Introduction
Gastric carcinoma is one of the most common types of
cancer and the main causes of cancer-related mortality worldwide
(1). According to the Japanese
Gastric Cancer Classification, gastric adenocarcinoma is
histologically subdivided into differentiated and undifferentiated
types, and patients with undifferentiated tumors generally have a
poorer prognosis (2-4).
Diffuse types of gastric carcinoma, consisting of infiltration by
single cells or small groups of tumor cells, correspond to poorly
differentiated gastric carcinoma in the World Health Organization
classification and include heterogeneous subtypes, such as signet
ring cell carcinoma (SRCC) and non-SRCC (NSRCC) (5). The prevalence of poorly
differentiated gastric carcinoma is higher compared with that of
well-differentiated gastric carcinoma (6). Furthermore, poorly differentiated
carcinoma and SRCC have a worse prognosis than differentiated
carcinoma (7,8).
Peroxisome proliferator-activated receptors (PPARs)
are nuclear hormone receptors that were initially described as
molecular targets for compounds that induce peroxisomal
proliferation (9). PPARs regulate
the transcription of several genes involved in lipid metabolism,
energy utilization and storage (10), and consist of three subtypes
(PPAR-α, PPAR-β/δ and PPAR-γ) (11,12).
These subtypes may be partially distinguished by their tissue
distribution, ligands and target specificities (13-16).
PPAR-α is predominantly expressed in tissues that catabolize large
amounts of fatty acids, such as the liver, kidneys, and heart
(17). Additionally, PPAR-α
regulates important cellular functions, including cell
proliferation, differentiation, energy metabolism, oxidative
stress, inflammation, circadian rhythm, immune responses and cell
differentiation. However, the relationship between the expression
of PPAR-α and the histological type of gastric carcinoma currently
remains unclear. Furthermore, the biological function of PPAR-α has
not yet been elucidated, and the role of PPAR-α expression in
gastric carcinoma has not been investigated to date.
Therefore, further studies are needed to clarify
these controversial findings and to fully elucidate the function of
PPAR-α. The aim of the present study was to examine the
associations between PPAR-α expression and clinicopathological
factors in gastric carcinoma and assess the usefulness of PPAR-α as
a new prognostic marker.
Materials and methods
Clinical samples
A total of 57 patients (42 men and 15 women, with a
mean age of 72.1±9.0 years; range, 50-91 years) who were diagnosed
with gastric carcinoma at Kagawa University Hospital (Kagawa,
Japan) between April 2012 and March 2014 were examined in the
present study. Clinicopathological factors were classified
according to sex, age, histological type, lymphatic invasion,
venous invasion, lymph node metastasis, depth of invasion and stage
based on the 15th Edition of Japanese Classification of Gastric
Carcinoma (18). Samples obtained
from surgical resection for curative treatment included 7 from
endoscopic submucosal dissection, 45 from partial gastrectomy and 5
from total gastrectomy. There was one case of distant metastasis.
All clinical samples were provided after obtaining written informed
consent from the patients. The present study was conducted with the
approval of the Institutional Research Ethics Committee of the
Kagawa Prefectural University of Health Sciences (Kagawa, Japan;
approval no. 215).
Immunohistochemistry
Immunohistochemistry was performed as previously
described (19). Briefly,
formalin-fixed paraffin-embedded tissues were cut into 4-µm
sections. The sections were deparaffined in xylene (Muto Pure
Chemicals Co., Ltd.) and rehydrated in ethanol (Muto Pure Chemicals
Co., Ltd.). Antigen retrieval was conducted by autoclave heating at
120˚C for 15 min in 0.01 M citrate buffer (pH 6.0) containing 38
mg/dl citric acid monohydrate and 241 mg/dl trisodium citrate
dehydrate (Wako Pure Chemical Industries, Ltd.). Endogenous
peroxidase activity was blocked using 3% hydrogen peroxide at room
temperature for 10 min (Wako Pure Chemical Industries, Ltd.) and
non-specific antibody binding using 0.1% skimmed milk at room
temperature for 10 min (Wako Pure Chemical Industries, Ltd.). An
HRP-labeled monoclonal anti-PPAR-α antibody (cat. no. sc-398394,
Santa Cruz Biotechnology, Inc.) was used for the primary antibody
reaction. The sections were incubated with primary antibody diluted
to 1:200 in PBS at room temperature for 2 h, rinsed three times
with PBS and stained with 3,3'-diaminobenzidine tetrahydrochloride
substrate (Nichirei Biosciences). The sections were then
counterstained with Meyer's hematoxylin, dehydrated,
transparentized with xylene, and mounted in malinol. The expression
of PPAR-α in cells was examined under a light microscope (BX53;
Olympus Corporation) at a magnification of x200. The classification
of PPAR-α expression was based on the criteria of Lin et al
(20). Nuclear PPAR-α expression
was assessed using the following scores: Unstained, 0; <25%
positive cells, 1+; 25-50% positive cells, 2+; 50-75% positive
cells, 3+; and >75% positive cells, 4+. PPAR-α expression levels
were measured in the negative (0, 1+ and 2+) and positive (3+ and
4+) groups.
Statistical analysis
The associations between immunohistochemical
staining and clinicopathological factors were examined using
Pearson's χ2 test or Fisher's exact test. P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were performed using SPSS 24.0 software (IBM
Corp.).
Results
Clinicopathological
characteristics
The characteristics of patients with gastric
carcinoma are summarized in Table
I. There were 57 patients (42 men and 15 women) with a mean age
of 72.1±9.0 years. There were 30 cases of differentiated carcinoma
and 27 of undifferentiated carcinoma (20 of poorly differentiated
carcinoma and 7 of SRCC). Lymphatic invasion was positive in 41
cases and negative in 16, venous invasion was positive in 36 cases
and negative in 21, and lymph node metastasis was positive in 22
cases. As regards the depth of invasion, T1a was detected in 6
cases, T1b in 18, T2 in 7, T3 in 14, T4a in 11 and T4b in 1 case.
As regards disease stage, 26 cases were stage I, 5 were stage IIA,
9 were stage IIB, 16 were stage III and 1 was stage IVA.
 | Table IClinical characteristics of 57
patients with gastric adenocarcinoma. |
Table I
Clinical characteristics of 57
patients with gastric adenocarcinoma.
| Parameters | Patients, n (%) |
|---|
| Sex | |
|
Male | 42 (73.7) |
|
Female | 15 (26.3) |
| Age (mean ± standard
deviation) | 72.1±9.0 |
| Histological
type | |
|
Differentiated
carcinoma | 30 (52.6) |
|
Undifferentiated
carcinoma | 27 (47.4) |
| Lymphatic
invasion | |
|
Positive | 41 (71.9) |
|
Negative | 16 (28.1) |
| Venous invasion | |
|
Positive | 36 (63.2) |
|
Negative | 21 (36.8) |
| Lymph node
metastasis | |
|
Positive | 22 (38.6) |
|
Negative | 35 (61.4) |
| Depth of
invasion | |
|
T1a | 6 (10.5) |
|
T1b | 18 (31.6) |
|
T2 | 7 (12.3) |
|
T3 | 14 (24.6) |
|
T4a | 11 (19.3) |
|
T4b | 1 (1.7) |
| Stage | |
|
I | 26 (45.6) |
|
IIA | 5 (8.8) |
|
IIB | 9 (15.8) |
|
III | 16 (28.1) |
|
IVA | 1 (1.7) |
PPAR-α expression in gastric
carcinoma
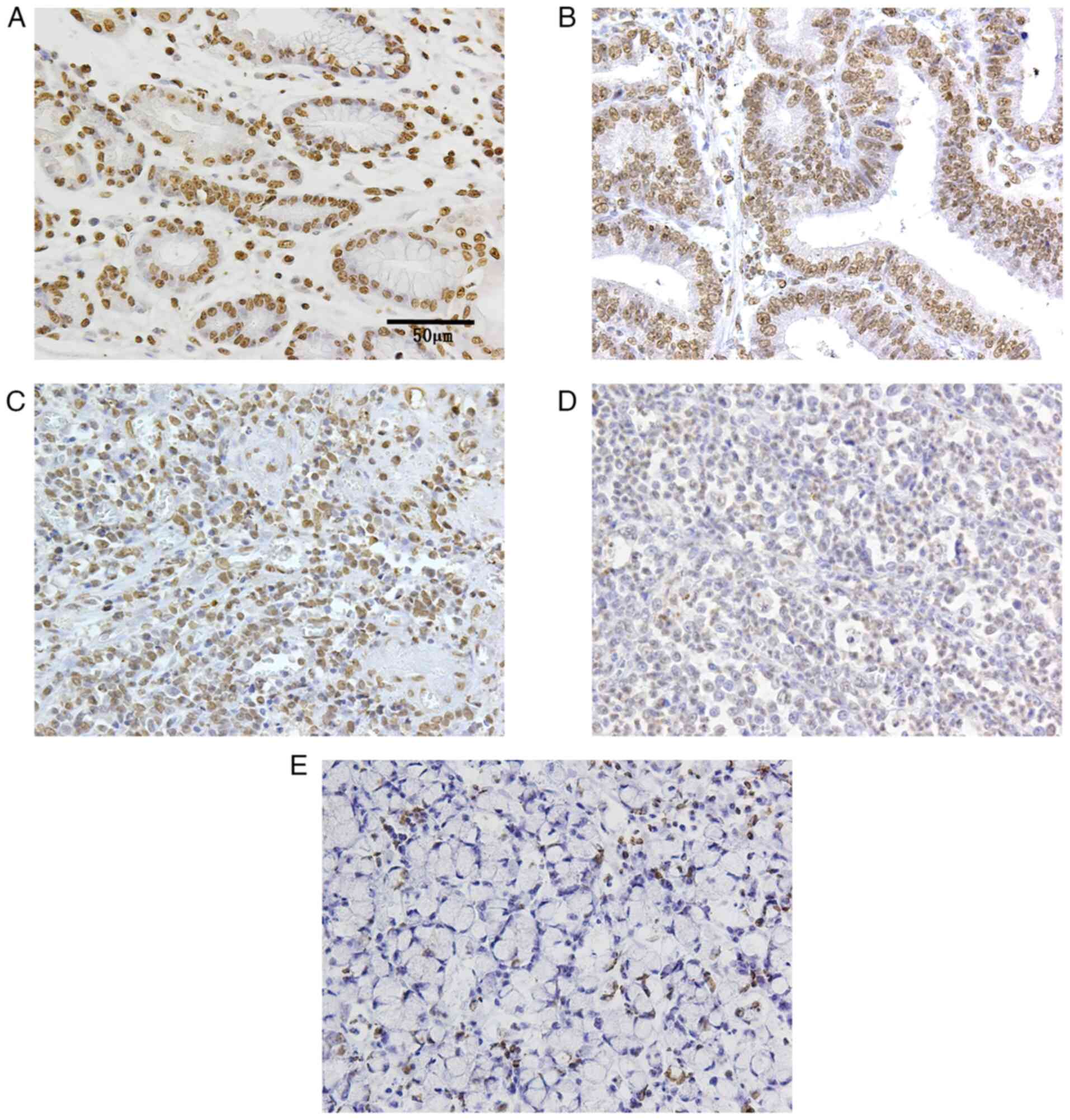
The expression of PPAR-α was mainly localized to the
nucleus and was present in all normal epithelial tissues (Fig. 1A). In terms of PPAR-α expression
and clinicopathological factors, it was expressed in all cases of
differentiated carcinoma (30/30, 100%; Fig. 1B), while positive and negative
expression was observed in cases of undifferentiated carcinoma
(14/27, 51.9%). In terms of PPAR-α expression and histological
subtype, PPAR-α expression was significantly higher in
differentiated carcinoma compared with undifferentiated carcinoma
(P<0.01; Table II).
Undifferentiated carcinoma included poorly differentiated carcinoma
and SRCC, and PPAR-α expression differed significantly between
poorly differentiated carcinoma (both positive and negative: 14/20,
70%; Fig. 1C and D) and SRCC (not expressed: 0/7, 0%;
Fig. 1E and Table III; P<0.01). PPAR-α expression
was not significantly affected by sex, age, lymphatic invasion,
venous invasion, lymph node metastasis, depth of invasion or stage
(Table II).
 | Table IIRelationship between PPAR-α
expression and clinicopathological parameters of gastric
carcinoma. |
Table II
Relationship between PPAR-α
expression and clinicopathological parameters of gastric
carcinoma.
| | PPAR-α
expression | |
|---|
| Parameters | Number of
cases | (-) | (+) | P-value |
|---|
| Sex | | | | 0.082 |
|
Male | 42 | 7 | 35 | |
|
Female | 15 | 6 | 9 | |
| Age, years | | | | 0.172 |
|
<72 | 27 | 4 | 23 | |
|
≥72 | 30 | 9 | 21 | |
| Histological
type | | | |
<0.010a |
|
Differentiated
carcinoma | 30 | 0 | 30 | |
|
Undifferentiated
carcinoma | 27 | 13 | 14 | |
| Lymphatic
invasion | | | | >0.999 |
|
Positive | 41 | 9 | 32 | |
|
Negative | 16 | 4 | 12 | |
| Venous
invasion | | | | >0.999 |
|
Positive | 36 | 8 | 28 | |
|
Negative | 21 | 5 | 16 | |
| Lymph node
metastasis | | | | 0.199 |
|
Positive | 22 | 7 | 15 | |
|
Negative | 35 | 6 | 29 | |
| Depth of
invasion | | | | 0.322 |
|
T1a | 6 | 0 | 6 | |
|
T1b | 18 | 5 | 13 | |
|
T2 | 7 | 1 | 6 | |
|
T3 | 14 | 3 | 11 | |
|
T4a | 11 | 3 | 8 | |
|
T4b | 1 | 1 | 0 | |
| Stage | | | | 0.279 |
|
I | 26 | 4 | 22 | |
|
IIA | 5 | 2 | 3 | |
|
IIB | 9 | 2 | 7 | |
|
III | 16 | 4 | 12 | |
|
IVA | 1 | 1 | 0 | |
 | Table IIIAssociation between undifferentiated
gastric carcinoma types and PPAR-α expression. |
Table III
Association between undifferentiated
gastric carcinoma types and PPAR-α expression.
| | PPAR-α
expression | |
|---|
| Histological
type | Number of
cases | (-) | (+) | P-value |
|---|
| Poorly
differentiated carcinoma | 20 | 6 | 14 |
<0.010a |
| Signet ring cell
carcinoma | 7 | 7 | 0 | |
Discussion
In the present study, the expression of PPAR-α was
investigated in 57 patients with gastric carcinoma.
Immunohistochemical staining was performed using an HRP-labeled
monoclonal anti-PPAR-α antibody to elucidate the relationship
between changes in PPAR-α expression and clinicopathological
factors. PPAR-α expression was found to be correlated with
histological type, with significantly higher expression levels
observed in differentiated carcinoma and lower expression levels in
undifferentiated carcinoma. These results provide evidence for the
development of useful molecular markers that may predict cancer
progression and outcome in patients with gastric carcinoma, as
PPAR-α expression was shown to be downregulated in undifferentiated
gastric carcinoma.
Gastric carcinoma is generally subdivided into
differentiated and undifferentiated types, with the latter mainly
including poorly differentiated carcinoma and SRCC, as defined by
the Japan Gastric Cancer Classification (21). Patients with SRCC have a higher
stage of progression and poorer prognosis compared with those with
other types of gastric carcinoma (22,23),
and poorly differentiated carcinoma has been associated with lymph
node metastasis, which carries a poor prognosis (24-26).
Poorly differentiated carcinoma and SRCC are generally considered
to have a poor prognosis and high malignant potential (27). Therefore, it is crucial to detect
undifferentiated carcinomas at an early stage and develop new
markers for histological subtypes.
The activation of PPAR-α is widely known to induce
cell metabolism, inflammation, differentiation, cell cycle arrest
and apoptosis in ovarian cancer (11), hepatocellular carcinoma (28-30),
colorectal carcinoma (31,32) and endometrial cancer (33). Furthermore, regarding the levels of
PPAR-α expression in cancer tissue, immunohistochemistry revealed
that PPAR-α expression levels were significantly low in clear cell
renal cell carcinoma specimens and were correlated with patient age
and sex, and cancer stage and grade (34). Although several studies have
examined the relationship between PPAR-α expression and cancer
outcomes (32-34),
there is currently no information on the association between PPAR-α
expression and clinicopathological factors in poorly differentiated
carcinoma and SRCC. The association between PPAR-α and gastric
cancer was also analyzed by cBioPortal (https://www.cbioportal.org), and the findings obtained
revealed that limited information is currently available on PPAR-α
and gastric cancer (data not shown). The results of the present
study demonstrated that PPAR-α expression was downregulated in
highly malignant undifferentiated carcinoma, suggesting that its
expression may serve a role in the degree of differentiation in
gastric carcinoma.
Undifferentiated carcinoma included poorly
differentiated carcinoma and SRCC in the present study. Therefore,
it was investigated whether PPAR-α expression differed between
poorly differentiated carcinoma and SRCC. A comparison between
poorly differentiated carcinoma and SRCC revealed that PPAR-α
expression was absent in SRCC (0/7, 0%), but present in poorly
differentiated carcinoma (14/20, 70%), and the difference was
statistically significant (P<0.01). As regards the expression of
PPAR-α and histology, no comparative study has been conducted to
date on the associations of PPAR-α expression with poorly
differentiated carcinoma and SRCC. However, PPAR-γ, a subtype of
PPARs, has been examined in relation to histological types
(35,36). Immunohistochemical staining for
PPAR-γ in gastric cancer tissues revealed that the frequency of
positive samples decreased as cancer transitioned from
differentiated to poorly differentiated carcinoma, and a gradual
decrease in PPAR-γ activity was found to contribute to the
histological differentiation of gastric cancer cells and tumor
progression (35). Furthermore,
the majority of SRCC samples lacked expression of PPAR-γ (37). These findings prompted us to
investigate whether PPAR-α expression is also lower in
undifferentiated compared with that in differentiated cancers. The
finding of the differential expression of PPAR-α in poorly
differentiated carcinoma and SRCC suggests similarities between
PPAR-α and PPAR-γ. Although PPAR-α has been shown to regulate lipid
energy metabolism, cancer cell differentiation and apoptosis
(38), its relationship with
differentiation, namely poorly differentiated carcinoma and SRCC,
remains unclear and requires further study.
In the present study, no significant differences
were observed in the expression of PPAR-α between normal epithelial
tissues and differentiated carcinomas, whereas its expression was
lower in the two undifferentiated, more malignant types compared
with that in the differentiated type. Since the relationship
between PPAR-α expression and histology has not yet been elucidated
in detail, further studies with a larger number of subjects are
needed to clarify the relationship between PPAR-α expression and
tumor progression and to analyze long-term clinical survival. The
relationship between PPAR-α and patient prognosis was not assessed
in this cohort as the hospital did not have post-treatment data on
the patients examined in the present study. Furthermore, no
cytology materials were available and, thus, additional experiments
could not be conducted. The findings of molecular biological
studies using cultured cells will be discussed in future studies.
In conclusion, the findings of the present study demonstrated that
the downregulated expression of PPAR-α may be involved in the
biological transformation of tumors, suggesting that PPAR-α is an
important protein associated with tumor histology and may hold
potential as a prognostic marker.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TMo and EH designed the study. TMo, YT, KK and SK
performed the experiments. TMa and EI collected the pathological
data. TMo, YT and EH analyzed all data. TMo, YT and EH wrote the
manuscript. TMo, YT, ST, HO and EH critically reviewed the
manuscript for important intellectual content. TAM, YT and EH
confirm the authenticity of the raw data. All the authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
All clinical samples were provided after obtaining
written informed consent from the patients. The present study was
conducted with the approval of the Institutional Research Ethics
Committee of the Kagawa Prefectural University of Health Sciences
(Kagawa, Japan; approval no. 215).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kepil N, Batur S and Goksel S:
Immunohistochemical and genetic features of mucinous and
signet-ring cell carcinomas of the stomach, colon and rectum: A
comparative study. Int J Clin Exp Pathol. 12:3483–3491.
2019.PubMed/NCBI
|
|
2
|
Katada T, Ishiguro H, Kuwabara Y, Kimura
M, Mitui A, Mori Y, Ogawa R, Harata K and Fujii Y: microRNA
expression profile in undifferentiated gastric cancer. Int J Oncol.
34:537–542. 2009.PubMed/NCBI
|
|
3
|
Adachi Y, Yasuda K, Inomata M, Sato K,
Shiraishi N and Kitano S: Pathology and prognosis of gastric
carcinoma: Well versus poorly differentiated type. Cancer.
89:1418–1424. 2000.PubMed/NCBI
|
|
4
|
Noda S, Soejima K and Inokuchi K:
Clinicopathological analysis of the intestinal type and diffuse
type of gastric carcinoma. Jpn J Surg. 10:277–283. 1980.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Henson DE, Dittus C, Younes M, Nguyen H
and Albores-Saavedra J: Differential trends in the intestinal and
diffuse types of gastric carcinoma in the United States, 1973-2000:
Increase in the signet ring cell type. Arch Pathol Lab Med.
128:765–770. 2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nakamura T, Yao T, Niho Y and Tsuneyoshi
M: A clinicopathological study in young patients with gastric
carcinoma. J Surg Oncol. 71:214–219. 1999.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pozos-Ochoa LI, Lino-Silva LS,
León-Takahashi AM and Salcedo-Hernández RA: Prognosis of signet
ring cell carcinoma of the colon and rectum and their distinction
of mucinous adenocarcinoma with signet ring cells. A comparative
study. Pathol Oncol Res. 24:609–616. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hyngstrom JR, Hu CY, Xing Y, You YN, Feig
BW, Skibber JM, Rodriguez-Bigas MA, Cormier JN and Chang GJ:
Clinicopathology and outcomes for mucinous and signet ring
colorectal adenocarcinoma: Analysis from the national cancer data
base. Ann Surg Oncol. 19:2814–2821. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Nolte RT, Wisely GB, Westin S, Cobb JE,
Lambert MH, Kurokawa R, Rosenfeld MG, Willson TM, Glass CK and
Milburn MV: Ligand binding and co-activator assembly of the
peroxisome proliferator-activated receptor-gamma. Nature.
395:137–143. 1998.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Pozzi A, Ibanez MR, Gatica AE, Yang S, Wei
S, Mei S, Falck JR and Capdevila JH: Peroxisomal
proliferator-activated receptor-alpha-dependent inhibition of
endothelial cell proliferation and tumorigenesis. J Biol Chem.
282:17685–17695. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yokoyama Y, Xin B, Shigeto T, Umemoto M,
Kasai-Sakamoto A, Futagami M, Tsuchida S, Al-Mulla F and Mizunuma
H: Clofibric acid, a peroxisome proliferator-activated receptor
alpha ligand, inhibits growth of human ovarian cancer. Mol Cancer
Ther. 6:1379–1386. 2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ramanan S, Kooshki M, Zhao W, Hsu FC and
Robbins ME: PPARalpha ligands inhibit radiation-induced microglial
inflammatory responses by negatively regulating NF-kappaB and AP-1
pathways. Free Radic Biol Med. 45:1695–1704. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang CY, Chao YJ, Chen YL, Wang TW, Phan
NN, Hsu HP, Shan YS and Lai MD: Upregulation of peroxisome
proliferator-activated receptor-α and the lipid metabolism pathway
promotes carcinogenesis of ampullary cancer. Int J Med Sci.
18:256–269. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Grygiel-Górniak B: Peroxisome
proliferator-activated receptors and their ligands: Nutritional and
clinical implications-a review. Nutr J. 13(17)2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pandey MK, Gupta SC, Nabavizadeh A and
Aggarwal BB: Regulation of cell signaling pathways by dietary
agents for cancer prevention and treatment. Semin Cancer Biol.
46:158–181. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Liu YL, Lin LC, Tung YT, Ho ST, Chen YL,
Lin CC and Wu JH: Rhododendron oldhamii leaf extract
improves fatty liver syndrome by increasing lipid oxidation and
decreasing the lipogenesis pathway in mice. Int J Med Sci.
14:862–870. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kliewer SA, Forman BM, Blumberg B, Ong ES,
Borgmeyer U, Mangelsdorf DJ, Umesono K and Evans RM: Differential
expression and activation of a family of murine peroxisome
proliferator-activated receptors. Proc Natl Acad Sci USA.
91:7355–7359. 1994.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Japanese Gastric Cancer Association:
Japanese Classification of Gastric Carcinoma. 15th ed. Tokyo,
Kanehara Shuppan, 2017 (In Japanese).
|
|
19
|
Tokuhara Y, Morinishi T, Matsunaga T,
Ohsaki H, Kushida Y, Haba R and Hirakawa E: Claudin-1, but not
claudin-4, exhibits differential expression patterns between well-
to moderately-differentiated and poorly-differentiated gastric
adenocarcinoma. Oncol Lett. 10:93–98. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lin MS, Huang JX, Chen WC, Zhang BF, Fang
J, Zhou Q, Hu Y and Gao HJ: Expression of PPARγ and PTEN in human
colorectal cancer: An immunohistochemical study using tissue
microarray methodology. Oncol Lett. 2:1219–1224. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Dicken BJ, Bigam DL, Cass C, Mackey JR,
Joy AA and Hamilton SM: Gastric adenocarcinoma: Review and
considerations for future directions. Ann Surg. 241:27–39.
2005.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liu X, Cai H, Sheng W, Yu L, Long Z, Shi Y
and Wang Y: Clinicopathological characteristics and survival
outcomes of primary signet ring cell carcinoma in the stomach:
Retrospective analysis of single center database. PLoS One.
10(e0144420)2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Pernot S, Voron T, Perkins G,
Lagorce-Pages C, Berger A and Taieb J: Signet-ring cell carcinoma
of the stomach: Impact on prognosis and specific therapeutic
challenge. World J Gastroenterol. 21:11428–11438. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Jinawath N, Furukawa Y, Hasegawa S, Li M,
Tsunoda T, Satoh S, Yamaguchi T, Imamura H, Inoue M, Shiozaki H and
Nakamura Y: Comparison of gene-expression profiles between diffuse-
and intestinal-type gastric cancers using a genome-wide cDNA
microarray. Oncogene. 23:6830–6844. 2004.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sipponen P: Gastric cancer: Pathogenesis,
risks, and prevention. J Gastroenterol. 37 (Suppl 13):S39–S44.
2002.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hwang CS, Ahn S, Lee BE, Lee SJ, Kim A,
Choi CI, Kim DH, Jeon TY, Kim GH, Song GA and Park DY: Risk of
lymph node metastasis in mixed-type early gastric cancer determined
by the extent of the poorly differentiated component. World J
Gastroenterol. 22:4020–4026. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chirieac LR, Swisher SG, Correa AM, Ajani
JA, Komaki RR, Rashid A, Hamilton SR and Wu TT: Signet-ring cell or
mucinous histology after preoperative chemoradiation and survival
in patients with esophageal or esophagogastric junction
adenocarcinoma. Clin Cancer Res. 11:2229–2236. 2005.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Maggiora M, Oraldi M, Muzio G and Canuto
RA: Involvement of PPARα and PPARγ in apoptosis and proliferation
of human hepatocarcinoma HepG2 cells. Cell Biochem Funct.
28:571–577. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Zhang N, Chu ES, Zhang J, Li X, Liang Q,
Chen J, Chen M, Teoh N, Farrell G, Sung JJ and Yu J: Peroxisome
proliferator activated receptor alpha inhibits hepatocarcinogenesis
through mediating NF-κB signaling pathway. Oncotarget. 5:8330–8340.
2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
You BJ, Hour MJ, Chen LY, Luo SC, Hsu PH
and Lee HZ: Fenofibrate induces human hepatoma Hep3B cells
apoptosis and necroptosis through inhibition of thioesterase domain
of fatty acid synthase. Sci Rep. 9(3306)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Gao J, Liu Q, Xu Y, Gong X, Zhang R, Zhou
C, Su Z, Jin J, Shi H, Shi J and Hou Y: PPARα induces cell
apoptosis by destructing Bcl2. Oncotarget. 6:44635–44642.
2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Morinishi T, Tokuhara Y, Ohsaki H, Ibuki
E, Kadota K and Hirakawa E: Activation and expression of peroxisome
proliferator-activated receptor alpha are associated with
tumorigenesis in colorectal carcinoma. PPAR Res.
2019(7486727)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Knapp P, Chabowski A, Błachnio-Zabielska
A, Jarząbek K and Wołczyński S: Altered peroxisome-proliferator
activated receptors expression in human endometrial cancer. PPAR
Res. 2012(471524)2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Luo Y, Chen L, Wang G, Qian G, Liu X, Xiao
Y, Wang X and Qian K: PPARα gene is a diagnostic and prognostic
biomarker in clear cell renal cell carcinoma by integrated
bioinformatics analysis. J Cancer. 10:2319–2331. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yu H and Xin Y: Down-regulated expressions
of PPARγ and its coactivator PGC-1 are related to gastric
carcinogenesis and Lauren's classification in gastric carcinoma.
Chin J Cancer Res. 25:704–714. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Theocharis S, Kanelli H, Politi E, Margeli
A, Karkandaris C, Philippides T and Koutselinis A: Expression of
peroxisome proliferator activated receptor-gamma in non-small cell
lung carcinoma: Correlation with histological type and grade. Lung
Cancer. 36:249–255. 2002.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Nomura S, Nakajima A, Ishimine S,
Matsuhashi N, Kadowaki T and Kaminishi M: Differential expression
of peroxisome proliferator-activated receptor in histologically
different human gastric cancer tissues. J Exp Clin Cancer Res.
25:443–448. 2006.PubMed/NCBI
|
|
38
|
Tan Y, Wang M, Yang K, Chi T, Liao Z and
Wei P: PPAR-α modulators as current and potential cancer
treatments. Front Oncol. 11(599995)2021.PubMed/NCBI View Article : Google Scholar
|