sheets, configuring the typical features of
MGA. No conventional ductal carcinoma in situ was
identified. Infiltrative elements with a more solid and nested
appearance were seen within <1 mm from the tumour margins. There
was a variable nuclear pleomorphism up to nuclear score 3 and
patchy high mitotic rate giving a mitotic score of 3. Overall, the
acinar score was of 2. These appearances were in keeping with
features of grade 3 disease (Fig.
1).
Despite some variation in tumour cellularity and
focal fibrosis compared to the initial biopsy analysis, there was
no obvious vascular fibrosis associated with any significant
decrease in cellularity present to indicate any definite response
to NACT.
Further analysis using immunohistochemistry showed
that myoepithelial cell markers (CK14, CK5/6, p63, SMA, Calponin)
were negative. The tumour showed immunopositivity for S100,
epithelial membrane antigen (EMA), amylase and gross cystic disease
fluid protein 15 (GCDFP-15), as well as focal positivity to CD68.
Neuroendocrine markers, CD56, chromogranin and synaptophysin were
all negative. Tumour cells were negative for ER and HER2 but
positive for PR with variable staining. Overall estimated PR score
was 4 but was up to 6 in some foci. All margins were found to be
positive of residual invasive carcinoma. Re-excision of all margins
revealed residual tumour elements, leading to an increase in tumour
volume. No therapeutic response to NACT was observed. The patient
subsequently underwent right mastectomy and the tissue analysis
reported further residual disease extending beyond all original
margins with similar appearances to the tumour in previous
specimens. The whole size of the lesion was estimated to be at
least 71 mm in the largest diameter.
After surgery, the patient proceeded with chest wall
adjuvant radiotherapy as per standard protocol. No further
treatment was offered at that time and she received annual surgical
follow up and contralateral annual mammography.
Unfortunately, 14 months later, the patient
presented with upper abdominal distention and pain. Gastroscopy
revealed Linitis Plastica. Peritoneal and gastric biopsies were
taken and confirmed infiltration by a carcinoma with similar
features to the primary breast cancer. Immunohistochemistry also
revealed an identical profile to that seen in the first breast core
biopsy with neoplastic cells showing immunopositivity for CK7,
GATA3, EMA, amylase, E-Cadherin, GCDFP and S-100 and
immunonegativity for ER, CK20 and CDX2. PR status was regarded as
negative since the cells displayed patchy aberrant cytoplasmic
staining. The patient had full staging with a CT scan of chest,
abdomen and pelvis (CAP) and a bone scan. CT CAP showed extensive
peritoneal and serosal disease with biliary tree dilatation,
pleural effusions and ascites. The bone scan did not reveal any
bone metastases.
In view of disease progression, performance status
(PS) deterioration and highly deranged liver enzymes (LFTs), the
patient was started initially on weekly Paclitaxel as first line
chemotherapy. After 2 weeks, all parameters including PS and LFTs
improved and weekly Carboplatin AUC2 was added to Paclitaxel.
Overall, the patient achieved an impressive response for 30 months
with several breaks in chemotherapy over the time, not due to
toxicities but for treatment holiday purposes. This is interesting
given that the patient had no response to neo-adjuvant
Docetaxel.
Two and a half years after the diagnosis of
metastatic AcCC, and while on Paclitaxel/Carboplatin and having an
excellent response with regards to her visceral disease, the
patient presented with excruciating headaches and a CT scan of the
head was performed. The CT showed no evidence of intracranial
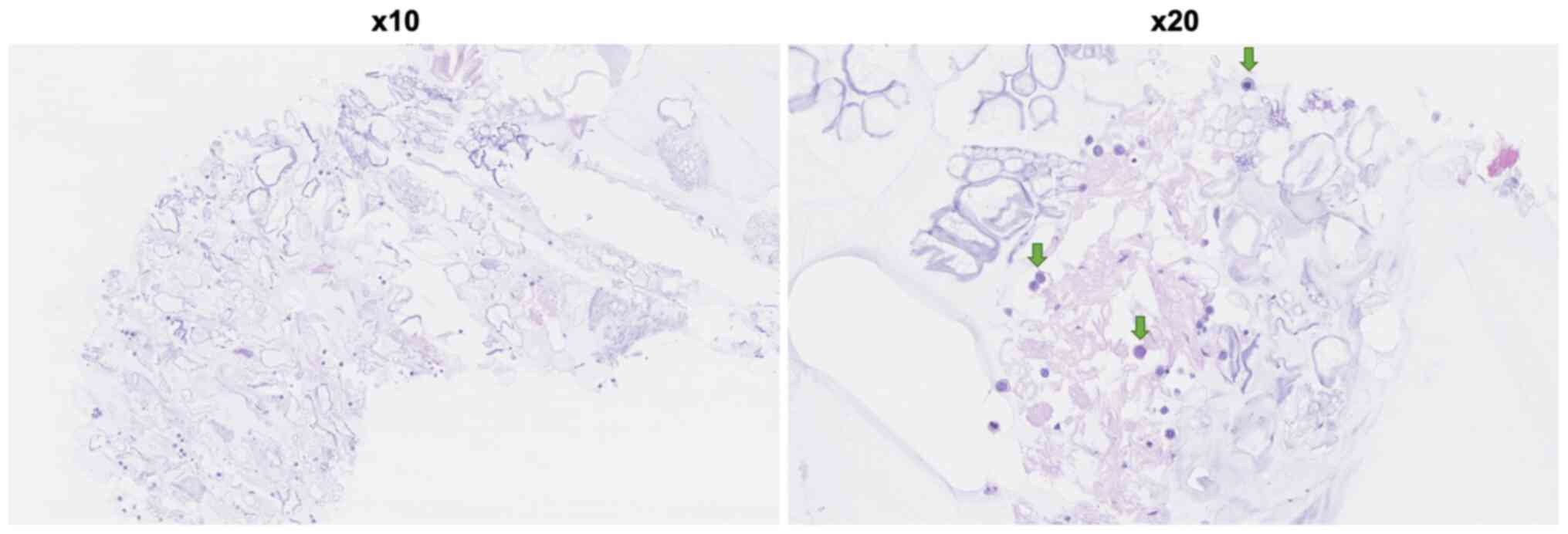
metastases. Subsequently a head MRI was requested and a lumbar
puncture was performed. The MRI head showed radiological evidence
of leptomeningeal carcinomatosis (Fig.
2). The lumbar puncture showed malignant cells in the
cerebrospinal fluid. Cytospin and cell block preparations revealed
pleomorphic cells as both adhesive and individual groups, which
immunoreacted with cytokeratin 7 and GATA3, but were negative for
cytokeratin 20. The patient's PS was very poor and decision against
further treatment was made. At that time, she was transferred to a
hospice setting where she died 6 weeks later.
Discussion
Breast and salivary glands are both composed of
tubuloacinar exocrine glands, and as such they share similar
cytology and morphology to normal healthy tissue. Therefore, in the
event of disease they may exhibit similar characteristics.
We have presented a case report of breast cancer
displaying characteristic salivary gland-like features. The
association of a microglandular and a solid growth pattern, the
presence of bright eosinophilic cytoplasmic granularity and the
immuno-profile are most in keeping with acinic cell-like carcinoma,
according to the criteria outlined by Roncaroli (1) and Damiani (3). The main immunohistochemical features
reported in the literature on breast AcCC in a subset of cases with
detailed immunohistochemical profile description (n=36) are
summarised in Table I. The presence
of coarse bright eosinophilic cytoplasmic granules in breast
epithelium is rare. It has mostly been described in MGA lesions and
in AcCC carcinoma (25). Both
present a similar morphology, positivity for S-100 and absence of
myoeptithelial markers. However, they can be differentiated by
immunohistochemistry since AcCC shows positivity for EMA, lysozyme,
alpha amylase and A1-ACT, whereas MGA does not. Additionally, MGA
typically features a basal lamina that is absent in AcCC. MGA is
known to be a benign breast lesion but can be associated with
breast carcinoma up to 27% (33,34).
Its relationship with AcCC remains unclear, but some authors have
postulated that MGA may be a precursor lesion of AcCC (35). In the present case, it is difficult
to be certain whether some of the acinar areas may represent
pre-existing MGA.
 | Table ISummary of the immunohistochemical
features reported in the literature on breast AcCC. |
Table I
Summary of the immunohistochemical
features reported in the literature on breast AcCC.
| Immunohistochemical
features of breast AcCC | Positivity, % (number
of cases/total cases) (present case included) | Feature present
(+)/Absent (-) in the present case |
|---|
| PAS (diastase
resistant) | 100 (24/24) | + |
| S-100 | 87 (27/31) | + |
| Lysozyme | 96 (23/24) | + |
| Epithelial membrane
antigen | 100 (21/21) | + |
| Amylase | 95 (19/20) | + |
| α-1-Antitrypsin | 54 (6/11) | NR |
|
α-1-Antichymotrypsin | 78 (11/14) | NR |
| Cytokeratin 7 | 100 (9/9) | NR |
| Neuroendocrine
markers (synaptophysin) | 15 (2/13) | - |
| Gross cystic disease
fluid protein 15 | 50 (9/18) | + |
| Estrogen
receptor | 13 (4/31) | - |
| Progesterone
receptor | 22 (7/31) | + |
| Androgen
receptor | 11 (1/9) | NR |
| HER2 | 0 (0/25) | - |
| Triple-negative
carcinoma | 72 (18/25) | - |
The present case showed immunopositivity for PAS,
S-100, lysozyme and EMA, as in almost all AcCC described in
literature, but also for GCDFP-15, a marker of apocrine
differentiation expressed in one-half of the cases. Moreover, the
current case displayed positivity of PR that was observed in only
20% of reported cases (Table I). To
date, as summarized in Table II,
nine cases of hormonal receptor positive AcCC have been described.
The mean age at diagnosis was 53 years with a single case involving
a male patient (2). Of these, only
one patient experienced local recurrence of disease and,
subsequently, lymph node metastases after radical surgery, adjuvant
radiotherapy and systemic therapies (25). Similarly to our case, tumour
exhibited parallel cytological spectrum where the solid and nested
infiltrative areas had all high-grade features of triple-negative
breast carcinomas whereas the well-differentiated acinar-like
components displayed lower mitotic rate and nuclear pleomorphism
score (25).
 | Table IIKey characteristics of the nine cases
of hormonal receptor-positive acinic cell carcinoma reported in the
literature. |
Table II
Key characteristics of the nine cases
of hormonal receptor-positive acinic cell carcinoma reported in the
literature.
| Case no. | First author,
year | Sex/age, years | ER status | PR status | AR status | HER2 status |
Recurrence/metastasis | Overall
survivala, months | (Refs.) |
|---|
| 1 | Shimao et
al, 1998 | M/23 | + | NR | NR | NR | No | 34 | (2) |
| 2 | Hirokawa et
al, 2002 | F/61 | + | NR | NR | NR | No | 24 | (8) |
| 3 | Hirokawa et
al, 2002 | F/59 | + | NR | NR | NR | No | 84 | (8) |
| 4 | Stolnicu et
al, 2010 | F/79 | - | + | + | - | No | 9 | (26) |
| 5 | Huo et al,
2011 | F/40 | + | + | NR | - | No | 12 | (13) |
| 6 | Sakuma et
al, 2013 | F/61 | + | + | NR | - | No | NR | (15) |
| 7 | Falleti et
al, 2013 | F/58 | - | + | - | - | No | 10 | (21) |
| 8 | Conlon et
al, 2016 | F/47 | + | + | - | - | Yes | 72 | (25) |
| 9 | Li et al,
2017 | F/52 | + | + | NR | - | No | 3 | (29) |
The majority of AcCC breast cancer cases reported in
the literature are triple negative tumours (36). However, recently, some authors
described rare cases of AcCC showing positivity for both estrogen
and progesterone receptors (Table
II).
In the present case, the biopsy sample showed a very
weak immunoreactivity for ER (Allred score 2) while the tissue from
WLE specimen following NACT displayed no signal for this receptor.
On the contrary, PR result was negative at the analysis of core
biopsy but positive with variable staining (Allred score 4) when
assessed on the WLE specimen. This could be explained by the
greater accuracy of the pathology assessment on a WLE specimen
rather than the biopsy. A change of the receptor status could also
be due to the chemotherapy, and perhaps this, rather than accuracy
of sample analysis, could underlie the difference in the expression
of HRs before and after NACT. Interestingly, biopsies of the
recurrent disease exhibited similar features to the diagnostic
biopsy rather than the surgical specimen.
Although the majority of AcCC are of the triple
negative subtype, early case reports and the first few reviews of
breast AcCC cases reported it to be a tumour with a mostly
favourable outcome. However, more recent reviews have identified
that this is not always the case, and suggest that there is a
sub-group of patients with higher-grade tumours who have a very
poor outcome (6,10).
As reported in Table
III, of the 52 cases available in the literature (including the
present case), nine patients experienced complications following
adjuvant treatment, such as local recurrence, metastases, or death.
Distant metastases have been reported in five cases, and death
consequent to tumour progression occurred in three patients.
However, follow up period is not available for all patients and for
some of them it is probably too short to detect local or distant
recurrences. It is noteworthy that most of the patients have been
treated with chemotherapy and radiotherapy in addition to
surgery.
 | Table IIISummary of the nine reported cases of
acinic cell carcinoma associated with recurrent or metastatic
disease. |
Table III
Summary of the nine reported cases of
acinic cell carcinoma associated with recurrent or metastatic
disease.
| Case no. | First author,
year | Sex/age, years | Breast
affected | Tumour size,
mm | ER status | PR status | HER2 status | No. of LN
involved/No. of LN removed | Neoadjuvant
treatment | Follow-up time,
months |
Recurrence/metastases | Death | (Refs.) |
|---|
| 1 | Damiani et
al, 2000 | F/63 | L | 50 | - | - | - | No biopsy | BCS, contralateral
mastectomy for IDC | 48 | Local recurrence 48
months after diagnosis → radically resected | No | (3) |
| 2 | Coyne and Dervan,
2002 | F/49 | R | 20 | NR | NR | NR | 2/11 | NACT (Adriamycin,
CPA, MTX, 5FU) → Mastectomy | 36 | Liver
metastases | Yes | (6) |
| 3 | Peintinger et
al, 2004 | F/36 | R | 35 | - | - | - | 0/15 | BCS + ACT + RT | 120 | Lung metastases 96
months after diagnosis → radically resected | No | (10) |
| 4 | Huo et al,
2011 | F/30 | R | 26 | - | - | - | 2/33 | BCS → ACT + RT | 34 | Bone
metastases | Yes | (13) |
| 5 | Guerini-Rocco et
al, 2015 | F/70 | NR | 14 | - | - | - | NR | NR | NR | Recurrence | NR | (24) |
| 6 | Guerini-Rocco et
al, 2015 | F/35 | NR | 18 | - | - | - | 2/22 | NR | 72 | Recurrence | No | (24) |
| 7 | Kawai et al,
2016 | F/49 | R | 35 | - | - | - | NR | Mastectomy → ACT
(UFT) | NR | Local recurrence 8
months after surgery | NR | (27) |
| 8 | Conlon et
al, 2016 | F/47 | R | 23 | + | + | - | 1/18 | BCS → ACT + RT +
ET | 72 | Local recurrence 48
months after surgery (radically resected + ACT) → lymph node
metastases within 1 year | No | (25) |
| 9 | Present case | F/59 | R | 71 | + | + | - | 0/2 | NACT (3 cycles FEC
→ 3 cycles Docetaxel) → Mastectomy + RT | 49 | Peritoneal
metastases 14 months after surgery | Yes | |
The current case is the third of AcCC in which
cancer-related death was registered. This unfavorable outcome could
be related to the presence of a grade 3 large cancer and the triple
negative nature of the tumour.
To date, there is a lack of consensus in terms of
the appropriate systemic treatment and the effect of chemotherapy
on tumour cells in breast AcCC is not currently well known. There
are only five reports in the literature of patients with AcCC that
have received NACT (3,6,13,19,25).
Comparative descriptions of specimens pre- and post-NACT have been
performed in 4 out of the 5 cases (3,6,13,19,25).
In these series, the response to NACT has not been specified,
except in the case published by Winkler et al (19) in which a lack of chemotherapeutic
effect was described in surgical specimen as well as at MRI
imaging, after four cycles of Adriamycin followed by four cycles of
Paclitaxel. As discussed above no response to anthracycline and
taxane based NACT was observed in this case either.
Furthermore, immunohistochemically, the omnipresence
of coarse bright eosinophilic cytoplasmic granules confirms the
fact that these are features of the tumour and not a chemotherapy
effect. Interestingly, a significant increase in presence of these
granules was observed after chemotherapy in three of reported cases
(6,19,25).
Conversely, in all cases where intraductal carcinoma (IDC) was
diagnosed with concurrent AcCC and in which NACT was given, the
solid poorly differentiated cells of the IDC, which were
predominant in the pretreatment core biopsy specimen, were residual
or absent after treatment. This leads the authors to postulate a
strong chemosensitivity of solid poorly differentiated carcinoma
cells, and a possible chemoresistance of the microglandular
component of these tumours.
In our case, the presence of eosinophilic granules
was also identified both in the breast biopsy and the mastectomy
specimen without any cellularity decrease seen after therapy.
Moreover, the apparent decrease in tumour volume observed at
follow-up mammogram following NACT could be due to clearance of the
infiltrative solid nests of cells, against which chemotherapy may
be more effective at cell kill than against microglandular
areas.
Finally, another point of interest is the prolonged
response (30 months) that the patient achieved by receiving
Carboplatin in combination with Paclitaxel as first line
chemotherapy, despite the marked chemoresistance of the tumour that
has been previously observed in neo-adjuvant setting.
Since ER and PR status was regarded as negative at
the time of peritoneal biopsy, the reason of this impressive and
unexpected response could lie in the well-known sensitivity of the
TNBC subtype to the Platinum salts and Taxanes (37).
In conclusion, primary AcCC is a rare type of breast
carcinoma and in the majority of cases is classified as triple
negative subtype. There is a growing body of evidence that AcCC is
not always associated with a good prognosis, as believed until now,
and further investigations are required to identify predictors of
poor outcome. Here, we have presented the case of a patient with ER
negative AcCC with no response to conventional NACT. Despite being
triple negative, AcCC are not considered as very chemosensitive
tumours. However, the combination of Carboplatin and Paclitaxel
might offer a therapeutic benefit and significantly prolong the
progression free survival as shown in this case. Our case also
highlights the importance of careful interpretation of follow-up
imaging, as an apparent positive response to treatment may not
always be a true representation of disease.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the current
study.
Authors' contributions
LS, GW, AT, AD and OO conducted the literature
review and wrote/edited the final manuscript. OO provided all
clinical information relevant to this case. OO and AT confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this case report before her death.
Additionally, the patient donated tissue and blood samples for
translational research.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Roncaroli F, Lamovec J, Zidar A and Eusebi
V: Acinic cell-like carcinoma of the breast. Virchows Arch.
429:69–74. 1996.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Shimao K, Haga S, Shimizu T, Imamura H,
Watanabe O, Kinoshita J, Nagumo H, Utada Y, Okabe T, Kajiwara T, et
al: Acinic cell adenocarcinoma arising in the breast of a male: A
clinicopathological, immunohistological and ultrastructural study.
Breast Cancer. 5:77–81. 1998.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Damiani S, Pasquinelli G, Lamovec J,
Peterse JL and Eusebi V: Acinic cell carcinoma of the breast: An
immunohistochemical and ultrastructural study. Virchows Arch.
437:74–81. 2000.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Schmitt FC, Riberio CA, Alvarenga S and
Lopes JM: Primary acinic cell-like carcinoma of the breast-a
variant with good prognosis? Histopathology. 36:286–289.
2000.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chang ED, Lee EJ, Lee AW, Kim JS and Kang
CS: Primary acinic cell carcinoma of the breast: A case report with
an immunohistochemical and ultrastructural studies. J Breast
Cancer. 14:160–164. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Coyne JD and Dervan PA: Primary acinic
cell carcinoma of the breast. J Clin Pathol. 55:545–547.
2002.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Elster EA, Markusic J, Ball R, Soballe P,
Henry M, Louie A and Clare S: Primary acinic cell carcinoma of the
breast. Am Surg. 68:993–995. 2002.PubMed/NCBI
|
|
8
|
Hirokawa M, Sugihara K, Sai T, Monobe Y,
Kudo H, Sano N and Sano T: Secretory carcinoma of the breast: A
tumour analogous to salivary gland acinic cell carcinoma?
Histopathology. 40:223–229. 2002.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kahn R, Holtveg H, Nissen F and Holck S:
Are acinic cell carcinoma and microglandular carcinoma of the
breast related lesions? Histopathology. 42:195–196. 2003.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Peintinger F, Leibl S, Reitsamer R and
Moinfar F: Primary acinic cell carcinoma of the breast: A case
report with long-term follow-up and review of the literature.
Histopathology. 45:645–648. 2004.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kinkor Z and Skálová A: Acinic cell-like
differentiation in invasive ductal carcinoma and in ductal
hyperplasia of the breast-report of two cases. Cesk Patol.
41:29–33. 2005.PubMed/NCBI(In Czech).
|
|
12
|
Tanahashi C, Yabuki S, Akamine N, Yatabe Y
and Ichihara S: Pure acinic cell carcinoma of the breast in an
80-year-old Japanese woman. Pathol Int. 57:43–46. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Huo L, Bell D, Qiu H, Sahin A, Wu Y and
Sneige N: Paneth cell-like eosinophilic cytoplasmic granules in
breast carcinoma. Ann Diagn Pathol. 15:84–92. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Choh CT, Komar V and Courtney SP: Primary
acinic cell carcinoma of the breast: A rare lesion with good
prognosis. Breast J. 18:610–611. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sakuma T, Mimura A, Tanigawa N and
Takamizu R: Fine needle aspiration cytology of acinic cell
carcinoma of the breast. Cytopathology. 24:403–405. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhao Y, Li W, Lang R, Yang Y, Gao X, Zheng
Y, Zhang C, Fu X and Fu L: Primary acinic cell carcinoma of the
breast: A case report and review of the literature. Int J Surg
Pathol. 22:177–181. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shingu K, Ito T, Kaneko G and Itoh N:
Primary acinic cell carcinoma of the breast: A clinicopathological
and immunohistochemical study. Case Rep Oncol Med.
2013(372947)2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Osako T, Takeuchi K, Horii R, Iwase T and
Akiyama F: Secretory carcinoma of the breast and its
histopathological mimics: Value of markers for differential
diagnosis. Histopathology. 63:509–519. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Winkler N, Morrell G and Factor R:
Invasive carcinoma with acinic cell-like features of the breast.
Breast J. 19:334–335. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ripamonti CB, Colombo M, Mondini P,
Siranoush M, Peissel B, Bernard L, Radice P and Carcangiu ML: First
description of an acinic cell carcinoma of the breast in a BRCA1
mutation carrier: A case report. BMC Cancer. 13(46)2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Falleti J, Coletti G, Rispoli E, Scarabeo
F, Cervasio M, Tornillo L, Pettinato G and Insabato L: Acinic cell
carcinoma of the breast arising in microglandular adenosis. Case
Rep Pathol. 2013(736048)2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Limite G, Di Micco R, Esposito E, Sollazzo
V, Cervotti M, Pettinato G, Varone V, Benassai G, Monda A, Luglio
G, et al: The first case of acinic cell carcinoma of the breast
within a fibroadenoma: Case report. Int J Surg. 12 (Suppl
1):S232–S235. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Piscuoglio S, Hodi Z, Katabi N,
Guerini-Rocco E, Macedo GS, Ng CK, Edelweiss M, De Mattos-Arruda L,
Wen HY, Rakha EA, et al: Are acinic cell carcinomas of the breast
and salivary glands distinct diseases? Histopathology. 67:529–537.
2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Guerini-Rocco E, Hodi Z, Piscuogloio S, Ng
CK, Rakha EA, Schultheis AM, Marchiò C, da Cruz Paula A, De Filippo
MR, Martelotto LG, et al: The repertoire of somatic genetic
alterations of acinic cell carcinomas of the breast: An
exploratory, hypothesis-generating study. J Pathol. 237:166–178.
2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Conlon N, Sadri N, Corben AD and Tan LK:
Acinic cell carcinoma of breast: Morphologic and
immunohistochemical review of a rare breast cancer subtype. Hum
Pathol. 51:16–24. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Stolnicu S, Dohan M, Preda O, Goez E,
Cabrero IA and Nogales FF: Primary acinic cell carcinoma of the
breast associated with an intraductal acinic cell component.
Patología. 48:204–207. 2010.
|
|
27
|
Kawai H, Sugimoto R, Iga N, Ikeda H,
Yoshida R, Waki N, Ishizaki M, Nishi H and Yamashita K: A case of
primary acinic cell carcinoma (ACC) of the breast. Gan To Kagaku
Ryoho. 43:2019–2021. 2016.PubMed/NCBI(In Japanese).
|
|
28
|
Sherwell-Cabello S, Maffuz-Aziz A,
Rios-Luna NP, Bautista-Piña V and Rodríguez-Cuevas S: Salivary
gland-like breast carcinomas: An infrequent disease. Pathol Res
Pract. 212:1034–1038. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Li H, Wang F, Shen P and Zhou F: Pure
acinic cell carcinoma of the breast: A case report and literature
review. Medicine (Baltimore). 96(e8866)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sen R, Bhutani N, Kamboj J and Dahiya S:
Primary acinic cell carcinoma of the breast: A case report with a
clinicopathological and immunohistochemical study of a rare breast
cancer subtype. Ann Med Surg (Lond). 35:137–140. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Tavassoli FA and Eusebi V (eds): Tumors of
the Mammary Gland. American Registry of Pathology, Washington, DC,
pp212-260, 2009.
|
|
32
|
Foschini MP, Morandi L, Asioli S, Giove G,
Corradini AG and Eusebi V: The morphological spectrum of salivary
gland type tumours of the breast. Pathology. 49:215–227.
2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Salarieh A and Sneige N: Breast carcinoma
arising in microglandular adenosis: A review of the literature.
Arch Pathol Lab Med. 131:1397–1399. 2007.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Khalifeh IM, Albarracin C, Diaz LK,
Symmans FW, Edgerton ME, Hwang RF and Sneige N: Clinical,
histopathologic, and immunohistochemical features of microglandular
adenosis and transition into in situ and invasive carcinoma. Am J
Surg Pathol. 32:544–552. 2008.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Shui R and Yang W: Invasive breast
carcinoma arising in microglandular adenosis: A case report and
review of the literature. Breast J. 15:653–656. 2009.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lakhani SR, Ellis IO, Schnitt SJ, Tan PH
and van de Vijver MJ (eds): WHO classification of tumors of the
breast. IARC, Lyon, 2012.
|
|
37
|
Curigliano G: Addition of platinum salts
to neoadjuvant chemotherapy in triple-negative breast cancer: A new
standard of care? Lancet Oncol. 19:434–436. 2018.PubMed/NCBI View Article : Google Scholar
|